Abstract
Background
Ischemia reperfusion (I/R) injury involves sterile inflammation and is commonly associated with diverse clinical situations such as hemorrhage followed by resuscitation, transient embolic events, and organ transplantation. I/R injury can induce lung dysfunction whether the I/R occurs in the lung itself or in a remote organ. Recently, evidence has emerged that receptors and pathways of the innate immune system are involved in recognizing sterile inflammation and overlap considerably with those involved in recognition and response to pathogens.
Methods
We used a mouse surgical model of transient unilateral left pulmonary artery occlusion without bronchial involvement to create ventilated lung I/R injury. Additionally, we mimicked nutritional I/R injury in vitro by transiently depriving cells of all nutrients.
Results
Compared with sham-operated mice, mice subjected to ventilated lung I/R injury had upregulated lung expression of inflammatory mediator messenger RNA for IL-1β, IL-6, and CXCL1 and 2, paralleled by histologic evidence of lung neutrophil recruitment, and increased plasma levels of IL-1β, IL-6 and HMGB1 proteins. This inflammatory response to I/R required toll-like receptor-4. Furthermore, we demonstrated in vitro cooperativity and cross-talk between macrophages and endothelial cells, resulting in augmented inflammatory responses to I/R. Remarkably, we found that selective depletion of alveolar macrophages rendered mice resistant to ventilated lung I/R injury.
Conclusions
Our data reveal that alveolar macrophages and the pattern recognition receptor, toll-like receptor-4 are required for the generation of the early inflammatory response to lung I/R injury.
Introduction
Trauma, sepsis, organ transplantation, and thromboembolic events can result in periods of diminished blood flow leading to ischemia. The restoration of blood flow (reperfusion) is often accompanied by injury to the affected organ(s) – termed ischemia reperfusion injury (I/R) – and can ultimately result in severe sterile inflammation.
The lung, as an organ, appears especially vulnerable to I/R injury perhaps due to the extent of its vascular tree and continuous physiologic demand for oxygen uptake and gas exchange. However, lung vasculature possesses unique characteristics, such as exposure to highly variable oxygen tensions and adaptive hypoxic pulmonary vasoconstriction that may make these vascular beds distinct in their response to I/R injury. Understanding the key components and processes involved in lung I/R injury could thus significantly alter how we care for mechanically ventilated patients with acute lung injury.
Toll-like receptors (TLRs) are a well-characterized group of immune receptors that recognize foreign molecules, such as those associated with pathogens1. Interestingly, in the past decade, evidence has emerged that a subset of TLRs along with other receptors can also sense cellular and tissue damage2,3. That the body can reliably distinguish between sterile and infectious stimuli using a common set of sensors reveals yet another example of the elegant economy employed by the innate arm of the immune system.
In situations of extreme cellular damage, an over-exuberant inflammatory response can result in organ injury and dysfunction, followed by a period of immune hypoactivity creating an environment where infectious pathogens can thrive4. I/R injury represents one such potentially maladaptive response of the innate immune system. Prior studies have implicated TLR4 as a key receptor involved in I/R injury in the lungs, brain, liver, and kidneys5–12 with TLR2 and TLR9 also potentially involved13,14. The NALP3 (NLRP3) inflammasome, active caspase-1, and downstream cytokines such as interleukin (IL) -1β may also mediate the response to damage markers and non-programmed cell death2,3,15,16. The final outcome of I/R injury involves the infiltration of neutrophils, whose activity causes further damage to targeted tissues9,10,12–14,17,18. The steps between the creation of the early post-I/R milieu and the later neutrophil infiltration are less well defined. Some studies have suggested that resident monocytic cell populations may be involved in mediating the response to hepatic I/R injury3,19.
In this study, we utilized a murine model of unilateral ventilated lung I/R injury in which left-sided lung blood flow, but not alveolar ventilation, is temporarily interrupted17. This model is an improvement over hilar clamping models in which interruption of ventilation and perfusion does not distinguish I/R injury from atelectatic injury. Using this model, we found TLR4 to be required for the response to lung I/R injury. Through in vitro studies, we established that interactions between endothelial cells (EC) and macrophages resulted in augmented inflammatory responses to simulated I/R conditions. This correlated with our observations that in vivo depletion of macrophages, specifically alveolar macrophages (AM), protected mice from I/R-generated lung inflammation. Our data overall indicates a critical role for AM in the response to lung I/R injury possibly through the sensing of released damage signatures by TLR4.
Materials & Methods
Animal Care
All studies were approved by the institutional animal care and use committee at University of California San Francisco. All mice were purchased (The Jackson Laboratory, Bar Harbor, ME) or bred in the animal facility at University of California San Francisco. Only male mice were used (8–16 weeks old, 20–35g) for in vivo studies. CD11c-DTR mice were a kind gift from Audrey Gerard, Max Krummel (both - Department of Pathology, University of California San Francisco, San Francisco, CA; date: May 20, 2011), Vicki Platz, and Zena Werb (both - Department of Anatomy, University of California San Francisco, San Francisco, CA; date: October 31, 2011).
Reagents
Liposome encapsulated clodronate was obtained from Dr. Nico Van Rooijen, Ph.D. (Department of Molecular Cell Biology, Free University Medical Center, Amsterdam, the Netherlands*). Cl2MDP (aka clodronate) was a gift of Roche Diagnostics GmbH (Mannheim, Germany). Diphtheria toxin (DTx) was purchased (Sigma-Aldrich, St. Louis, MO).
Cell lines
Human EC were incubated at 37°C under humidified 5% CO2. Human umbilical vein EC (HUVEC, passage 2–6) and human microvascular EC – lung (HMVEC-L, passage 2–6) were purchased (Lonza, Walkersville, MD). HUVEC were grown in EGM-2 (Lonza) and HMVEC-L in EGM-2-MV (Lonza). Endothelial growth medium was supplemented with 2% fetal calf serum. The monocytic cell line THP-1 was grown and maintained in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal calf serum, L-glutamine, and antibiotics20. THP1 monocytes were differentiated into THP1 macrophages by adding phorbyl 12-myristate 13-acetate (100 nM) to the medium for 3 days, after which the medium was replaced with medium without phorbyl 12-myristate 13-acetate and the cells allowed to rest for 5 additional days20.
In vivo mouse I/R procedure
A murine model that temporarily interrupts pulmonary arterial flow while maintaining alveolar ventilation was employed as perviously reported17. In short, mice were anesthetized with avertin (intraperitoneal), intubated, mechanically ventilated and buprenorphine was administered (intraperitoneal) for analgesia. A left thoracotomy was performed and the left pulmonary artery isolated from the left bronchus. A slip knot (8-0 prolene) placed around the pulmonary artery was used to occlude pulmonary arterial flow for time periods ranging from 30 minutes to 2 h (ischemia time). One end of the suture utilized to make the slip knot was externalized to allow the tie to be released at the end of the ischemic period. The left lung was reinflated using positive pressure, the thoracotomy closed, the mouse extubated, and allowed to recover from anesthesia. The period of reperfusion lasted 1–3 h, after which the lungs were harvested and divided for RNA preparation and hematoxylin and eosin (H&E) staining.
For the sham procedure, mice received a thoracotomy but the left pulmonary artery was not isolated. The left lung was reinflated using positive pressure, after which the thoracotomy was closed and the mouse extubated. The mouse was sacrificed after a cumulative period of 30 minutes (analogous to the ischemic period) and additional 1–3 h (analogous to the reperfusion period).
Plasma and lung collection and processing
Prior to sacrificing mice, blood was obtained when possible by cardiac puncture. Blood was collected in heparinized tubes and stored on ice prior to centrifuging at 14,000g for 10min at 4C. The plasma upper layer was carefully separated and flash frozen in liquid N2 before storing at −80C. After sacrificing the mice, lungs were removed and placed in Trizol® (Invitrogen/Life technologies, Grand Island, NY) at 4°C and rapidly transferred to −80°C for future processing and analysis. As reported previously for this surgical model, the upper segment of the left lung was excluded since the thoracotomy and left pulmonary artery isolation procedures create direct damage to this part of the left lung and this occurs both in sham and I/R surgeries but does not occur in the right lungs – specifically in the right lung sham RNA sample used as the internal standard (RQ=1) for quantitative polymerase chain reaction (qPCR) analyses17. In cases where the lower half of the left lung was collected for both RNA preparation and histology, the lowest portion/lung base (furthest away from the surgical incision) was selected for histology and the other portion for RNA preparation.
