Abstract
Background
To evaluate whether medullary breast cancer has a better prognosis compared with invasive ductal tumors.
Methods
Among 12 409 patients, 127 were recorded as invasive medullary tumors and 8096 invasive ductal tumors. Medullary and ductal invasive tumors were compared with regard to stage, age at diagnosis, grade, hormone receptor status, peritumoral vascular invasion, and local and systemic treatment. Pattern of relapse, distant recurrence-free interval (DRFI), and overall survival (OS) were determined for both histological groups. Two cohorts were investigated: a full cohort including the pathologist-determined medullary histology without regard to any other tumor features and a cohort restricted to patients with ER-negative grade 3 tumors.
Results
Fourteen-year DRFI and OS percents for medullary tumors (n = 127) and invasive ductal tumors (n = 8096) of the full cohort were 76% and 64% [hazard ratio (HR) 0.52, P = 0.0005] and 66% and 57% (HR = 0.75, P = 0.03), respectively. For the restricted cohort, 14-year DRFI and OS percents for the medullary (n = 47) and invasive ductal tumors (n = 1407) were 89% and 63% (HR 0.24, P = 0.002) and 74% and 54% (HR = 0.55, P = 0.01), respectively. Competing risk analysis for DRFI favored medullary tumors (HR medullary/ductal = 0.32; 95% confidence interval = 0.13–0.78, P = 0.01).
Conclusion
Medullary tumors have a favorable prognosis compared with invasive ductal tumors.
Keywords: breast cancer, histology, invasive ductal, medullary, prognosis
introduction
Medullary carcinomas are rare breast tumors that account for <5% of invasive breast carcinomas [1–3]. The diagnosis of medullary carcinoma is usually defined by histologic diagnostic criteria proposed by Ridolfi et al. [4]. These histopathologic features include: lymphoplasmacytic infiltration, noninvasive microscopic circumscription, syncytial growth pattern >75%, and grade 2 or 3 nuclei. Despite these well-defined morphological features, medullary tumor diagnoses have poor reproducibility. Although several simplified classifications of a medullary phenotype have been proposed in order to increase reproducibility, the Ridolfi criteria remain the most generally accepted [5, 6]. Results of gene expression profiling show that medullary carcinoma may be a subtype of basal breast cancers, and a more modern definition would consider immunohistochemical results indicating negative estrogen, progesterone, and HER2 receptors. However, positive estrogen receptors (ER) and progesterone receptors (PgR) have been reported in up to 30%–40% of cases, and HER2 overexpression in ∼10% of tumors diagnosed as medullary subtype [7–14], leaving the diagnosis of medullary breast cancer an area of controversy.
Data on the prognosis of medullary breast cancer are also conflicting. Some studies have indicated that this histologic type is associated with a favorable prognosis despite its association with biological features, which usually characterize a more aggressive subtype [2, 4, 15–18]. Other studies do not confirm this observation and some report survival rates similar to the invasive ductal type ‘not otherwise specified’ (NOS) [19–22].
In order to clarify the prognosis of patients diagnosed with medullary breast carcinoma, we compared the clinicopathological features and outcomes of patients diagnosed with medullary carcinoma with those having invasive ductal tumors NOS. Data were obtained from 13 International Breast Cancer Study Group (IBCSG) trials conducted from 1978 through 1999. Recognizing the changing criteria over time, we defined two cohorts. The ‘full cohort’ includes the pathologist-determined histology without regard to any other tumor features, and the ‘restricted cohort’ is a more pure classification restricted to patients with ER-negative grade 3 tumors. In studies describing both ER and grade in medullary subtypes, all, or at least the vast majority, of the tumors were characterized as ER negative and of poor grade [9, 13, 23, 24]. Thus, we considered the classification using these additional features as more appropriate. We further limited the description of our restricted cohort according to ER status, since almost all ER-negative tumors are without PgR expression and PgR expression has only been described in a subset of reports [25]. In order to have the proper comparator group, we matched the full cohort to all invasive ductal tumors and the restricted cohort to the ER-negative, poor grade invasive ductal tumors. In each cohort, we identified patients as having tumor histology of medullary breast carcinoma or invasive ductal carcinoma NOS.
patients and methods
patients
Among the 12 409 patients enrolled in 13 IBCSG trials (conducted from 1978 to 1999) [26–38], 127 were recorded as having medullary invasive tumors, 8096 invasive ductal tumors NOS, and 4186 other tumor types (including atypical medullary) (supplemental Table S1, available at Annals of Oncology online; Table 1). All 13 trials included patients with early breast cancer and studied the timing and duration of chemoendocrine treatments. Histology was determined by central pathology review of submitted hematoxylin- and eosin-stained slides in 11 of the 13 trials (trials I–V, VIII, IX, 11–14) and by local review for the remaining two (trials VI and VII, n = 2687) where central review was not available. The ‘full cohort’ includes all patients recorded as either having medullary or ductal NOS invasive tumors. The ‘restricted cohort’ is a subset of the full cohort restricted to those with grade 3 and ER-negative tumors: 47 patients with medullary invasive tumors and 1407 with invasive ductal NOS tumors (supplemental Figure S1, available at Annals of Oncology online).
