Abstract
Claudins are tight junction proteins with claudin-6 (CLDN6) expression mostly restricted to embryonic and fetal life. Previously reported gene expression microarray analysis showed an increased level of CLDN6 in atypical teratoid rhabdoid tumors (AT/RT) compared to other CNS tumors and sarcomas. However, there is conflicting data on expression of CLDN6 by immunohistochemistry in CNS tumors. We established membranous saining as a specific and reproducible method for evaluating CLDN6 expression based on fetal and adolescent controls. We then evaluated a large group (257) of pediatric tumors using tissue microarrays (TMA) including: 47 malignant rhabdoid tumors (MRT), (31 AT/RT and 16 non-CNS MRT); 67 small round blue cell tumors (10 Wilms tumors, 10 embryonal rhabdomyosarcomas, 10 neuroblastomas, 10 synovial sarcomas, 9 hepatoblastomas, 9 alveolar rhabdomyosarcomas and 9 Ewings sarcomas); and 143 CNS tumors (24 medulloblastomas, 21 pilocytic astrocytomas, 14 astrocytomas grade II/III, 13 gangliogliomas, 12 glioblastomas, 12 ependymal tumors, 11 choroid plexus tumors, 10 meningiomas, 8 dysembryoplastic neuroepithelial tumors, 8 oligodendrogliomas, 4 craniopharyngiomas, 2 germinomas, 2 PNET, 2 central neurocytomas). CLDN6 expression was seen in 12/31 (39%) AT/RT and 7/16 (44%) non-CNS MRT, 5/10 (50%) Wilms tumor, 1/9 (11%) hepatoblastomas, 2/2 (100%) germinomas, 1/2 (50%) CNS PNET, 1/24 (4%) medulloblastomas and 1/10 (10%) meningiomas. Ten of 11 (91%) choroid plexus tumors showed apical staining, but no concentric membranous staining. While CLDN6 is expressed in both AT/RT and MRT, it is not a specific biomarker as it is expressed in a variety of other pediatric CNS and soft tissue tumors.
Keywords: Claudin-6, malignant rhabdoid tumor, atypical teratoid rhabdoid tumor, pediatric tumor, small round blue cell tumor
Introduction
Malignant rhabdoid tumor (MRT) is a highly aggressive childhood neoplasm that occurs in the kidney, liver and soft tissue as well as the central nervous system where it is referred to as atypical teratoid/rhabdoid tumor (AT/RT). MRT is characterized by a polyphenotypic immunohistochemical profile, often expressing mesenchymal, epithelial and/or neuronal markers within the same tumor. MRT and AT/RT are associated with mutations and/or deletions of the SMARCB1 tumor suppressor gene (4,33). The loss of SMARCB1 protein expression in these tumors is associated with loss of immunohistochemical (IHC) staining in the proper context (16,18). However, with the recognition of other tumors with altered SMARCB1 protein expression by IHC, as well as rare otherwise typical MRT with retained SMARCB1 expression, there is a need for additional diagnostic biomarkers (5).
In a recent study of gene expression microarray analysis, Birks et al showed an increased level of claudin-6 (CLDN6) in AT/RT as compared to 100 CNS tumors and 15 sarcomas, suggesting a role for CLDN6 as a positive diagnostic marker for MRT in addition to SMARCB1 loss (5). Claudins are a family of transmembrane proteins that are essential to the structure and function of tight junctions. As such, they help to regulate paracellular transport as well as establish and maintain cell polarity in epithelial and endothelial cells (2,14,22). CLDN6 is one of the earliest molecules expressed in embryonic stem cells committed to epithelial differentiation and its expression is almost entirely restricted to embryonic and fetal life (1,8,12,30). As increasing research is performed on the claudin gene family, more information is being discovered on the role of claudins in the etiology of medical diseases in addition to the aggressiveness and survival of neoplasms (10,15,25).
Despite the promising gene expression data, there is conflicting data on the specificity and sensitivity of CLDN6 IHC for the diagnosis of AT/RT versus other CNS tumors (3,5). Birks et al reported positive staining in 7/7 AT/RTs and no staining in 26/27 other brain tumor samples including medulloblastomas, supratentorial primitive neuroectodermal tumors (PNET), glioblastomas and a choroid plexus carcinoma, all of which are in the differential diagnosis of AT/RT in the pediatric population. The only non-AT/RT case to demonstrate staining was a single choroid plexus papilloma. By contrast, Antonelli et al reported CLDN6 positivity in only 17/59 (29%) of AT/RT with additional staining reported in medulloblastomas, gliomas and choroid plexus papillomas. To address these conflicting results and to better evaluate the potential value of CLDN6 as a diagnostic biomarker, we characterized the expression of CLDN6 in normal human tissue, and used a tissue microarray (TMA) approach to evaluate expression of CLDN6, in a group of AT/RT and MRT, as well as in a large number of pediatric CNS tumors and non-CNS small round blue cell tumors.