In vitro model of nutritional I/R injury
HUVEC and HMVEC-L were seeded on a 48-well plate (at 5–10,000 cells/well) and then grown either alone or with varying ratios of THP1 monocytes or freshly prepared peripheral blood mononuclear cell (PBMC) for 12–24 h in Roswell Park Memorial Institute 1640 with 10% serum at which time the HUVEC were at confluence (~20,000 cells/well). The THP-1 monocytes were grown initially separately in suspension and one day before using for experiments, 20,000 cells were added to plated HUVEC in designated wells in a 48 well dish when HUVEC approached confluence. The THP-1 macrophages, in contrast to the monocytes, attach to cell culture surfaces; so monocytes were first seeded in the densities noted (20,000 cells/well in a 48 well dish); after the 3 days of phorbyl 12-myristate 13-acetate (PMA) treatment, these differentiated macrophages attached to the plate and HUVEC were then seeded in these same wells at 2,500–5,000 cells/well; 2–3 days later, confluent HUVEC cultures were present in co-culture with the initially seeded numbers of differentiated THP-1 macrophages. For HMVEC-L/PBMC co-cultures, HMVEC-L were seeded similarly to HUVEC and grown for 48–72 h with media changed every 48 h. PBMCs were obtained from freshly drawn human blood (from healthy volunteers) using the Lymphoprep™ kit (Axis-Shield, Dundee, United Kingdom) per manufacturer’s instructions, counted and subjected to in vitro nutritional I/R as described in the following paragraph. We chose a 1:1 ratios of EC to macrophages/PBMC based on approximations of the total number of lung EC (3–5 × 106/mouse)21 and AM in mice (1–2 × 106/mouse).
In vitro nutritional I/R conditions were established by first washing the respective cells 3 times with phosphate buffered saline (PBS) or Hank’s balanced salt solution (HBSS) and then replacing the medium with PBS for 2 h. Under these conditions the THP-1 monocytes transiently adhered to the well surface. We did not include hypoxic conditions for the period of in vitro nutritional I/R injury because in our in vivo I/R model lung EC are not subjected to hypoxia given the continued ventilation of the left lung during the period of ischemia. Following the period of in vitro “ischemia”, Roswell Park Memorial Institute with 10% calf serum was added for “reperfusion” time periods noted (in the corresponding figures) and supernatants were collected at denoted times for ELISA analysis. Similar conditions have been used by Egan and colleagues on pulmonary microvascular EC to mimic in vitro “warm” ischemia reperfusion injury6.
Sandwich ELISAs
Confluent monolayers of HUVEC was grown and treated with in vitro I/R conditions as described in the previous section. Supernatant were collected after centrifugation to remove cell debris, flash frozen in liquid N2, and stored in aliquots at −80°C. Concentrations of IL-6, IL-1β, and tumor necrosis factor-alpha (TNFα) in these supernatants were detected by ELISA (Human DuoSet or Quantikine kits, R&D Systems, Minneapolis, MN). IL-1β, IL-6 and high-mobility group protein B1 (HMGB1) levels were measured from mouse plasma samples by ELISA (mouse DuoSet or Quantikine kits, R&D Systems for IL-1β and IL-6; and HMGB1 ELISA kit from IBL International GmBH, Hamburg, Germany). All assays were performed according the manufacturer’s supplied protocol. Error bars depicted are the standard deviations for the values from triplicate wells of each condition.
Flow cytometry
Lungs and spleens were digested with prewarmed collagenase solution (Worthington Biochemical Corportaion, Lakewood, NJ) and then filtered through a 0.45 µM cell filter. The cells were then washed using Flow Cytometry Staining Buffer (R&D Systems) and incubated with 10 µg of human IgG (R&D Systems) in 0.2 mL of Flow Cytometry Staining Buffer for 15 minutes at 4°C. After washing twice with Flow Cytometry Staining Buffer, the cells were incubated for 45 minutes at 4°C with primary antibodies, which included phycoerythrin (PE), fluorescein isothiocyanate (FITC), perCyP-conjugated mouse anti-human CD11b, F4/80, and CD68 (all R&D Systems; 1:10 dilution). All samples were washed two more times with Flow Cytometry Staining Buffer and then were analyzed on a BD LSRII Flow Cytometer (BD Biosciences, San Jose, CA). Data analysis was done using FloJo software (Treestar, Ashland, OR).
Real time qPCR
TaqMan specific gene primers -– Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), intercellular adhesion molecule-1 (ICAM1), IL6, IL-1β, TNFα, chemokine (C-X-C motif) ligand-1 (CXCL1), CXCL2, IL-17, interferon (IFN) beta, IFNα4, IFNα7 – and the manufacturer’s suggested assay reagents were purchased (Applied Biosystems, Foster City, CA). Lung tissue was homogenized using a Tissue Tearor™ tissue homogenizer (Biospec products, Bartlesville, OK) and messenger RNA (mRNA) isolated using Trizol® according to the manufacturer’s instructions (Invitrogen/Life technologies). mRNA concentrations were determined with a NanoDrop® (Thermo Fisher Scientific, Waltham, MA) and mRNA to complementary DNA (cDNA) conversion was performed with the “High Capacity RNA-to-cDNA Kit” using 1–2 µg of mRNA per reaction (Applied Biosystems). 50 ng of complementary DNA (cDNA) in 10 µL total reaction volume per well containing TaqManÆ Fast Advanced Master Mix (Applied Biosystems) was used in all qPCR experiments and qPCR was performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems). Run method: Polymerase chain reaction (PCR) activation at 95°C for 20 s was followed by 40 cycles of 1 s at 95°C and 20 s at 60°C. The average threshold count (Ct) value of 2–3 technical replicates was used in all calculations. The Ct value of the internal control mRNA, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to calculate ΔCt values for the array samples since this gene displayed the lowest standard deviation amongst groups compared to other housekeeping genes tested. Initial data analysis was performed using the 2−ΔΔCt method and the data was corrected for statistical analysis using log transformation, mean centering, and autoscaling as decribed by Willems et al.22,23. The method of calculation using the 2−ΔΔCt method utilized assumes an amplification efficiency of 100% between successive cycles. Relative mRNA data derived from the Ct values are expressed as mean ± SD. For all qPCR results an internal standard (right lung upper lobe sham surgery) was included for comparison from experiment to experiment and set as RQ=1.
Liposome Encapsulated Clodronate Treatment
Liposome encapsulated clodronate was stored at 4°C, and was gently resuspended and allowed to reach room temperature before using. The liposome encapsulated clodronate suspension (~7mg/ml) was administered intraperitoneally to mice at 48 h (70mg/kg), 24 h (35mg/kg), and 6 h (35mg/kg) prior to conducting the experiment/procedure of interest in order to deplete macrophage and dendritic cell populations19. Left lung lower segments were collected for RNA preparation or histology as described earlier.
CD11c-DTR Mouse Experiments
DTx (Sigma) was administered intraperitoneally (4ng/g body weight) to CD11c-DTR mice once 24 h prior to conducting the experiment/procedure of interest. Left lung lower segments were collected for RNA preparation or histology.
Microscopy
Bright field and Immunofluorescence microscopy of hematoxylin and eosin (H&E) stained and unstained paraffin mounted sections of lung was carried out using a Zeiss Axiocam™ microscope and images were collected using Zeiss Axiovision™ software (Carl Zeiss Microscopy, LLC, Thornwood, NY).
Statistical Analysis
Data are expressed as mean values ± SD. Data from in vivo studies comparing 2 conditions were analyzed using two-tailed non-parametric Mann-Whitney analyses. Data from in vitro studies comparing more than 2 conditions (time course and coculture conditions) were analyzed using two-way ANOVA with Bonferroni’s posttest to generate p values. GraphPad Prism™ was used for statistical analyses (GraphPad Software, Inc., La Jolla, CA). For all in vivo experiments, exact p values are reported and for in vitro ANOVA analyses, p values < 0.05 were considered significant and p values are represented as follows in the figures: * < 0.05; ** < 0.01; *** < 0.001. If any samples were excluded from analysis, the number of samples excluded and the reasons for exclusion are included in the figure legends for the appropriate figures.