Table 1.
Incidence of medullary and invasive ductal carcinomas among the 13 International Breast Cancer Study Group trials analyzed
| All patients (full cohort) |
Patients with grade 3 and estrogen receptor-negative tumors (restricted cohort) |
|||
|---|---|---|---|---|
| Medullary | Ductal | Medullary | Ductal | |
| Total patients (% total trial accrual) | 127 (1) | 8096 (65) | 47 (0.4) | 1407 (11) |
| Trials with central pathology review | ||||
| I | 13 | 306 | 5 | 34 |
| II | 4 | 216 | 0 | 29 |
| III | 19 | 289 | 3 | 24 |
| IV | 8 | 206 | 3 | 10 |
| V | 18 | 1701 | 11 | 350 |
| VIII | 8 | 693 | 6 | 135 |
| IX | 5 | 1065 | 5 | 178 |
| 11 | 1 | 98 | 0 | 1 |
| 12 | 0 | 277 | 0 | 2 |
| 13 | 0 | 799 | 0 | 247 |
| 14 | 3 | 622 | 3 | 198 |
| Trials with local pathology review | ||||
| VI | 26 | 991 | 7 | 120 |
| VII | 22 | 833 | 4 | 79 |
Patients with medullary and invasive ductal tumors were compared within the two defined cohorts with regard to age at diagnosis, menopausal status, local and systemic treatment, nodal status, tumor size, peritumoral vessel invasion (PVI), grade, and hormone receptor status.
The trials were conducted according to good clinical practice and in accordance with human investigation laws in the participating countries at the time of patient enrollment.
statistical methods
For the 13 trials, the protocol-defined primary end point was disease-free survival (DFS), defined as the time from randomization to the first occurrence of a breast event (local, regional, distant recurrence; contralateral breast event), a second (non-breast) malignancy, or a death before a cancer event. For this report with long-term follow-up, the more relevant end points used were distant recurrence-free interval (DRFI) and overall survival (OS). DRFI was defined as the time from randomization to first distant recurrence. Local and regional recurrences, contralateral breast and second non-breast events were ignored and follow-up continued until the first distant recurrence. Deaths without distant recurrences were censored. OS was defined as the time from randomization to death. DFRI and OS were presented using Kaplan–Meier curves. Log-rank P values of DFRI and OS were stratified by pathologist. There were four strata: one for each of the three central laboratories (the central laboratory changed three times over the 20-year period covered by these trials) and one strata for trials VI and VII (trials without central pathologic review). Competing risk regression models [39] were used to account for the competing risk of distant breast cancer events with other DFS events (i.e. local and regional recurrences, contralateral breast cancers, second non-breast malignancies, and deaths without recurrence). The competing risk multivariate models included covariates for ER status, grade, nodal status, and tumor size. Patient and tumor characteristics were compared according to tumor type using the Fisher's exact test. No adjustment was made for multiple comparisons.
results
patient and tumor characteristics
The median follow-up for both the full and the restricted cohorts was 14 years. In both cohorts, medullary and ductal carcinomas differed in their presentation of tumor characteristics: nodal status, tumor size, grade, PVI, and hormone receptor status, with medullary tumors being associated with less favorable prognostic features with the single exception that medullary tumors were less likely to have PVI (Table 2). The majority of patients had some type of adjuvant systemic treatment: 67% of medullary cases and 77% of ductal cases in the full cohort received chemotherapy, and corresponding numbers in the restricted cohort were 68% versus 84% (Table 2). Of the 127 medullary cases in the full cohort, 64 were enrolled in trials with a chemotherapy randomization and 34 were assigned chemotherapy; corresponding numbers for the restricted cohort were 26 randomized and 14 assigned chemotherapy.
Table 2.