Materials and Methods
Cases
This study was conducted under the auspices of the Children’s Hospital of Philadelphia (CHOP) Institutional Review Board approval for the use of human tissues. Cases used for both fetal and post-natal controls, as well as pediatric tumors were selected from the files of the Department of Pathology and Laboratory Medicine, CHOP. For CLDN6 antibody characterization and control tissues, cases of fetal and adolescent autopsies were utilized. Inclusion criteria for the fetal cases included normally formed fetuses without structural or genetic abnormalities and minimal to no maceration or tissue autolysis. For the adolescent cases, inclusion criteria were ages 13 years and older with minimal tissue autolysis. Five adolescent cases (13-19 years) and 7 fetal cases (17-22 weeks gestation) were included which met the above criteria. Whole sections of liver, lung, kidney and heart were selected from these cases for evaluation.
Pediatric tumor cases were studied using TMAs. A previously existing TMA comprised of 36 AT/RT and 16 non-CNS MRT cases was utilized (32). Three additional TMAs were constructed: one comprising 72 cases of pediatric small round blue cell tumors and two comprising 148 non-AT/RT pediatric CNS tumors. Molecular data were reviewed to confirm the pathologic diagnosis when available. The TMAs were constructed following previously described methods (34). Briefly, representative tumor areas were delineated on H&E sections and matched directly to corresponding blocks. A cylindrical portion (0.6 mm) of tumor tissue was transferred into the recipient block at specific positions to form a TMA. Evaluation of the TMA confirmed that the appearance of tumor tissue cores corresponded to the original blocks. Cores with artifact, low cellularity, cautery effect or viable tumor less than 90% per core were not analyzed. The specific distribution of cases meeting these criteria is summarized, by tumor type in Table 1. The expected pattern of SMARCB1 expression was confirmed for all TMA cases by immunohistochemical staining (16,18,20).
Table 1.
Summary of pediatric tumors studied
| Category | Total | Tumor Type | Number |
|---|---|---|---|
| Rhabdoid tumors | 47 | AT/RT | 31 |
| Non-CNS MRT | 16 | ||
| CNS tumors | 143 | Medulloblastoma | 24 |
| Pilocytic Astrocytoma | 21 | ||
| Astrocytoma (Grade II/III) | 14 | ||
| Ganglioglioma | 13 | ||
| Glioblastoma | 12 | ||
| Ependymal Tumors | 12 | ||
| Choroid Plexus Tumors | 11 | ||
| Meningioma | 10 | ||
| Dysembryoplastic neuroepithelial tumor | 8 | ||
| Oligodendroglioma | 8 | ||
| Craniopharyngioma | 4 | ||
| Germinoma | 2 | ||
| Primitive neuroectodermal tumor | 2 | ||
| Central Neurocytoma | 2 | ||
| Non-CNS tumors | 67 | Wilms Tumor | 10 |
| Neuroblastoma | 10 | ||
| Synovial Sarcoma | 10 | ||
| Embryonal rhabdomyosarcoma | 10 | ||
| Alveolar rhabdomyosarcoma | 9 | ||
| Ewings Sarcoma | 9 | ||
| Hepatoblastoma | 9 | ||
| Total 257 |
Abbreviations: atypical teratoid/rhabdoid tumor (AT/RT), central nervous system (CNS), malignant rhabdoid tumor (MRT)
Immunohistochemical staining
TMAs were heated in a 60°C oven for 5-10 minutes. CLDN6 antibody (American Research Products, Belmont, MA) was used to stain formalin fixed paraffin embedded TMA and autopsy slides in an identical manner. Slides were rinsed in 2 changes of xylene for 5 minutes each then rehydrated in a series of descending concentrations of ethanol. They were treated with 0.3% H2O2/methanol for 30min and placed in a pressure cooker with 0.01M Citrate buffer pH 7.6. After cooling, they were rinsed in 0.1M Tris Buffer then blocked with 2% fetal bovine serum for 15 minutes. Avidin/Biotin blocking was performed using a commercial blocking kit (Vector Laboratories, Burlingame, CA). Slides were then incubated with CLDN6 antibody at a 1:100 dilution overnight at 4 degrees Celsius, rinsed and then incubated with biotinylated anti-Rabbit IgG (Vector Laboratories Burlingame, CA) at a 1:200 dilution for 30 minutes at room temp. After rinsing, slides were incubated with the avidin biotin complex (Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature, rinsed and incubated with DAB (DAKO, Carpinteria, CA) for 10 minutes at room temperature. Counterstaining was performed for 1 minute in Harris Hematoxylin (Fisher Scientific, Pittsburgh, PA). Slides were then rinsed, dehydrated through a series of ascending concentrations of ethanol and xylene, then coverslipped.