Results
Unilateral pulmonary artery reversible ligation causes I/R injury resulting in an early induction of inflammatory markers and later influx of neutrophils
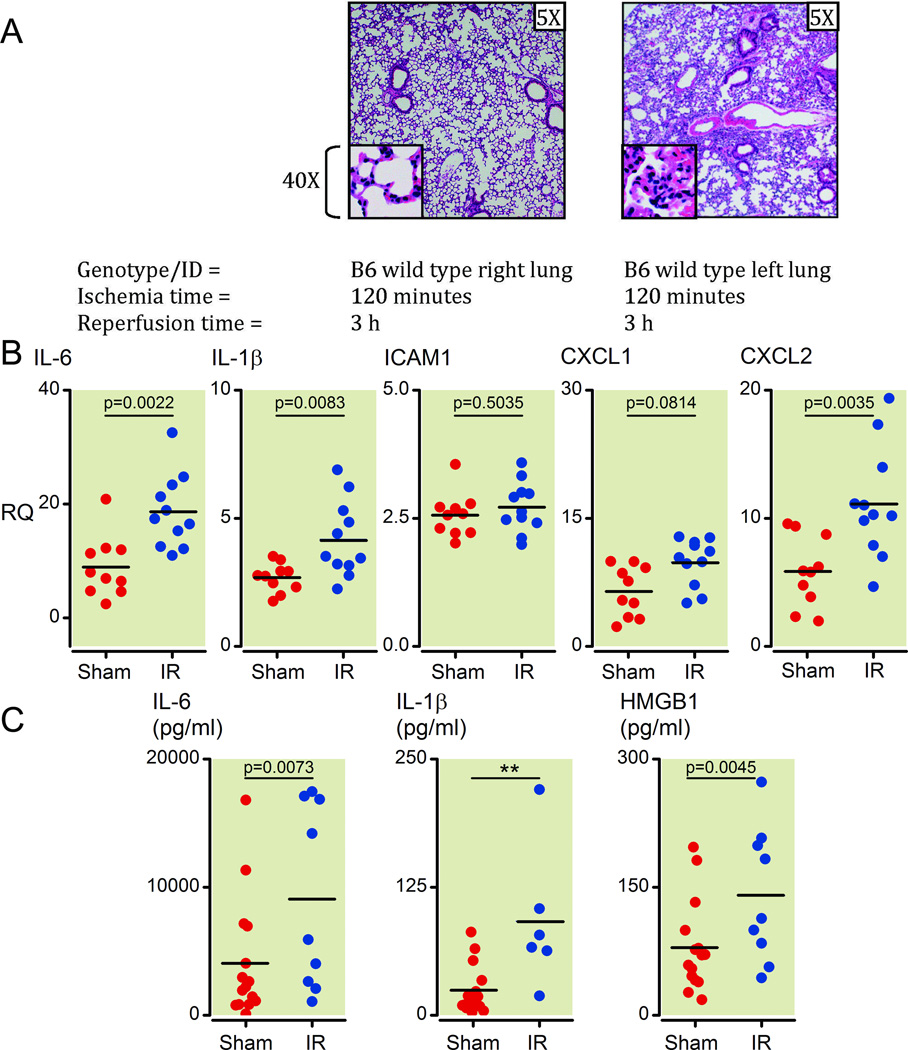
We used a previously described but technically challenging surgical method of lung ischemia reperfusion injury, that specifically and reversibly interrupts blood flow but not ventilation to the left lungs of mice17. This period of ischemia (120 minutes) was followed by reestablishment of blood flow for defined periods of reperfusion. Initially, we sought to confirm that this procedure resulted in I/R-type injury with cellular infiltration in the affected lung tissue. We observed a marked increase in infiltrating leukocytes after restoration of blood flow (3 h later) accompanied by obfuscation of the normal architecture specifically of the affected (left) lung (Figure 1A). Higher magnification (40X) identified these leukocytes to be neutrophils, which are stereotypically seen in I/R injury. The infiltration of neutrophils along with histological and gross visualization of blood accumulation in lungs post reperfusion along with previous published results confirm the absence of the ‘no-reflow’ phenomenon24 in this model of ventilated lung I/R injury17.
Figure 1. Unilateral pulmonary artery occlusion as a model of ventilated lung ischemia reperfusion (I/R) injury in mice results in inflammatory marker upregulation and neutrophil infiltration.
(A) Histology from prolonged ventilated left lung ischemia (2 h) followed by reperfusion (3 h) in the left versus right lung in C57/BL6 wild type mice (H&E staining, 5X and 40X magnifications). Each image is representative of histology images from 4 independent surgeries.
(B) Inflammatory cytokines and neutrophil-specific chemokines (mRNA) measured early (1 h) after reperfusion (Ischemia time=30 minutes). Lower segments of left lungs were collected and relative mRNA levels measured by Q-PCR. All measurements were normalized to GAPDH message levels and RQ values obtained using the relative ΔΔCt method with an independent sample (right lung sham surgery) used to set as an internal reference RQ of 1. Values from sham surgery left lungs are higher compared to sham surgery right lungs because of the injury generated during the thoracotomy procedure that affects the left and not right lungs (see materials and methods section for more details). Each point represents RNA from left lung lower segments of individual mice that underwent either sham or I/R surgery. Mice that died before completion of surgery or collection of lungs or had inadvertent esophageal intubation were excluded from analysis (2 sham and 6 I/R mice).
(C) Inflammatory cytokine and HMGB1 protein levels measured at 1 h after reperfusion (ischemia time = 30 minutes). Plasma from blood collected from mice prior to collection of lungs was analyzed by ELISA for protein levels of IL-1β, IL-6 and HMGB1. Each point represents plasma from blood collected from individual mice that underwent either sham or I/R surgery. Mice that died before completion of surgery or collection of lungs, yielded insufficient blood volumes, from whom blood was not collected or had inadvertent esophageal intubation were excluded from analysis (2 sham and 8 I/R mice).
H&E: hematoxylin and eosin; mRNA: messenger RNA; Q-PCR: real-time quantitative polymerase chain reaction; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase;
RQ: relative quantification; Ct: threshold count; HMGB1: high-mobility group protein B1; IL: interleukin; ICAM: intercellular adhesion molecule; CXCL: Chemokine (C-X-C motif) ligand.
Even a shorter ischemic time of 30 minutes was able to generate neutrophil infiltrates in the I/R-affected (left) lungs as compared to contralateral (right) lungs as well as compared to left lungs from mice that received a sham operation (data not shown, and see Supplemental Digital Content 1, figure 1A, which compares neutrophil infiltration levels by histology between sham left and I/R left lungs). We chose this shorter ischemic period for all subsequent experiments. Of note, the contralateral right lungs that did not under go I/R displayed detectable neutrophilic infiltrates consistent with the period of hyperperfusion experienced by that lung (Figure 1A and data not shown). Thus we chose not to use contralateral right lungs as our controls and instead used left lungs from mice that received a sham operation as controls for all experiments from then on.
We next analyzed the expression of specific markers of inflammation early in the reperfusion period. After 30 minutes of left pulmonary artery ligation followed by 1 h of reperfusion, lung tissue was assayed for levels of inflammatory cytokines, chemokines and adhesion molecules. We chose the 1 h reperfusion time point because we wanted to observe early changes in gene expression that were generated by I/R injury prior to the secretion of, and response to, cytokines and chemokines and prior to the entry of infiltrating neutrophils into the lung. We observed an upregulation of IL-6, IL-1β, CXCL1, and CXCL2 in the left lungs of mice following I/R relative to lungs from mice that underwent a sham procedure (Figure 1B, p = 0.0022, 0.0083, 0.0184, 0.0035, respectively). ICAM1 and vascular cell adhesion molecule-1 (VCAM1) were not induced at this early time point (Figure 1B, p = 0.5035 and data not shown). This is consistent with the reported delayed kinetics of ICAM1 induction (>1–2 h) in response to other stimulations, such as lipopolysaccharide (LPS), which requires locally or systemically secreted cytokines, like IFNγ, TNFα or IL1β (25,26 and reviewed in 27). Nonetheless, evidence of direct activation of ICAM1 has been reported in some circumstances28. Other inflammatory, immune, and neutrophil-specific cytokines and effector molecules, such as TNFα, IFNα, IFNβ, IL-10, IL-17, and inducible nitric oxide synthaste (iNOS) were not induced at this 1 h time point while chemokine (C-C motif) ligand 2 was induced (data not shown). This upregulation of message level of inflammatory cytokines was confirmed by examining plasma samples from these mice, which showed elevated IL-1β and IL-6 as well as the damage marker HMGB1 (Figure 1C, p = 0.0072, 0.0064, and 0.0045). Additionally, we confirmed that these early inflammatory processes preceeded perturbations in lung architecture with infiltration of neutrophils not seen by histology at 1 h following reperfusion (See Supplemental Digital Content 1, figure 1B, which compares histology sections of lungs that received the sham surgery or the I/R surgery 1 h after reperfusion).
We also examined traditional lung injury end points of edema fluid accumulation and found that at these early time points (1 h and 3 h), significant fluid accumulation was not observed by high resolution computed tomography scanning and wet to dry lung weight measurements (data not shown).