Patient and tumor characteristics according to histologic type and cohort
| All patients (full cohort) |
Patients with ER- and grade 3 tumors (restricted cohort) |
|||||
|---|---|---|---|---|---|---|
| Medullary | Ductal | P value* | Medullary | Ductal | P value* | |
| Total patients | 127 | 8096 | 47 | 1407 | ||
| Mean age at study entry | 52.2 | 52.0 | 50.7 | 50.2 | ||
| N (%) | N (%) | N (%) | N (%) | |||
| Menopausal status | 0.86 | 0.66 | ||||
| Pre | 62 (49) | 4046 (50) | 24 (51) | 767 (55) | ||
| Post | 65 (51) | 4050 (50) | 23 (49) | 640 (45) | ||
| Surgery | 0.06 | 0.74 | ||||
| Mastectomy | 100 (79) | 5748 (71) | 35 (74) | 1003 (71) | ||
| BCS | 27 (21) | 2348 (29) | 12 (26) | 404 (29) | ||
| Radiotherapy | 0.07 | 1.00 | ||||
| Yes | 25 (20) | 2197 (27) | 12 (26) | 369 (26) | ||
| No | 102 (80) | 5899 (73) | 35 (74) | 1038 (74) | ||
| Nodal group | 0.002 | 0.55 | ||||
| Node negative | 26 (20) | 2572 (32) | 19 (40) | 463 (33) | ||
| 1–3+ nodes | 72 (57) | 3382 (42) | 17 (36) | 555 (39) | ||
| 4+ nodes | 29 (23) | 2142 (26) | 11 (23) | 389 (28) | ||
| Tumor size | 0.002 | |||||
| 0–2 cm | 44 (35) | 3891 (49) | 11 (23) | 498 (36) | 0.09 | |
| >2 cm | 83 (65) | 4085 (51) | 36 (77) | 895 (64) | ||
| Missing | 0 | 120 | 0 | 14 | ||
| Grade | <0.0001 | |||||
| 1 | 4 (4) | 950 (13) | ||||
| 2 | 9 (9) | 3276 (45) | ||||
| 3 | 83 (86) | 3053 (42) | 47 (100) | 1407 (100) | ||
| Missing | 31 | 817 | ||||
| PVI | <0.0001 | <0.0001 | ||||
| Present | 18 (19) | 2963 (43) | 6 (15) | 586 (46) | ||
| Absent | 77 (81) | 3995 (57) | 34 (85) | 695 (54) | ||
| Missing | 32 | 1138 | 7 | 126 | ||
| ER status | <0.0001 | |||||
| Positive | 18 (19) | 4998 (68) | ||||
| Negative | 77 (81) | 2387 (32) | 47 (100) | 1407 (100) | ||
| Missing | 32 | 711 | ||||
| PgR status | <0.0001 | 0.10 | ||||
| Positive | 17 (19) | 4124 (59) | 3 (7) | 220 (17) | ||
| Negative | 74 (81) | 2899 (41) | 43 (93) | 1112 (83) | ||
| Missing | 36 | 1073 | 1 | 75 | ||
| Adjuvant systemic therapy | 0.007** | 0.008** | ||||
| No adjuvant Rx | 10 (8) | 503 (6) | 4 (9) | 64 (5) | ||
| ET alone | 32 (25) | 1317 (16) | 11 (23) | 162 (12) | ||
| CT alone | 60 (47) | 3196 (39) | 23 (49) | 572 (41) | ||
| CT + ET | 25 (20) | 3080 (38) | 9 (19) | 609 (43) | ||
Percentages sum within columns.
*P values are calculated using Fisher's exact test. Missing categories are not included in the calculation of the P value.
**P values compare chemotherapy versus no chemotherapy percents.
BCS, breast-conserving surgery; CT, chemotherapy; ER, estrogen receptor; ET, endocrine therapy; PgR, progesterone receptor; PVI, peritumoral vascular invasion; Rx, treatment.
sites of first DFS event
Patients with ductal tumors had more local and distant sites of first DFS event, whereas those with medullary tumors had more second non-breast malignancies and deaths without prior cancer event (Table 3). These observations were similar in the two cohorts.
Table 3.