Scoring
Stained slides were scanned on an Aperio OS Slide Scanner (Aperio Technologies, Vista, CA) and scored independently by two pathologists. CLDN6 staining in autopsy tissue was characterized for tissue distribution, cellular localization (cytoplasmic, membranous or both) and staining intensity on a semiquanititavite scale (negative, weak, moderate and strong). Based on this data, appropriate scoring criteria for tumor cases was determined to be percent of tumor with membranous staining (0-100%) and intensity. Tumor positivity was then defined as the presence of membranous staining identified at 10x in at least 10% of tumor cells in TMA cores.
Results
Claudin 6 in fetal and adolescent autopsy sections
Examples of CLDN6 IHC are shown in Figures 1A-D. Fetal tissues showed the expected distribution of membranous staining within developing epithelial structures and endothelial cells. Sections of liver showed moderate to strong CLDN6 positivity in developing bile ducts within the ductal plate region while bile ducts incorporated into the portal tracts were negative to weak. In the lungs, cells lining the alveolar spaces showed strong membranous staining with mild to moderate staining in more proximal bronchial structures. Sections of heart showed mild cytoplasmic staining with no membranous component in cardiomyocytes. In the kidney, the zone of nephrogenesis demonstrated moderate to strong membranous staining along a spectrum with the more primitive structures staining more intensely than the better-differentiated tubules. Endothelial cells and lymphatics in all fetal tissues sections showed varying degrees of membranous positivity. In the adolescent cases, mild to strong cytoplasmic staining was seen in peripheral nerve, macrophages, smooth muscle, cardiac muscle and skeletal muscle. Focal membranous staining was seen in endothelial cells. Based upon the control tissue evaluation, membranous staining was regarded as most specific for CLDN6. Any potential aberrant cytoplasmic expression of CLDN6 in tumors could not be differentiated from background staining and as a result was not scored.
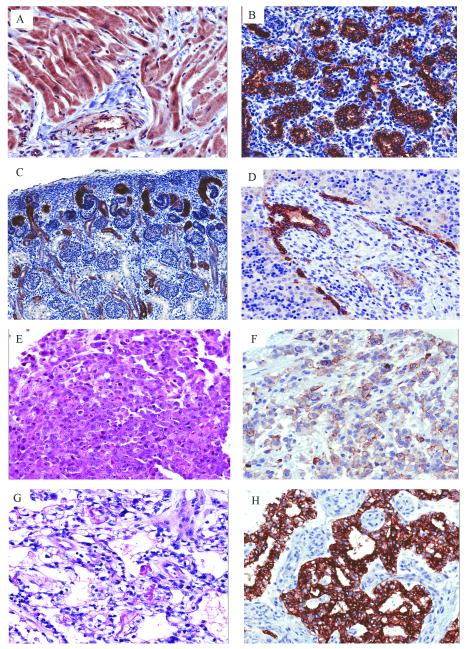
Figure 1.
Pattern of CLDN6 expression in developing human tissues and MRT: (A) Heart (14-years-old) showing diffuse cytoplasmic staining of cardiomyocytes and smooth muscle surrounding vessels (20x). (B) Lung (22 week fetus) demonstrating strong membranous staining of alveolar lining cells (20x). (C) Kidney (19 week fetus) zone of nephrogenesis showing variable membranous staining in developing tubules (10x). (D) Liver (18 week fetus) with edge of portal triad showing strong membranous staining in developing bile ducts (20x). MRT of the liver showing (E) classic rhabdoid morphology (H&E, 20x) and (F) membranous CLDN6 staining (20x). AT/RT of the cervical spinal cord with (G) prominent vacuolar cytoplasmic changes (H&E, 20x) and (H) strong diffuse membranous CLDN6 staining (20x).