Toll-like receptor 4 is required for the full inflammatory response to lung I/R injury
Since both TLR4 and TLR2 have been implicated in I/R injury in various organ systems, including TLR4 in the lung specifically5–7,9–13,29–33, and TLR4 has been reported to be the receptor for HMGB134, we tested mice that were TLR4 −/− or signaling defective and TLR2 −/− in our model of surgically induced ventilated lung I/R injury.
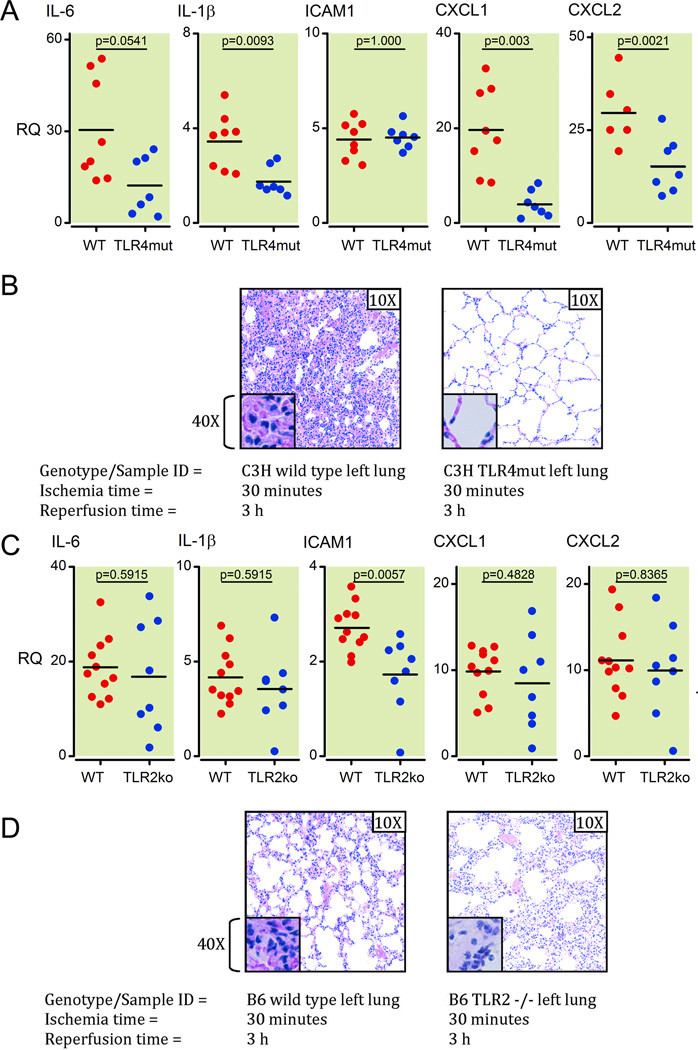
We used C3H/HeJ mice that carry a point mutation in TLR4 and do not respond to lipopolysaccharide (LPS) and analyzed their expression of specific markers of inflammation early in the reperfusion period following lung I/R surgery. We observed that 30 minutes of ischemia followed by 1 h of reperfusion in the left lung induced robust inflammatory markers in the wild type control mice (C3H/HeOuJ) but not in the C3H/HeJ TLR4 mutant mice (Figure 2A). In fact, relative levels of cytokines and chemokines detected post I/R were lower in these TLR4 mutant mice (Figure 2A; p = 0.0541 (IL-6), 0.0093 (IL-1β), 0.0003 (CXCL1) and 0.0221 (CXCL2) with ICAM1 p = 1.000). Moreover, this lack of induction of cytokines and chemokines was accompanied by an inability of the I/R-subjected TLR4 signaling defective C3H/HeJ mice to recruit neutrophils to their left lungs after 3 h of reperfusion (Figure 2B). Of note, the C3H murine background has been reported to have the most severe pathologic response to I/R injury compared to other mouse strains35 and this was reflected in our results where the C3H/HeOuJ control mice displayed greater lung neutrophil infiltrates with I/R as compared to C57BL/6 mice (Figures 2B and see Supplemental Digital Content 1, figure 1A, which compares neutrophil infiltration levels by histology between sham left and I/R left lungs)
Figure 2. TLR4 presence and signaling capability is required for full pathologic response to ventilated lung I/R injury.
(A) Inflammatory cytokines and chemokine induction (mRNA) at 1 h post reperfusion measured in lung samples from C3H TLR4mut versus wild type mice using Q-PCR. All measurements were normalized to GAPDH message levels. Each point represents RNA from left lung lower segments of individual WT or C3H TLR4mut mice that underwent I/R surgery. Mice that died before completion of surgery or collection of lungs, or had inadvertent esophageal intubation and required multiple reintubation attempts were excluded from analysis (2 WT and 1 C3H TLR4mut mouse).
(B) H&E histopathology of C3H TLR4 mutant mice and C3H wild type mice left lungs at 3 h post reperfusion after I/R surgery. Each image is representative of histology images from 3 independent surgeries.
(C) Analysis of relative mRNA levels of inflammatory markers (as noted) for left lower lung segments from C57BL/6 wild type mice versus C57BL/6 TLR2 −/− mice at 1 h post reperfusion after I/R surgery. Each point represents RNA from left lung lower segments of individual WT or TLR2ko mice that underwent I/R surgery. One wild type mouse died before completion of surgery and was excluded from analysis.
(D) Histological analysis of C57/BL6 wild type versus TLR2 −/− left lungs following 30 minutes of ischemia and 3 h of reperfusion. Each image is representative of histology images from 2 independent surgeries.
TLR: toll-like receptor; mRNA: messenger RNA; Q-PCR: real-time quantitative polymerase chain reaction; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase;
H&E: hematoxylin and eosin; I/R: ischemia-reperfusion; IL: interleukin; ICAM: intercellular adhesion molecule; CXCL: Chemokine (C-X-C motif) ligand; WT: wild type.
The role of TLR4 in mediating early periods in the response to I/R injury was confirmed by performing the I/R surgery on the C57BL/6 TLR4 −/− mouse. Compared to matched wild type mice, C57BL/6 TLR4 −/− mice (similar to the C3H/HeJ TLR4 mutant mice) did not experience neutrophil infiltration following I/R injury (See Supplemental Digital Content 1, figure 2, which compares neutrophil infiltration levels by histology between I/R left lungs of wild type and TLR4 −/− mice). Thus, TLR4 plays an important and early role in lung I/R injury arguably as a sensor of cell damage signatures.
Lung inflammation is not reduced in Toll-like receptor 2-deficient mice following lung ischemia reperfusion injury
We observed that compared to wild type (C57BL/6) mice, TLR2 −/− mice have higher baseline levels of inflammatory markers (Figure 2C). However, TLR2 −/− mice that underwent the I/R procedure did not express different levels of inflammatory markers as compared to either matched wild type controls or sham operation (Figure 2C – p values: 0.9551 (IL-6), 0.8665 (IL-1β), 0.1206 (ICAM1), 0.8665 (CXCL1), 0.9551 (CXCL2) and data not shown for sham operation). The degree of neutrophil infiltration in affected lung tissue in TLR2 −/− mice that received the I/R procedure was similar to that seen for wild type C57BL/6 mice (Figure 2D) and unlike that seen for TLR4 −/− mice on C57BL/6 background (See Supplemental Digital Content 1, figure 2, which compares neutrophil infiltration levels by histology between I/R left lungs of wild type and TLR4 −/− mice). These data suggest that TLR2 does not play a major role in the genesis of lung I/R injury, in contrast to TLR4.