Sites of first disease-free survival (DFS) event according to histologic type and cohort
| All patients (full cohort) |
Patients with estrogen receptor- and grade 3 tumors (restricted cohort) |
|||
|---|---|---|---|---|
| Medullary | Ductal | Medullary | Ductal | |
| Total patients | 127 | 8096 | 47 | 1407 |
| No DFS event | 58 (46) | 3577 (44) | 26 (55) | 642 (46) |
| Breast cancer-related DFS events | 44 (35) | 3770 (47) | 14 (30) | 674 (48) |
| Local | 6 (5) | 666 (8) | 0 | 94 (7) |
| Contralateral breast | 7 (6) | 333 (4) | 4 (9) | 44 (3) |
| Regional | 8 (6) | 493 (6) | 5 (11) | 116 (6) |
| Distant (as first site) | 23 (18) | 2278 (28) | 5 (11) | 420 (30) |
| Distant soft tissue | 4 (3) | 129 (2) | 0 | 33 (2) |
| Bone | 6 (5) | 880 (11) | 1 (2) | 105 (7) |
| Viscera | 13 (10) | 1269 (16) | 4 (9) | 282 (20) |
| Non-breast cancer-related events | 25 (9) | 749 (29) | 7 (6) | 91 (15) |
| Second primary non-breast | 12 (9) | 362 (4) | 3 (6) | 49 (3) |
| Death without prior cancer event | 11 (9) | 360 (4) | 3 (6) | 39 (3) |
| Unknown | 2 (2) | 27 (0.3) | 1 (2) | 3 (0.2) |
distant recurrence-free interval
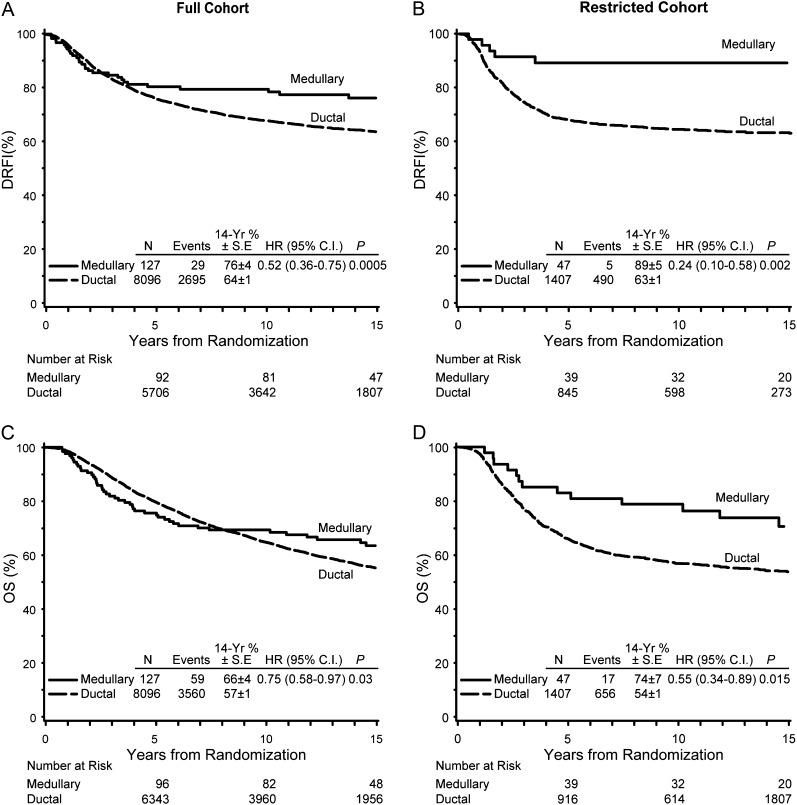
The Kaplan–Meier curves in the full cohort show that the two histologic types had similar DRFI for the first 4 years, but thereafter, the curves split with consistently better outcome for the medullary subtype and a statistically significant overall DRFI [stratified log-rank test hazard ratio (HR) medullary/ductal = 0.52, 95% confidence interval (CI) = 0.36–0.75, P = 0.0005; Table 4, Figure 1A]. An even more pronounced difference was observed in the restricted cohort (DRFI HR medullary/ductal = 0.24, 95% CI = 0.10–0.58, P = 0.002; Table 4, Figure 1B) with the Kaplan–Meier curves diverging earlier. We note that because the hazards are not proportional, the hazard rates are not constant over time. Therefore, the hazard rates reported represent an average over the entire follow-up period and the P values reflect the statistical significance of these HRs. In any case, the overall outcome for the medullary cohort is superior to that of the ductal cases. When the two subtypes were compared according to nodal status, similar results were observed in node-negative and node-positive subgroups, although the differences were statistically significant only among the patients with node-positive disease. DRFI was significantly better for medullary tumors in both cohorts both with and without adjuvant chemotherapy (Table 4). Among medullary cases in the full cohort, 10 of 34 randomly assigned chemotherapy had a distant recurrence compared with 7 of 34 not assigned chemotherapy; corresponding numbers for the restricted cohort were 2 of 14 compared with 1 of 12.
Table 4.