Claudin 6 in tumors
CLDN6 positivity was seen in 12/31 AT/RT cases (39%) and 7/16 non-CNS MRT cases (44%). Of the positive MRT cases, the staining ranged from clusters of contiguous cells preferentially surrounding vessels and areas of necrosis to half or more of cells showing positivity (Figures 1E-H). The latter more robust staining pattern was seen in 7/12 (58%) AT/RTs and 4/7 (57%) non-CNS MRTs. The majority of CLDN6 positivity was identified in cells with rhabdoid histology. However, other tumors without classic rhabdoid morphology also demonstrated staining, as shown in Figures 2G and 2H.
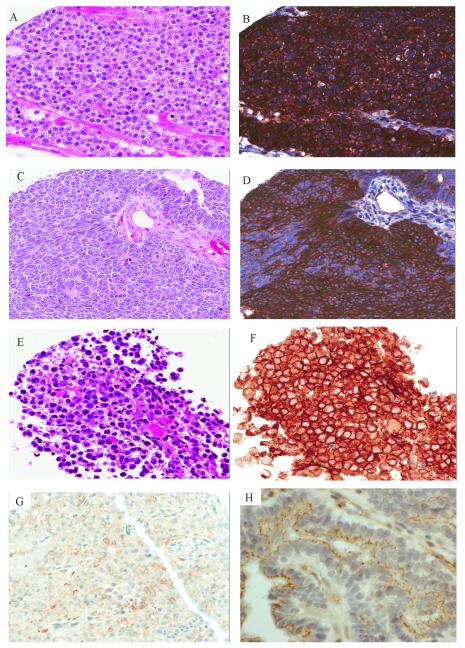
Figure 2.
Pattern of CLDN6 expression in non-MRT pediatric neoplasms: Hepatoblastoma with (A) fetal histology (H&E, 20x) and (B) strong diffuse membranous CLDN6 (20x). Wilms tumor showing (C) primitive epithelial differentiation (H&E, 20x) and (D) strong membranous CLDN6 positivity (20x). CNS germinoma with (E) classic morphology (20x, H&E) and (F) strong diffuse membranous CLDN6 staining (20x). (G) Anaplastic meningioma with focal CLDN6 staining (20X). (H) Choroid plexus papilloma showing apical CLDN6 staining simulating circumferential membranous staining in some cells cut in cross section (60x).
Results of CLDN6 staining in the non-CNS tumors are shown in Table 2. All 9 alveolar rhabdomyosarcomas (ARM), 10 embryonal rhabdomyosarcomas (ERM), 10 synovial sarcomas (SS), 10 neuroblastomas (NB) and 9 Ewing sarcomas were negative for CLDN6 staining. Eight epithelial-type hepatoblastomas (HB) were negative while a single case (11%) showed strong membranous staining in 100% of tumor cell (Figures 2A and 2B). Five of 10 Wilms tumors (50%) showed positivity restricted to the epithelial tumor component with various degrees of staining intensity both between and within the same tumor (Figures 2C and 2D). In general, the better-differentiated tubules showed less staining than the more primitive epithelial elements.
Table 2.
CLDN6 staining in Non-CNS tumors
| Tumor Type | Positive | Negative | Total |
|---|---|---|---|
| MRT | 7 | 9 | 7/16 (44%) |
| Wilms Tumor | 5 | 5 | 5/10 (50%) |
| Hepatoblastoma | 1 | 8 | 1/9 (11%) |
| ARMS | 0 | 9 | 0/9 |
| ERMS | 0 | 10 | 0/10 |
| Synovial Sarcoma | 0 | 10 | 0/10 |
| Ewings Sarcoma | 0 | 9 | 0/9 |
| Neuroblastoma | 0 | 10 | 0/10 |
Abbreviations: claudin-6 (CLDN6), central nervous system (CNS), malignant rhabdoid tumor (MRT), alveolar rhabdomyosarcoma (ARMS), embryonal rhabdomyosarcoma (ERMS)
Results of CLDN6 staining in the CNS tumors are shown in Table 3. All 21 pilocytic astrocytomas, 14 astrocytomas grade II/III, 13 gangliogliomas, 12 glioblastomas, 12 ependymal tumors, 8 dysembryoplastic neuroepithelial tumors (DNET), 8 oligodendrogliomas, 4 craniopharyngiomas and 2 central neurocytomas were negative. Strong membranous staining was encountered in all cells in 2/2 germinomas (Figures 2E and 2F). One of 2 (50%) CNS PNETs showed membranous staining in 20% of cells. Nine meningiomas, including 2 rhabdoid subtypes were negative with a single anaplastic lymphoplasmacyte-rich meningioma showing membranous staining in 15% of cells (Figure 2G). Of the 24 medulloblastomas, one case (4%) showed strong membranous staining in 60% of cells scattered throughout the tissue core. While negative by our staining criteria (expression in at least 10% of cells) an additional 3 medulloblastoma cases (12%) showed membranous staining in less than 5% of tumor cells. None of the choroid plexus tumors showed convincing concentric membranous staining. However, 10/11 (91%) had a unique pattern of positivity with staining limited to the apical pole of the cell perhaps corresponding to the basal lamina (Figure 2H), which in cross section simulated concentric membranous staining. The single case without apical or membranous staining was a choroid plexus carcinoma. Also, significant cytoplasmic positivity was seen, most commonly in reactive and neoplastic astrocytes and glial processes including Rosenthal fibers and eosinophilic granular bodies. Weak to strong membranous staining was seen in endothelial cells in a variety of tumor types.