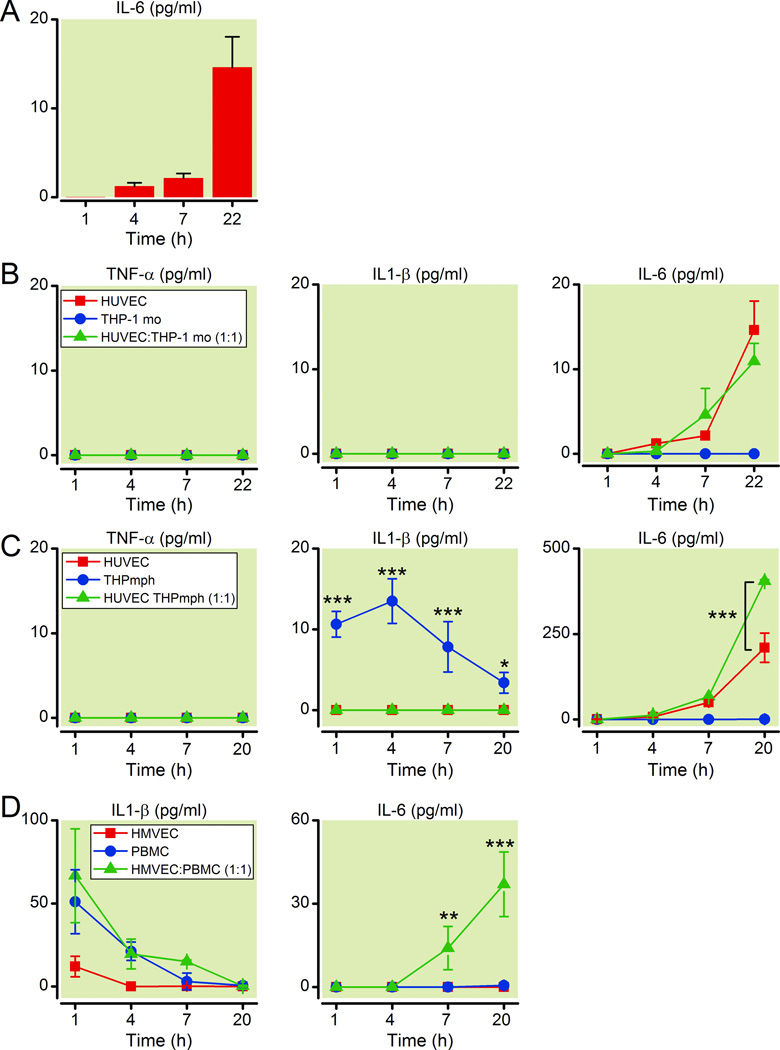
EC in vitro nutritional I/R injury induces IL-6 but not IL-1β
To identify the cell types that were responsible for the inflammatory cytokine production described above in the in vivo model of I/R injury, we simulated I/R nutritional deprivation conditions in vitro for EC in culture. Nutrients (including serum, glucose, and growth factors) were omitted from cell culture media for a fixed time period (2 h) and following replenishment with rich/complete media, cytokine production was assessed by ELISA. While this does not recreate the blood-flow dependent shear stress changes experienced by cells in in vivo I/R injury, in vitro nutritional I/R induced IL-6, but not TNFα or IL-1β protein from human EC (Figure 3A). These findings are consistent with data from our lab showing that EC are generally incapable of making TNFα or IL-1β even when exposed to bacterial pathogen-associated molecular patterns such as lipopolysaccharide (LPS) and N-Palmitoyl-S-[2, 3-bis(palmitoyloxy)-(2RS)-propyl]-[R]-cysteinyl-[S]-seryl-[S]-lysyl-[S]-lysyl- [S]-lysyl-[S]-lysine (pam3cys), which can potently induce EC production of other cytokines, including IL-6 and IL-836.
Figure 3. Generation of inflammatory cytokines from EC exposed to in vitro nutritional I/R conditions is amplified by the presence of macrophages.
(A) IL-6 protein levels were measured by ELISA at indicated time points in human umbilical vein endothelial cells (HUVEC) after they were exposed to serum-, nutrient- and glucose-free conditions for 2 h, followed by replenishment with rich complete media. Data are representative of 3 independent experiments.
(B) HUVEC were co-cultured with THP-1 human monocytes (THPmo) as noted (HUVEC or THPmo alone or HUVEC:THPmo 1:1). TNFα, IL-1β, and IL-6 protein levels were quantified from cell culture supernatants by ELISA. Data are representative of 3 independent experiments.
(C) HUVEC were co-cultured with THP-1 human differentiated macrophages (THPmph) as noted (HUVEC or THPmo alone or HUVEC:THPmo 1:1). TNFα, IL-1β, and IL-6 protein levels were assessed by ELISA from supernatants at times indicated. Data are representative of 3 independent experiments.
(D) Lung HMVEC were co-cultured with peripheral blood mononuclear cells (PBMC) as noted (HMVEC or PBMC alone or HMVEC:PBMC 1:1). IL-1β, and IL-6 protein levels were assessed by ELISA from supernatants at times indicated. Data are representative of 2 independent experiments.
EC: endothelial cells; I/R: ischemia-reperfusion; IL: interleukin; THP-1: human monocytic leukemia cell line; TNFα: tumor necrosis factor-alpha.
Differentiated macrophages and not monocytes enhance the ability of EC specifically to produce IL-6 in response to in vitro nutritional I/R
The lack of IL-1β production by EC under in vitro nutritional I/R conditions suggested that another cell type was potentially contributing to the I/R injury response in our mouse lung I/R model (Figure 1B), so we assessed the effects of co-culturing monocytes with EC on the inflammatory response to in vitro nutritional I/R injury. As seen in figure 3B, the presence of THP-1 monocytes in co-culture with EC during in vitro nutritional I/R did not induce or upregulate EC expression of TNFα, IL-1β or IL-6 (Figure 3B).
On the other hand, when THP-1 monocytes were differentiated into macrophages (THP-1 macrophages) by treatment with phorbyl 12-myristate 13-acetate (PMA), the resulting macrophages produced IL-1β (but not TNFα) when exposed to in vitro nutritional I/R. Additionally, while these macrophages did not produce IL-6 under these conditions, they enhanced the ability of EC to produce IL-6 (Figure 3C, p <0.001 at 20 h time point). The levels of IL-1β produced by THP-1 macrophages were relatively modest. However, our data suggest that even very small amounts of IL-1β, produced by 10-fold fewer THP-1 macrophages as compared to EC, (undetectable by ELISA) was sufficient to amplify EC IL-6 production (data not shown) perhaps either via cell-cell contact between EC and macrophages or an alternative mechanism by which macrophage secreted IL-1β is sequestered at the surface of EC could be important in this process. In contrast, when co-cultured under similar conditions with a lung epithelial cell line (A549), neither THP-1 monocytes nor differentiated THP-1 macrophages were capable of amplifying IL-6 production (data not shown).
To confirm that these findings applied to human lung EC and naturally occuring human macrophages, we repeated these experiments using HMVEC-L and PBMC and found that PBMC also made IL-1β early in response to in vitro nutritional I/R and their presence in co-culture with HMVEC-L significantly enhanced production of IL-6 (Figure 3D, p <0.01 for IL-1β at 1 h time point and p <0.001 at 20h).
Overall these data suggest that secreted factors, such as IL-1β, from macrophages in response to nutritional deprivation may act on EC and enhance their ability to produce IL-6 under these in vitro nutritional I/R conditions.
Macrophages are required for the generation of a full inflammatory response to I/R injury in vivo
Resident phagocytes, namely macrophages, have been reported to be involved in the initial sensing of microbial or damage signatures (reviewed in 37). Based on our in vitro data presented above, we hypothesized that lung macrophages may act as sentinels or sensor cells that initiate and/or mediate early steps in the inflammatory process generated by I/R.