Distant relapse-free interval and overall survival according to histologic type
| Distant recurrence-free interval | |||||
|---|---|---|---|---|---|
| N | Distant recurrence | 14-year DRFI % ± SE | HR (95% CI) | P value* | |
| Full cohort | 0.52 (0.36–0.75) | 0.0005 | |||
| Medullary | 127 | 29 | 76 ± 4 | ||
| Ductal | 8096 | 2695 | 64 ± 1 | ||
| Node-negative | 0.53 (0.17–1.65) | 0.27 | |||
| Medullary | 26 | 3 | 88 ± 6 | ||
| Ductal | 2572 | 486 | 80 ± 1 | ||
| Node-positive | 0.54 (0.37–0.80) | 0.002 | |||
| Medullary | 101 | 26 | 73 ± 5 | ||
| Ductal | 5524 | 2209 | 57 ± 1 | ||
| Chemotherapy | 0.57 (0.37–0.87) | 0.009 | |||
| Medullary | 85 | 22 | 72 ± 5 | ||
| Ductal | 6276 | 2190 | 63 ± 1 | ||
| No chemotherapy | 0.41 (0.19–0.87) | 0.02 | |||
| Medullary | 42 | 7 | 85 ± 6 | ||
| Ductal | 1820 | 505 | 70 ± 1 | ||
| Restricted cohort | 0.24 (0.10–0.58) | 0.002 | |||
| Medullary | 47 | 5 | 89 ± 5 | ||
| Ductal | 1407 | 490 | 63 ± 1 | ||
| Node-negative | 0.40 (0.10–1.62) | 0.20 | |||
| Medullary | 19 | 2 | 89 ± 7 | ||
| Ductal | 463 | 107 | 76 ± 2 | ||
| Node-positive | 0.22 (0.07–0.69) | 0.01 | |||
| Medullary | 28 | 3 | 89 ± 6 | ||
| Ductal | 944 | 383 | 57 ± 2 | ||
| Chemotherapy | 0.28 (0.10–0.76) | 0.01 | |||
| Medullary | 32 | 4 | 88 ± 6 | ||
| Ductal | 1181 | 415 | 63 ± 1 | ||
| No chemotherapy | 0.14 (0.02–0.98) | 0.05 | |||
| Medullary | 15 | 1 | 93 ± 6 | ||
| Ductal | 226 | 75 | 65 ± 3 | ||
|
Overall survival (OS) | |||||
| N | Deaths | 14-year OS % ± SE | HR (95% CI) | P value* | |
| Full cohort | 0.75 (0.58–0.97) | 0.03 | |||
| Medullary | 127 | 59 | 66 ± 4 | ||
| Ductal | 8096 | 3560 | 57 ± 1 | ||
| Node-negative | 0.88 (0.44–1.76) | 0.71 | |||
| Medullary | 26 | 8 | 80 ± 8 | ||
| Ductal | 2572 | 738 | 73 ± 1 | ||
| Node-positive | 0.76 (0.57–1.00) | 0.05 | |||
| Medullary | 101 | 51 | 62 ± 5 | ||
| Ductal | 5524 | 2822 | 49 ± 1 | ||
| Chemotherapy | 0.71 (0.51–1.00) | 0.05 | |||
| Medullary | 85 | 35 | 65 ± 5 | ||
| Ductal | 6276 | 2742 | 57 ± 1 | ||
| No chemotherapy | 0.67 (0.44–1.02) | 0.06 | |||
| Medullary | 42 | 24 | 67 ± 7 | ||
| Ductal | 1820 | 818 | 58 ± 1 | ||
| Restricted cohort | 0.55 (0.34–0.89) | 0.01 | |||
| Medullary | 47 | 17 | 74 ± 7 | ||
| Ductal | 1407 | 656 | 54 ± 1 | ||
| Node-negative | 0.86 (0.38–1.94) | 0.71 | |||
| Medullary | 19 | 6 | 78 ± 10 | ||
| Ductal | 463 | 149 | 70 ± 2 | ||
| Node-positive | 0.53 (0.29–0.98) | 0.04 | |||
| Medullary | 28 | 11 | 71 ± 9 | ||
| Ductal | 944 | 507 | 46 ± 2 | ||
| Chemotherapy | 0.52 (0.29–0.95) | 0.03 | |||
| Medullary | 32 | 11 | 74 ± 8 | ||
| Ductal | 1181 | 554 | 53 ± 2 | ||
| No chemotherapy | 0.52 (0.22–1.21) | 0.13 | |||
| Medullary | 15 | 6 | 73 ± 11 | ||
| Ductal | 226 | 102 | 58 ± 3 | ||
Log-rank P values are stratified by pathologist review.