Table 3.
CLDN6 staining in CNS tumors
| Tumor Type | Positive | Negative | Total |
|---|---|---|---|
| AT/RT | 12 | 19 | 12/31 (39%) |
| Germinoma | 2 | 0 | 2/2 (100%) |
| PNET | 1 | 1 | 1/2 (50%) |
| Meningioma | 1 | 9 | 1/10 (10%) |
| Medulloblastoma | 1 | 23 | 1/24 (4%) |
| Choroid Plexus Tumors | 0* | 11 | 0/11* |
| Pilocytic Astrocytomas | 0 | 21 | 0/21 |
| Astrocytomas (Grade II/III) | 0 | 14 | 0/14 |
| Ganglioglioma | 0 | 13 | 0/13 |
| Glioblastoma | 0 | 12 | 0/12 |
| Ependymal Tumors | 0 | 12 | 0/12 |
| DNET | 0 | 8 | 0/8 |
| Oligodendrogliomas | 0 | 8 | 0/8 |
| Craniopharyngioma | 0 | 4 | 0/4 |
| Central Neurocytoma | 0 | 2 | 0/2 |
Ten of 11 showed apical staining, see text for discussion.
Abbreviations: claudin-6 (CLDN6), central nervous system (CNS), atypical teratoid/rhabdoid tumor (AT/RT), primitive neuroectodermal tumor (PNET), dysembryoplastic neuroepithelial tumors (DNET)
Discussion
The diagnosis of MRT was revolutionized by the discovery of SMARCB1 alterations in these tumors with subsequent loss of protein expression detectable by IHC (16,18). However, it is increasingly clear that loss of SMARCB1 is not exclusively limited to rhabdoid tumor, a growing number of tumors have been described which also show loss of expression of SMARCB1 by IHC including renal medullary carcinoma, epithelioid sarcoma, epithelioid malignant peripheral nerve sheath tumor, myoepithelial carcinoma of soft tissue, pediatric undifferentiated sarcomas, some hepatobloastomas, and some schwannomas in patients with schwannomatosis (7,9,17,21,28). Conversely, rare MRT do not show loss of SMARCB1 implicating a possible second locus in the pathogenesis of these tumors (6,11). Examples of AT/RT without mutation or deletion of SMARCB1 but with abnormalities of other components of the SWI/SNF chromatin remodeling complex have recently been described (13,26). In this context, detailed characterization of both normal and neoplastic expression of CLDN6 is important to evaluate its potential utility as a diagnostic biomarker to differentiate MRT from other brain, soft tissue and renal tumors encountered in the differential diagnosis.
CLDN6 is one of the earliest molecules expressed in embryonic stem cells committed to epithelial differentiation in both murine and human tissues (15,22,29,30). However, the expression pattern of CLDN6 immunoperoxidase staining in human tissues has not previously been fully characterized. Our results confirmed that membranous expression is observed in a variety of epithelial and endothelial cells in fetal lung, liver, kidney and heart with minimal cytoplasmic staining in other fetal tissue types. The distinct membranous expression pattern in fetal tissues supports the use of this staining as the criteria for positivity with the antibody for CLDN6.