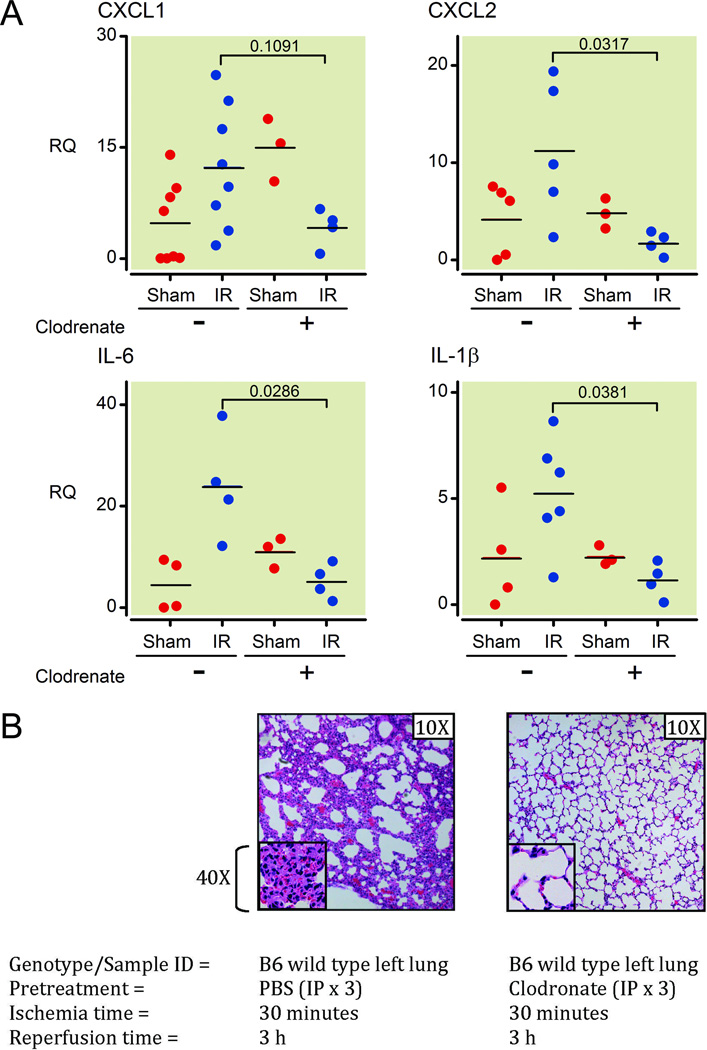
To test this hypothesis, we used liposome encapsulated clodronate to deplete macrophages in C57BL/6 mice38,39. We found that macrophage-depleted mice had reduced induction of inflammatory markers (IL-6, IL-1β, CXCL1, CXCL2: p = 0.0286, 0.0381, 0.1091, 0.0317, respectively) in lungs following the I/R procedure, when compared with mice that had not undergone macrophage depletion (Figure 4A). Furthermore and in concordance with the qPCR data, there was markedly decreased neutrophil recruitment to the left lungs of the macrophage-depleted mice after the I/R surgery (Figure 4B). Flow cytometry analysis of spleens and lungs of liposome encapsulated clodronate treated mice confirmed the depletion of CD11b+, F4/80+ and CD68+ populations (See Supplemental Digital Content 1, figure 3, which shows flow cytometry analyses of spleen and lung cell populations with or without liposome encapsulated clodronate administration). The liposome encapsulated clodronate treatment (intraperitoneally – 48 h, 24 h, and 6 h before surgery) depleted conventional macrophages (CD11b+, F4/80+) that were accessible to systemic blood circulation as well as interstitial lung macrophages, including AM (CD68+, CD11c+), that were not accessible to systemic blood circulation but did not deplete other CD11b+ cells (such as granulocytes/neutrophils/NK cells/etc.) (See Supplemental Digital Content 1, figure 3, which shows flow cytometry analyses of spleen and lung cell populations with or without liposome encapsulated clodronate administration). The depletion of AM and interstitial macrophages that were not in contact with blood flow was likely due to the depletion of precursors of these populations, which in turn resulted in a defect in repopulation of the lung macrophages and is consistent with other published data40.
Figure 4. In vivo depletion of macrophages results in protection from ventilated lung I/R injury.
(A) Mice were pretreated with clodronate liposomes (IP treatment × 3 at day -2, -1, and 12 hours before I/R surgery) or PBS and relative IL-6, IL-1β, CXCL1, and CXCL2 levels were measured by QPCR from mRNA extracted from left lower lung samples 1 h after the mice underwent the I/R procedure and ischemic period (30 minutes) was complete (normalized to GAPDH and quantified using the relative ΔΔCt method). Each point represents RNA from left lung lower segments of individual mice that underwent either sham or I/R surgery. Mice that died before completion of surgery or collection of lungs or had inadvertent esophageal intubation were excluded from analysis (2 WT mice not treated with clodronate – 1 sham and 1 I/R; and 1 clodronate treated I/R mouse).
(B) Histopathology of left lower lung segments of mice pretreated with clodronate liposomes or PBS 3 h following a 30min ischemic period. Each image is representative of histology images from 2 independent surgeries.
I/R: ischemia-reperfusion; PBS: phosphate buffered saline; IP: intraperitoneal; IL: interleukin; CXCL: Chemokine (C-X-C motif) ligand; Q-PCR: real-time quantitative polymerase chain reaction; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase;
mRNA: messenger RNA; RQ: relative quantification; WT: wild type.
Alveolar macrophages are the likely sensor cell for lung I/R injury
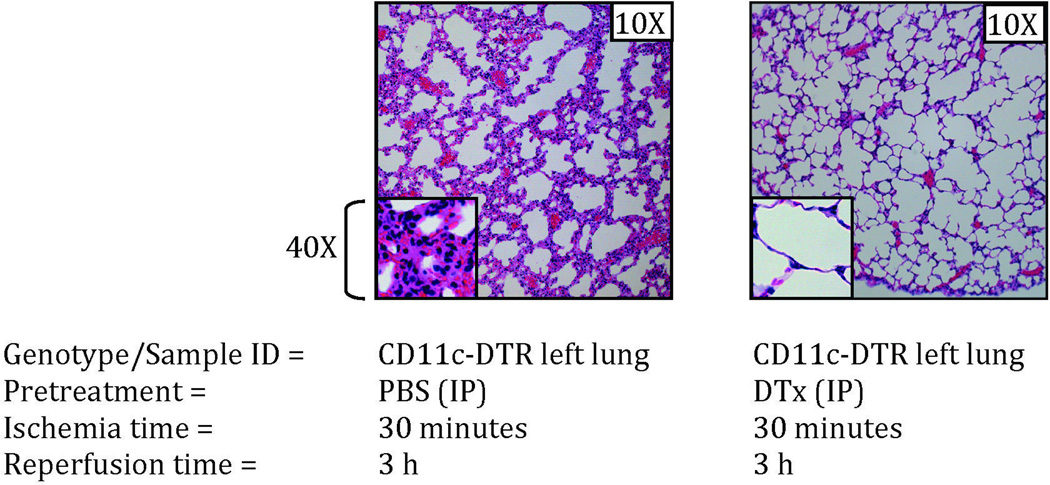
To further identify the subset of macrophages/phagocytic cells that may act as the sensor cell in the lung for sterile injury, we depleted CD11c+ AM by treating CD11c-DTR transgenic mice41 with DTx. This treatment also targets conventional dendritic cells. The absence of the AM was confirmed by fluorescent microscopy which showed the absence of large ovoid green fluorescent protein-positive cells in the lungs of mice pre-treated with DTx (See Supplemental Digital Content 1, figure 4, which shows fluorescence images of unstained lung sections from mice that either did or did not have their CD11c+ cells depleted by pretreatment with DTx intraperitoneally). The depletion of AM caused markedly diminished neutrophil trafficking to the left lung after left-sided I/R surgery (Figure 5). Our liposome encapsulated clodronate treatment of wild type mice depleted most macrophage subsets (including AM) while DTx treatment of CD11c-DTR mice depletes CD11c+ cells (including AM). Since our results demonstrate that both treatments resulted in profound protection against lung I/R injury (by lack of neutrophil infiltration), we propose AM to be involved in sensing I/R damage signals and a key component of the innate immune response to lung I/R injury.
Figure 5. CD11c+ alveolar macrophages are required for the full pathophysiologic response to ventilated lung I/R injury.
Alveolar macrophages (CD11c+) were depleted by treating transgenic CD11c-DTR mice with diphtheria toxin (DTx) (intraperitoneally at day -1) and these mice (or control mice that received PBS carrier) underwent I/R surgery and lungs were assessed for neutrophil infiltration by histopathology 3 h after reperfusion. Each image is representative of histology images from 3 independent surgeries.
I/R: ischemia-reperfusion; PBS: phosphate buffered saline; IP: intraperitoneal; DTx: diphtheria toxin.
Discussion
In patients that experience severe trauma, undergo lung transplantation, have pulmonary emboli, or have severe sepsis, lung I/R injury can be severe enough to require mechanical ventilatory support, which in turn can contribute to overall morbidity and mortality (reviewed in 42).
In this study we utilized a precise and sophisticated murine model to study isolated lung I/R injury. As opposed to the majority of published reports on lung I/R injury in which hilar clamping is employed5,6 we focus on direct lung injury from I/R alone. The contribution of atelectasis, lung collapse and mechanical ventilation to lung injury is well documented43–46 and by allowing spontaneous ventilation throughout the majority of the I/R period in our model, we are able to minimize the deletrious effects of lung collapse and mechanical ventilation. In addition we chose to study I/R injury in mice, so as to take advantage of available gene knockout and transgenic animals. Overall, we hope to understand the molecular and cellular basis of this pathophysiological response. In our mouse I/R model, we recapitulated neutrophil trafficking - a hallmark of clinical I/R injury - and this correlated well with the induction of inflammatory markers early on in the reperfusion period. IL-6 and IL-1β were found to be specifically upregulated both at the message and protein levels early in this process while TNFα, type I IFN, IL-10, IL-17, and inducible nitric oxide synthase (iNOS) were not. We observed early upregulation of specific chemokines that guide neutrophils to the site of injury (CXCL1 and 2). This appeared to be an innate immune process since IL-17, an adaptive immune T cell cytokine that results in neutrophil chemotaxis, was not induced by I/R in the lung. We were also able to detect a significant increase in HMGB1 levels in our I/R mice and this damage marker could play a key role in ventilated lung I/R injury. Future work will attempt to identify the key damage markers released and their roles in mediating I/R injury. It is likely that reactive oxygen species generation plays an important role in this process as inhibition of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase has been shown to reduce I/R injury in this model47.