DRFI, distant recurrence-free interval; CI, confidence interval; HR, hazard ratio; OS, overall survival; SE, standard error.
Figure 1.
Kaplan–Meier plots of distant recurrence-free interval (DRFI) for patients with medullary and ductal tumors in the full cohort (A) and the cohort restricted to ER-negative grade 3 tumors (B); and overall survival (OS) in the full (C) and restricted (D) cohorts. CI, confidence interval; HR, hazard ratio; SE, standard error.
Comparisons between medullary and invasive ductal cohorts in terms of DFS were similar to those based on DRFI (data not shown).
overall survival
There also was a statistically significant difference in OS between the tumor histologic types for both the full cohort (OS HR medullary/ductal = 0.75, 95% CI = 0.58–0.97, P = 0.03; Table 4, Figure 1C) and for the restricted cohort (OS HR medullary/ductal = 0.55, 95% CI = 0.34–0.89, P = 0.01; Table 4, Figure 1D). When patients with medullary tumors were compared with those with ductal tumors in subgroups defined by nodal status, the OS for the medullary category was better in all cases, significantly so for the node-positive cohort (Table 4). When negative PgR status was added to the characterization of the restricted medullary cohort, the outcome did not change (data not shown).
competing risks
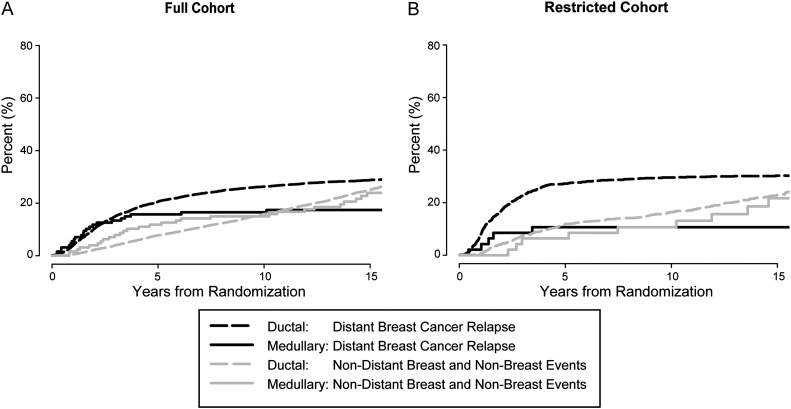
A secondary analysis was carried out focusing on the competing risk of distant breast cancer events with non-distant DFS events. While the competing risk curves for the full cohort were nonproportional (i.e. the curves cross) (Figure 2A), the curves restricted to patients with ER-negative grade 3 tumors were approximately proportional (Figure 2B). The result of the competing risk modeling in this restricted cohort indicated that medullary tumors had a significantly better prognosis than ductal carcinoma (HR medullary/ductal = 0.32, 95% CI = 0.13–0.78, P = 0.01). The result did not change after controlling for nodal status and tumor size (HR medullary/ductal = 0.32, 95% CI = 0.13–0.78, P = 0.01).
Figure 2.
Cumulative incidence plots of competing risk of distant and non-distant events for full cohort for medullary versus ductal tumors: competing causes of failure with the full (A) and restricted cohorts (B).
discussion
In our analysis of 12 409 patients, we identified only 127 (1.0%) medullary carcinomas, a frequency similar or slightly lower than in other published reports [1–3]. In both the full cohort and the restricted cohort, patients with medullary carcinomas had better outcomes overall compared with patients with invasive ductal carcinomas, despite medullary tumors being associated with biological features usually considered unfavorable. In fact, the 14-year DRFI of 89% in the restricted cohort of ER-negative grade 3 tumors was surprisingly good in this supposedly poor prognostic population. Our results thus confirm other reports that observed a superior outcome of tumors with medullary histology compared with invasive ductal tumors [2, 4, 15–18].
In subgroups defined by nodal status, a reduction in risk of distant recurrence for medullary cancers was observed for both node-negative and node-positive groups, although the differences were only statistically significant in the node-positive group. The lack of significance in the node-negative group may be due to the smaller number of cases, as several other reports describe better outcomes for medullary tumors irrespective of the nodal status [4, 13].