Significant cytoplasmic staining was observed in certain adolescent tissues including peripheral nerve, macrophages, smooth muscle, cardiac muscle and skeletal muscle while focal membranous staining was seen in endothelial cells. The high degree of presumed non-specific binding of antibody to intermediate filaments in muscle and macrophage debris in non-fetal tissues could not be resolved despite changes to antibody staining conditions and dilutions. Unexpectedly, some degree of membranous staining was seen in endothelial cells in adolescent tissues. Possible explanations include continued CLDN6 expression outside of fetal life or more likely cross reactivity of the CLDN6 antibody with other claudin proteins as a result of the high homology between different claudins and the polyclonal nature of the CLDN6 antibody. The main candidate for cross reactivity would be claudin-5 which is known to be abundantly expressed in endothelial cells and take part in the formation of the blood-brain barrier (23,24). This is further supported by the finding of endothelial positivity in a number of brain tumor cases.
Regardless of the explanation, it was apparent that cytoplasmic positivity was not specific for CLDN6 expression which lead us to revise previously used staining interpretation so that only the membranous component of staining was scored for our tumor types. Due to the inherent difficulty in distinguishing weak membranous from cytoplasmic background staining, a cut off at least 10% of cells showing membranous positivity at 10x magnification was established prior to evaluation of tumor samples. This method of interpretation was validated by the fact that there was 100% concordance between reviewers in determining if a case should be classified as positive or negative.
Examination of MRT showed 39% of AT/RT and 44% of the non-CNS MRT to be positive. Compared to previously evaluated AT/RT, these results are intermediate between those of Birks (100%) and Antonelli (29%). Due to the focal nature of staining in approximately half of the positive MRT cases and the use of a TMA in our study, the number of positive cases could be higher in the evaluation of whole mount sections. The similarity in percentage of positive cases for AT/RT versus other MRT suggests that overall CLDN6 expression is similar regardless of CNS versus non-CNS location. This is further supported by a recently reported case of a congenital MRT presenting in the axilla that showed CLDN6 positivity (27). No staining differences were identified between the supratentorial and infratentorial AT/RT. While the number of cases is limited, both renal rhabdoid tumors in our series were negative while all 3 of those in the liver were positive. We also compared the age at diagnosis of CLDN6 positive and negative MRT cases. While there was a correlation between positive staining with younger age at diagnosis, it was not statistically significant. Birks, et al. previously reported a lack of correlation between CLDN6 expression and survival. However, the range of treatments employed for patients in this study precluded our ability to correlate survival data with expression of CLDN6.
The majority of small round blue cell tumors evaluated were negative including all rhabdomyosarcomas, neuroblastomas, synovial sarcomas and Ewings family of tumors. These results are consistent with the CLDN6 mRNA expression data from Birks et al showing low levels in 2 Ewings sarcomas and 8 rhabdomyosarcomas. Half of the Wilms tumor cases showed positivity restricted to the epithelial component with the stromal and blastemal elements showing no membranous staining. This staining pattern is not surprising in light of the known expression of CLDN6 at early stages of epithelial differentiation and the positivity in developing kidney shown in our study.
The most robust staining within the small round blue cell tumors was observed in a single hepatoblastoma with strong, diffuse membranous staining in both tumor cores while the other 8 hepatoblastomas and hepatocytes within fetal liver cases were completely negative. All hepatoblastomas studied were of the epithelial type including fetal or fetal and embryonal, collected between 2004-2010 and received similar pre-resection chemotherapy. The positive case was from a 3-year-old girl with stage III unresectable hepatoblastoma with pure fetal histology and retained SMARCB1 expression on biopsy. Despite receiving chemotherapy including cisplatin, vincristine and 5-FU, she returned 3 months later with an increase in tumor size and extension of the tumor into the retroperitoneum. Subsequent to resection, the patient was lost to follow up; however, this was the only patient with tumor growth and extension during the course of chemotherapy.
Most, but not all of the CNS tumors evaluated show no expression of CLDN6. Conflicting results for astrocytic tumors have been reported, as Birks et al showed no staining in 3 glioblastomas and Kleinschmidt-Demasters reported no staining in 10 cases (19). On the contrary Antonelli reported staining in 1/6 low grade gliomas and 7/33 high grade gliomas. The conflicting data may result from differences in interpretation due to cytoplasmic positivity seen in some astrocytes and astrocyte processes in these tumors as well as membranous staining identified in vessels. Of the 10 meningiomas, including 2 rhabdoid meningiomas, only one anaplastic lymphoplasmacyte-rich case showed limited CLDN6 positivity.
Among non-MRT embryonal CNS tumors, we observed positive staining in 1/24 (4%) of medulloblastomas and extremely focal staining (<5% of cells) in 3 other cases while Birks et al reported no staining in 13 cases and Antonelli showed staining in 6/10 (60%). We also observed positive staining in 1/2 supratentorial PNETs. Taken together, these data support limited expression in a subset of non-MRT CNS embryonal tumors.