Examining mice that were defective or deficient for TLR4 signaling revealed an important role for TLR4 in lung I/R injury. In contrast, TLR2 −/− mice did not demonstrate similar levels of resistance to I/R injury. Our results are consistent with other published data that implicate TLR4 as a key receptor in lung I/R injury. However, these prior studies employed hilar clamping and thus did not focus on I/R injury alone because the atelectasis associated with occluding the left main bronchus along with the left pulmonary artery likely generated an additional source of inflammation which could also be TLR4-dependent5,6. We focused specifically on ventilated I/R injury and showed that TLR4 signaling was involved early in lung I/R injury. We hypothesize that TLR4 directly or indirectly senses damage patterns released by EC in reperfused vasculature.
EC express TLR4 and TLR2. Specifically, EC can respond to both TLR4 and TLR2 ligands to produce IL-6. As demonstrated by our in vitro data, simulated nutritional I/R injury in the absence of shear stress changes that accompany flow alterations generated EC IL-6, but not IL-1β production soon after reperfusion (Figure 3). The lack of IL-1β production by EC led us to create co-culture systems of EC with macrophages to identify the cell type producing IL-1β. We hypothesized that IL-1β made by macrophages acted on EC, amplifying IL-6 produciton under nutritional I/R conditions (Figure 3C and 3D). In studies examining EC responses to lipopolysaccharide (LPS) in the presence of PBMC monocytes, Sabroe and colleagues showed that IL-1β production fed back on EC to augment inflammatory cytokine production48. We suggest that this paradigm of monocytic cell types serving as sensors/amplifiers of pathogen-mediated inflammatory responses may apply to conditions of sterile inflammation in the presence of damage markers.
Macrophages have been implicated in some I/R injury models of the heart, liver, and even lung (in the setting of lung transplantation)19,49–53. To assess the in vivo role of macrophages in the lung I/R mediated inflammatory process, we used liposome encapsulated clodronate to deplete all macrophage cells in the mouse38 and demonstrated a failure in neutrophil recruitment following lung I/R. Liposome encapsulated clodronate treatment eliminates macrophage populations accessible to the blood stream (splenic macrophages, for example) and can also eliminate resident tissue macrophage populations (such as AM and interstitial lung macrophages) by targeting circulating blood monocyte precursors38,40,54,55.
AM are a major subset of macrophages that reside in the lung. AM make up 80+% of phagocytic cells in the alveoli and are important for responding to inhaled particles and pathogens56. In this study, we show that AM play a key role in the response to lung I/R injury. AM depletion with DTx in CD11c-DTR mice resulted in a near-complete absence of neutrophil trafficking to the lung following I/R (Figure 5). Our data suggest that these cells, which do not reside inside the pulmonary vasculature, may participate in the sensing of I/R and communicate with EC leading to the production of inflammatory mediators. Although lung EC and AM may not physically be in contact with each other, their close proximity would allow them to communicate via secreted factors such as IL-1β. Furthermore, AM may perform this I/R sensing role possibly through TLR4-mediated uptake of markers released by I/R-damaged EC or epithelial cells that diffuse into the alveoli.
While reports from others have suggested a role for monocytic cells in the process of neutrophil infiltration in a murine lung transplant model19, our data using a more focused and precise lung I/R mouse model show that a specific subset – AM – likely act as the primary sensor cell type that responds to I/R injury. One group reported results contradictary to ours with worsening of lung I/R injury with AM depletion57. However, their rat model involved ex vivo mechanical perfusion of isolated lungs and measurement of intrapulmonary neutrophil accumulation. In contrast, in our model we did not observe neutrophil trafficking at their early (1 h) reperfusion times (See Supplemental Digital Content 1, figure 1B, which examines histological lung sections 1 h after reperfusion).
Two other groups demonstrated a protective effect of AM-depletion in lung I/R injury. The first also used an ex-vivo model to study the role of AM in lung I/R injury58. Mice were ex-sanguinated and reperfused with a buffered solution to mimic mixed venous blood. The second used hilar clamping in a rat model of non-ventilated I/R injury59. We believe that our in vivo model better and more closely replicates clinical I/R scenarios. Furthermore, having validated this model in mice, we can further identify and dissect the key pathways important in lung I/R injury using available genetic knockouts and transgenic mice. Through in vivo cell depletion and reconstitution experiments, future experiments can examine the role of AM and specific signaling pathways within AM in this physiologically relevant model of ventilated lung I/R.
Two of the studies referred to previously also used liposome encapsulated clodronate to deplete AM and examined lung vascular permeability58,59. However, liposome encapsulated clodronate may affect lung vascular permeability independent of other treatments or procedures perhaps making this AM-depletion method unsuitable for examining changes in vascular permeability.
AM frequency in the lung, their location close to the vasculature, and reported functionality in consuming dead cells and debris, arguably make them ideally suited to perform the role of sterile damage or I/R sensor. However, some published reports have characterized AM as being immunosuppressive rather than proinflammatory54,57,60. Experiments are currently ongoing to directly address whether alveolar macrophages are necessary and sufficient for the generation of a full I/R inflammatory response. However, at this time we cannot formally rule out the possibility that another phagocytic CD11c+ population, such as a dendritic cell population, may contribute to initiating the response to lung I/R injury. It is also entirely possible that both dendritic cells and AM are immunomodulatory in distinct ways depending on the type of sterile insult, the branch of immune system activated (adaptive versus innate), and the co-presence of pathogen.
In summary, this study employs a murine model of lung injury that does not involve airway collapse and specifically focuses on ventilated I/R by isolating the blood flow to a single lung. The data provide compelling evidence that AM serve the role of sensing and/or amplifying lung I/R injury and that TLR4 plays a critical part in this process. Manipulating the activity and presence of TLR4 and AM could thus potentially permit control of the clinical response to lung I/R injury. In the future, one could envision this role of manipulating or modulating the immune system and its inflammatory responses falling to the anesthesiologist or perioperative physician in rapidly evolving clinical situations encountered in the operating theater and intensive care unit.
Supplementary Material
Summary Statement.
What We Already Know about This Topic
Ischemia-reperfusion of the lung can induce sterile inflammation in the lung
What This Article Tells Us That Is New
Mouse experiments were performed where unilateral pulmonary artery occlusion to generate ventilated lunch ischemia-reperfusion produced inflammation that required toll-like receptor 4 and the presence of macrophages
Acknowlegements
We would like to thank Clifford Lowell, M.D., Ph.D. (Professor and Chair, Department of Laboratory Medicine, University of California San Francisco), Michael Matthay, M.D. (Professor of Medicine and Anesthesia, University of California San Francisco), Mervyn Maze, MB, ChB (Professor and Chair, Department of Anesthesia and Perioperative Care, University of California San Francisco), Priya Budde, Ph.D. (Brookline, MA), Ruby Hsu, Ph.D. (San Francisco, CA), and Marta Sabbadini, Ph.D. (San Francisco, CA) for helpful discussions, guidance, and editorial assistance with the manuscript.
Funding Sources: Support was provided solely from institutional and/or departmental sources. A.P. is supported by a Severinghaus Award (Department of Anesthesia and Perioperative Care, University of California San Francisco) and by the Department of Anesthesia, San Francisco General Hospital, San Francisco, CA. J.H. is supported by the National Institute of Allergy and Infectious Diseases (R01AI058106), Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Department to which work is to be attributed: Department of Anesthesia and Perioperative Care, San Franciso General Hospital, University of California, San Francisco.