The lower risks of distant recurrence and death for medullary tumors compared with invasive ductal carcinomas were seen irrespective of the application of adjuvant chemotherapy. However, our data do not permit reliable conclusions regarding the role of adjuvant chemotherapy for patients with medullary tumors. Although patients with medullary tumors appear to have a relatively good prognosis even without chemotherapy, those with invasive ductal disease also have a better outcome without chemotherapy, indicating a selection bias to enroll patients with better prognosis in trials with a no chemotherapy option. Chemotherapy was a randomized option for very few patients with medullary tumors. Furthermore, several different chemotherapy regimens were given with or without endocrine therapy without considering ER status in the earlier trials. Thus, because the role of chemotherapy for medullary carcinomas in the restricted cohort is less certain than for invasive ductal carcinomas, further studies are needed to clarify this issue.
In our study, we were able to report on the sites of first recurrences, a feature not commonly reported by others. The majority of first recurrences were distant and we found that both local relapse and distant relapse were less frequently observed in the medullary type than in the invasive ductal type in both cohorts.
Although the main conclusions were similar in the full and restricted cohorts (i.e. improved outcome and association with poorer disease characteristics), we found differences between the two cohorts. The reduction in the risk of a distant recurrence in medullary tumors compared with invasive ductal was higher in the restricted cohort (76%) than in the full cohort (48%). In the full cohort, the incidence of distant recurrence separated after 4 years, with few events occurring thereafter in the medullary group, whereas events in the invasive ductal group continued to be observed beyond 4 years. In the restricted cohort, the separation of events occurred earlier and after 4 years, there were very few distant recurrences in either the medullary or invasive ductal (G3, ER −) groups. The distribution of events over time is typical for ER-negative tumors and thus not surprising [40]. Nevertheless, there was a substantial advantage in the control of distant recurrence in the medullary type during the first 5 years after diagnosis, which persisted over time.
These outcome distinctions between the two cohorts, with much clearer differences in the restricted group compared with its control group, support the suggestion that the definition of the medullary subtype seems to be most informative when restricted to ER-negative and poor grade tumors. In addition, the pattern of relapse occurring almost exclusively in the first 4 years in the restricted cohort further confirms this view.
These observations support recent reports linking the medullary tumors to myoepithelial features and the basal-like phenotype [14, 23, 41, 42], which in most cases is immunohistochemically characterized by negative expression of ER, PgR, and HER2 (triple-negative tumors). Interestingly, microarray-based analyses showed that medullary carcinomas and invasive ductal tumors with a basal-like phenotype have distinct molecular characteristics [9, 23] even though they share similar biological features. In medullary breast cancer, genes involved in Th1 immune response including interleukins, interferon regulatory factors, and Th1 cytokines and genes related to the apoptosis pathway were upregulated. By contrast, genes involved in the remodeling of the cytoskeleton and genes associated with cell invasiveness were downregulated in medullary carcinomas [43]. These different molecular characteristics may account for the favorable outcome of medullary carcinomas and suggest that the group of basal-like tumors constitutes a heterogeneous group of carcinomas.
In conclusion, our analysis, based on a compilation of data from 13 trials conducted by a single cooperative group, demonstrates an improved outcome for patients with medullary breast carcinomas compared with invasive ductal carcinomas despite the former's unfavorable biologic features. These differences in outcome were most pronounced within the restricted cohort confined to patients with ER-negative grade 3 tumors and we suggest that lack of ER expression and poor grade should be part of the definition of medullary breast cancer. The definition of medullary subtype used in our restricted cohort does not include information on the HER2 status. However, as most medullary cancers lack HER2 overexpression/amplification, our conclusions would most likely not change if HER2 was known. Currently, ER-negative and in particular triple-negative tumors (used as surrogate for basal-like phenotype even though not completely concordant) will be treated on average with more intensive chemotherapy due to their prognosis and the observation that these tumors are more sensitive to chemotherapy than others [44, 45]. However, the favorable prognosis of medullary tumors and the different molecular pattern of these tumors, compared with others linked to the basal-like phenotype, raise questions about this treatment approach. Clinical data are lacking on the efficacy of adjuvant chemotherapy in this patient population and whether less adjuvant treatment should be given is still an area of controversy. The NCCN guidelines recommend to treat early medullary cancers as other infiltrating ductal tumors [46], whereas the St Gallen Consensus recommendations suggest that medullary carcinomas may not require adjuvant cytotoxics if node-negative [47]. Based on the excellent 14-year DRFI in the medullary-restricted cohort, our data support this recommendation. Thus, we suggest that considering the histologic subtype may be helpful when deciding the appropriate adjuvant treatment assuming that the breast tumor is reliably classified as a medullary carcinoma. Lessons learned from rare tumors will improve our understanding of the biology of breast cancer and may help in further refinement and individualizing adjuvant treatment.