Choroid plexus tumors showed a unique pattern of staining limited to the apical portion of the cell in a linear pattern in 8 papillomas, 1 atypical papilloma and 1 carcinoma. The other choroid plexus carcinoma was completely negative. Immunohistochemical staining for CLDN6 by Antonelli revealed 2 positive papillomas and a negative carcinoma, while Birks showed positivity in 1/7 papillomas and a negative carcinoma. Birks et al also showed an increased level of CLDN6 mRNA expression in a choroid plexus papilloma, but not in a choroid plexus carcinoma. This data suggests that CLDN6 staining in choroid plexus tumors, although in an incompletely membranous pattern, may reflect upregulated CLDN6 protein expression, most commonly seen in lower grade tumors.
The most striking examples of CNS tumor positivity were in the 2 cases of germinoma, which showed strong diffuse membranous positivity in 100% of cells. This was an unexpected result based on previous evaluation of CLDN6 in germ cell tumors of the testis which demonstrated no positivity in 13 seminomas, but weak to moderate membranous positivity in a subset of embryonal carcinomas, teratocarcinomas, teratomas and choriocarcinomas (31). The significance of discordant expression of CLDN6 in seminomas and CNS germinomas is uncertain but warrants further evaluation.
In conclusion, based on our characterization of CLDN6 expression in non-neoplastic tissues we believe that a membranous pattern of expression is most likely to be of biological significance. While CLDN6 expression can be identified in both AT/RT and non-CNS MRT, it is of limited sensitivity, showing expression in only a subset of these tumors. CLDN6 expression is not specific to AT/RT and non-CNS MRT, and is observed both in other CNS and non-CNS pediatric tumors. In the CNS, CLDN6 was expressed in choroid plexus tumors and, less frequently, CNS PNET and meningioma. Most striking was the unexpected pattern of expression in CNS germinomas, suggesting the need for future studies to further characterize the role of CLDN6 in these tumors. Outside of the CNS, expression of CLDN6 was observed in Wilms tumor and hepatoblastoma. These results suggest that while CLDN6 expression may have potential utility when combined with other more sensitive and specific biomarkers of AT/RT and non-CNS MRT, CLDN6 is not reliable as a primary diagnostic biomarker for these tumors.
Acknowledgments
Sources of Support and Funding: This work was supported in part by a grant from the National Institutes of Health (RO146274) to J.A.B.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abuazza G, Becker A, Williams SS, et al. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol. 2006;291:F1132–41. doi: 10.1152/ajprenal.00063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–76. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonelli M, Hasselblatt M, Haberler C, et al. Claudin-6 is of Limited Sensitivity and Specificity for the Diagnosis of Atypical Teratoid/Rhabdoid Tumors. Brain Pathol. 2011 doi: 10.1111/j.1750-3639.2011.00478.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biegel JA, Zhou JY, Rorke LB, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 5.Birks DK, Kleinschmidt-Demasters BK, Donson AM, et al. Claudin 6 Is a Positive Marker for Atypical Teratoid/Rhabdoid Tumors. Brain Pathol. 2010;20:140–150. doi: 10.1111/j.1750-3639.2008.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourdeaut F, Freneaux P, Thuille B, et al. hSNF5/INI1-deficient tumours and rhabdoid tumours are convergent but not fully overlapping entities. J Pathol. 2007;211:323–330. doi: 10.1002/path.2103. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JX, Tretiakova M, Gong C, et al. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008;21:647–652. doi: 10.1038/modpathol.2008.44. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza T, Sherman-Baust CA, Poosala S, et al. Age-related changes of claudin expression in mouse liver, kidney, and pancreas. J Gerontol A Biol Sci Med Sci. 2009;64:1146–1153. doi: 10.1093/gerona/glp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton KW, Tooke LS, Wainwright LM, et al. Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer. 2011;56:7–15. doi: 10.1002/pbc.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escudero-Esparza A, Jiang WG, Martin TA. The Claudin family and its role in cancer and metastasis. Front Biosci. 2011;16:1069–1083. doi: 10.2741/3736. [DOI] [PubMed] [Google Scholar]