Meetings at which this work was partially presented: Thirty-fourth Annual Conference on Shock, Norfolk, VA – June 11–14, 2011.
clodronateliposomes.org (last accessed April 18, 2012)
References
- 1.Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 2.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–42. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 4.Adib-Conquy M, Asehnoune K, Moine P, Cavaillon JM. Long-term-impaired expression of nuclear factor-kappa B and I kappa B alpha in peripheral blood mononuclear cells of trauma patients. J Leukoc Biol. 2001;70:30–38. [PubMed] [Google Scholar]
- 5.Shimamoto A, Pohlman TH, Shomura S, Tarukawa T, Takao M, Shimpo H. Toll-like receptor 4 mediates lung ischemia-reperfusion injury. Ann Thorac Surg. 2006;82:2017–2023. doi: 10.1016/j.athoracsur.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 6.Zanotti G, Casiraghi M, Abano JB, Tatreau JR, Sevala M, Berlin H, Smyth S, Funkhouser WK, Burridge K, Randell SH, Egan TM. Novel critical role of Toll-like receptor 4 in lung ischemia-reperfusion injury and edema. Am J Physiol Lung Cell Mol Physiol. 2009;297:L52–L63. doi: 10.1152/ajplung.90406.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q, Lin S, Xiang J, Li JC, Fang CQ, Wang JZ. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab. 2011;31:593–605. doi: 10.1038/jcbfm.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai Y, Qiao B, Shen XD, Gao F, Busuttil RW, Cheng G, Platt JL, Volk HD, Kupiec-Weglinski JW. Evidence for the pivotal role of endogenous toll-like receptor 4 ligands in liver ischemia and reperfusion injury. Transplantation. 2008;85:1016–1022. doi: 10.1097/TP.0b013e3181684248. [DOI] [PubMed] [Google Scholar]
- 10.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 11.Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodd-o JM, Hristopoulos ML, Faraday N, Pearse DB. Effect of ischemia and reperfusion without airway occlusion on vascular barrier function in the in vivo mouse lung. J Appl Physiol. 2003;95:1971–1978. doi: 10.1152/japplphysiol.00456.2003. [DOI] [PubMed] [Google Scholar]
- 18.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: A brief review. Mol Med. 2008;14:337–345. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong QG, Bernasconi S, Lostaglio S, De Calmanovici RW, Martin-Padura I, Breviario F, Garlanda C, Ramponi S, Mantovani A, Vecchi A. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol. 1997;17:1599–1604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Willems E, Leyns L, Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal Biochem. 2008;379:127–129. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Lee KW, Norell MS. Management of 'no-reflow' complicating reperfusion therapy. Acute Card Care. 2008;10:5–14. doi: 10.1080/17482940701744318. [DOI] [PubMed] [Google Scholar]
- 25.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: Tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 26.Sawa Y, Ueki T, Hata M, Iwasawa K, Tsuruga E, Kojima H, Ishikawa H, Yoshida S. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J Histochem Cytochem. 2008;56:97–109. doi: 10.1369/jhc.7A7299.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long EO. ICAM-1: Getting a grip on leukocyte adhesion. J Immunol. 2011;186:5021–5023. doi: 10.4049/jimmunol.1100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen JSSTT, Schrieber L, King NJ. Early E-selectin, VCAM-1, ICAM-1, and late major histocompatibility complex antigen induction on human endothelial cells by flavivirus and comodulation of adhesion molecule expression by immune cytokines. J Virol. 1997;71:9323–9332. doi: 10.1128/jvi.71.12.9323-9332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Xiang M, Yuan Y, Xiao G, Zhang J, Jiang Y, Vodovotz Y, Billiar TR, Wilson MA, Fan J. Hemorrhagic shock augments lung endothelial cell activation: Role of temporal alterations of TLR4 and TLR2. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1670–R1680. doi: 10.1152/ajpregu.00445.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004;128:170–179. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 32.Mollen KP, Levy RM, Prince JM, Hoffman RA, Scott MJ, Kaczorowski DJ, Vallabhaneni R, Vodovotz Y, Billiar TR. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83:80–88. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- 33.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–I274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodd-o JM, Hristopoulos ML, Welsh-Servinsky LE, Tankersley CG, Pearse DB. Strain-specific differences in sensitivity to ischemia-reperfusion lung injury in mice. J Appl Physiol. 2006;100:1590–1595. doi: 10.1152/japplphysiol.00681.2005. [DOI] [PubMed] [Google Scholar]
- 36.Wilhelmsen K, Mesa KR, Prakash A, Xu F, Hellman J. Activation of endothelial TLR2 by bacterial lipoprotein upregulates proteins specific for the neutrophil response. Innate Immun. 2011 Dec 20; doi: 10.1177/1753425911429336. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 38.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 39.van Rooijen N, van Kesteren-Hendrikx E. "In vivo" depletion of macrophages by liposome-mediated "suicide". Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 40.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 41.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299:H1283–H1299. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 43.Featherstone RL, Chambers DJ, Kelly FJ. Ischemic preconditioning enhances recovery of isolated rat lungs after hypothermic preservation. Ann Thorac Surg. 2000;69:237–242. doi: 10.1016/s0003-4975(99)01134-0. [DOI] [PubMed] [Google Scholar]
- 44.Dodd OJ, Hristopoulos M, Scharfstein D, Brower R, Hassoun P, King LS, Becker P, Liu M, Wang W, Hassoun HT, Rabb H. Interactive effects of mechanical ventilation and kidney health on lung function in an in vivo mouse model. Am J Physiol Lung Cell Mol Physiol. 2009;296:L3–L11. doi: 10.1152/ajplung.00030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kao SJ, Wang D, Yeh DY, Hsu K, Hsu YH, Chen HI. Static inflation attenuates ischemia/reperfusion injury in an isolated rat lung in situ. Chest. 2004;126:552–558. doi: 10.1378/chest.126.2.552. [DOI] [PubMed] [Google Scholar]
- 46.Lamarche Y, Gagnon J, Malo O, Blaise G, Carrier M, Perrault LP. Ventilation prevents pulmonary endothelial dysfunction and improves oxygenation after cardiopulmonary bypass without aortic cross-clamping. Eur J Cardiothorac Surg. 2004;26:554–563. doi: 10.1016/j.ejcts.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Dodd OJ, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol Heart Circ Physiol. 2000;279:H303–H312. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- 48.Ward JR, Francis SE, Marsden L, Suddason T, Lord GM, Dower SK, Crossman DC, Sabroe I. A central role for monocytes in Toll-like receptor-mediated activation of the vasculature. Immunology. 2009;128:58–68. doi: 10.1111/j.1365-2567.2009.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bremer C, Bradford BU, Hunt KJ, Knecht KT, Connor HD, Mason RP, Thurman RG. Role of Kupffer cells in the pathogenesis of hepatic reperfusion injury. Am J Physiol. 1994;267:G630–G636. doi: 10.1152/ajpgi.1994.267.4.G630. [DOI] [PubMed] [Google Scholar]
- 50.Shibuya H, Ohkohchi N, Seya K, Satomi S. Kupffer cells generate superoxide anions and modulate reperfusion injury in rat livers after cold preservation. Hepatology. 1997;25:356–360. doi: 10.1053/jhep.1997.v25.pm0009021947. [DOI] [PubMed] [Google Scholar]
- 51.Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S. Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Lab Invest. 2000;80:1127–1136. doi: 10.1038/labinvest.3780119. [DOI] [PubMed] [Google Scholar]
- 52.Fiser SM, Tribble CG, Long SM, Kaza AK, Cope JT, Laubach VE, Kern JA, Kron IL. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121:1069–1075. doi: 10.1067/mtc.2001.113603. [DOI] [PubMed] [Google Scholar]
- 53.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Kron IL. Pulmonary macrophages are involved in reperfusion injury after lung transplantation. Ann Thorac Surg. 2001;71:1134–1138. doi: 10.1016/s0003-4975(01)02407-9. discussion 1138–9. [DOI] [PubMed] [Google Scholar]
- 54.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thepen T, McMenamin C, Oliver J, Kraal G, Holt PG. Regulation of immune response to inhaled antigen by alveolar macrophages: Differential effects of in vivo alveolar macrophage elimination on the induction of tolerance vs. immunity. Eur J Immunol. 1991;21:2845–2850. doi: 10.1002/eji.1830211128. [DOI] [PubMed] [Google Scholar]
- 56.MacLean JA, Xia W, Pinto CE, Zhao L, Liu HW, Kradin RL. Sequestration of inhaled particulate antigens by lung phagocytes. A mechanism for the effective inhibition of pulmonary cell-mediated immunity. Am J Pathol. 1996;148:657–666. [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura T, Abu-Dahab R, Menger MD, Schafer U, Vollmar B, Wada H, Lehr CM, Schafers HJ. Depletion of alveolar macrophages by clodronate-liposomes aggravates ischemia-reperfusion injury of the lung. J Heart Lung Transplant. 2005;24:38–45. doi: 10.1016/j.healun.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, Kron IL, Laubach VE. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1018–L1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 59.Naidu BV, Krishnadasan B, Farivar AS, Woolley SM, Thomas R, Van Rooijen N, Verrier ED, Mulligan MS. Early activation of the alveolar macrophage is critical to the development of lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2003;126:200–207. doi: 10.1016/s0022-5223(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 60.Bilyk N, Holt PG. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993;177:1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.