- 11.Fruhwald MC, Hasselblatt M, Wirth S, et al. Non-linkage of familial rhabdoid tumors to SMARCB1 implies a second locus for the rhabdoid tumor predisposition syndrome. Pediatr Blood Cancer. 2006;47:273–278. doi: 10.1002/pbc.20526. [DOI] [PubMed] [Google Scholar]
- 12.Hashizume A, Ueno T, Furuse M, et al. Expression patterns of claudin family of tight junction membrane proteins in developing mouse submandibular gland. Dev Dyn. 2004;231:425–431. doi: 10.1002/dvdy.20142. [DOI] [PubMed] [Google Scholar]
- 13.Hasselblatt M, Gesk S, Oyen F, et al. Nonsense Mutation and Inactivation of SMARCA4 (BRG1) in an Atypical Teratoid/Rhabdoid Tumor Showing Retained SMARCB1 (INI1) Expression. Am J Surg Pathol. 2011;35:933–935. doi: 10.1097/PAS.0b013e3182196a39. [DOI] [PubMed] [Google Scholar]
- 14.Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic. 2001;2:93–98. doi: 10.1034/j.1600-0854.2001.020203.x. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoot AC, Russo P, Judkins AR, et al. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol. 2004;28:1485–1491. doi: 10.1097/01.pas.0000141390.14548.34. [DOI] [PubMed] [Google Scholar]
- 17.Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542–550. doi: 10.1097/PAS.0b013e3181882c54. [DOI] [PubMed] [Google Scholar]
- 18.Judkins AR, Mauger J, Ht A, et al. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol. 2004;28:644–650. doi: 10.1097/00000478-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Kleinschmidt-Demasters BK, Alassiri AH, Birks DK, et al. Epithelioid Versus Rhabdoid Glioblastomas Are Distinguished by Monosomy 22 and Immunohistochemical Expression of INI-1 but not Claudin 6. Am J Surg Pathol. 2010;34:341–354. doi: 10.1097/PAS.0b013e3181ce107b. [DOI] [PubMed] [Google Scholar]
- 20.Kohashi K, Oda Y, Yamamoto H, et al. Reduced expression of SMARCB1/INI1 protein in synovial sarcoma. Mod Pathol. 2010;23:981–990. doi: 10.1038/modpathol.2010.71. [DOI] [PubMed] [Google Scholar]
- 21.Kreiger PA, Judkins AR, Russo PA, et al. Loss of INI1 expression defines a unique subset of pediatric undifferentiated soft tissue sarcomas. Mod Pathol. 2009;22:142–150. doi: 10.1038/modpathol.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita K, Furuse M, Fujimoto K, et al. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morita K, Sasaki H, Furuse M, et al. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawada N, Murata M, Kikuchi K, et al. Tight junctions and human diseases. Med Electron Microsc. 2003;36:147–156. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- 26.Schneppenheim R, Fruhwald MC, Gesk S, et al. Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet. 2010;86:279–284. doi: 10.1016/j.ajhg.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth G, Zraly CB, Thomson TL, et al. Congenital anomalies and rhabdoid tumor associated with 22q11 germline deletion and somatic inactivation of the SMARCB1 tumor suppressor. Genes Chromosomes Cancer. 2011;50:379–388. doi: 10.1002/gcc.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trobaugh-Lotrario AD, Tomlinson GE, Finegold MJ, et al. Small cell undifferentiated variant of hepatoblastoma: adverse clinical and molecular features similar to rhabdoid tumors. Pediatr Blood Cancer. 2009;52:328–334. doi: 10.1002/pbc.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turksen K, Troy TC. Permeability barrier dysfunction in transgenic mice overexpressing claudin 6. Development. 2002;129:1775–1784. doi: 10.1242/dev.129.7.1775. [DOI] [PubMed] [Google Scholar]
- 30.Turksen K, Troy TC. Claudin-6: a novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Dev Dyn. 2001;222:292–300. doi: 10.1002/dvdy.1174. [DOI] [PubMed] [Google Scholar]
- 31.Vare P, Soini Y. Twist is inversely associated with claudins in germ cell tumors of the testis. APMIS. 2010;118:640–647. doi: 10.1111/j.1600-0463.2010.02638.x. [DOI] [PubMed] [Google Scholar]
- 32.Venneti S, Le P, Martinez D, et al. p16INK4A and p14ARF tumor suppressor pathways are deregulated in malignant rhabdoid tumors. J Neuropathol Exp Neurol. 2011 doi: 10.1097/NEN.0b013e31822146ca. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 34.Winter C, Pawel B, Seiser E, et al. Neural cell adhesion molecule (NCAM) isoform expression is associated with neuroblastoma differentiation status. Pediatr Blood Cancer. 2008;51:10–16. doi: 10.1002/pbc.21475. [DOI] [PubMed] [Google Scholar]