funding
This work was supported by the Ludwig Institute for Cancer Research and the Cancer League of Ticino and the continuing support for central coordination, data management, and statistics provided by the Swedish Cancer Society; the Cancer Council Australia; Australian New Zealand Breast Cancer Trials Group (National Health and Medical Research Council); the Frontier Science and Technology Research Foundation; the Swiss Group for Clinical Cancer Research (SAKK); the Swiss Cancer League; and the United States National Institutes of Health (CA-75362).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgments
We thank the patients, physicians, nurses, and data managers who participate in the International Breast Cancer Study Group trials. We thank Joie Celano for data management. Presented in part elsewhere: American Society of Clinical Oncology, June, 2010, general poster session. We also acknowledge support for the Cape Town participants from the Cancer Association of South Africa, for the St Gallen participants from the Foundation for Clinical Research of Eastern Switzerland (OSKK), and for the Gothenburg participants from the Swedish Society for Cancer Research (Cancerfonden).
references
- 1.Dendale R, Vincent-Salomon A, Mouret-Fourme E, et al. Medullary breast carcinoma: prognostic implications of p53 expression. Int J Biol Markers. 2003;18:99–105. doi: 10.5301/jbm.2008.1279. [DOI] [PubMed] [Google Scholar]
- 2.Reinfuss M, Stelmach A, Mitus J, et al. Typical medullary carcinoma of the breast: a clinical and pathological analysis of 52 cases. J Surg Oncol. 1995;60:89–94. doi: 10.1002/jso.2930600205. [DOI] [PubMed] [Google Scholar]
- 3.Rakha EA, Putti TC, Abd El-Rehim DM, et al. Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J Pathol. 2006;208:495–506. doi: 10.1002/path.1916. [DOI] [PubMed] [Google Scholar]
- 4.Ridolfi RL, Rosen PP, Port A, et al. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40:1365–1385. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen I, Holck S, Mouridsen HT, et al. Prognostic comparison of three classifications for medullary carcinoma of the breast. Histopathology. 1999;34:175–186. doi: 10.1046/j.1365-2559.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- 6.Eichhorn JH. Medullary carcinoma, provocative now as then. Semin Diagn Pathol. 2004;21:65–73. doi: 10.1053/j.semdp.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Rosen PP, Lesser ML, Arroyo CD, et al. Immunohistochemical detection of HER2/neu in patients with lymph node negative breast cancer. Cancer. 1995;75:1320–1326. doi: 10.1002/1097-0142(19950315)75:6<1320::aid-cncr2820750614>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Foschini MP, Eusebi V. Rare (new) entities of the breast and medullary carcinoma. Pathology. 2009;41:48–56. doi: 10.1080/00313020802563528. [DOI] [PubMed] [Google Scholar]
- 9.Bertucci F, Finetti P, Cervera N, et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;66:4636–4644. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 10.Pertschuk LP, Kim DS, Nayer K, et al. Immunocytochemical estrogen and progestin receptor assays in breast cancer with monoclonal antibodies. Cancer. 1990;66:1663–1670. doi: 10.1002/1097-0142(19901015)66:8<1663::aid-cncr2820660802>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Jensen ML, Kier H, Melsen F. Medullary breast carcinoma vs. poorly differentiated ductal carcinoma: an immunohistochemical study with keratin 19 and oestrogen receptor staining. Histopathology. 1996;29:241–245. doi: 10.1111/j.1365-2559.1996.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 12.Orlando L, Renne G, Rocca A, et al. Are all high grade breast cancers with no steroid receptor hormone expression alike? The special case of the medullary phenotype. Ann Oncol. 2005;16:1094–1099. doi: 10.1093/annonc/mdi213. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen L, Zedeler K, Holck S, et al. Medullary carcinoma of the breast. Prevalence and prognostic importance of classical risk factors in breast cancer. Eur J Cancer. 1995;31A:2289–2295. doi: 10.1016/0959-8049(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 14.Flucke U, Flucke MT, Hoy L, et al. Distinguishing medullary carcinoma of the breast from high-grade hormone receptor-negative invasive ductal carcinoma: an immunohistochemical approach. Histopathology. 2010;56:852–859. doi: 10.1111/j.1365-2559.2010.03555.x. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen L, Zedeler K, Holck S, et al. Medullary carcinoma of the breast, proposal for a new simplified histopathological definition. Br J Cancer. 1991;63:591–595. doi: 10.1038/bjc.1991.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen L, Holck S, Schiødt T, et al. Medullary carcinoma of the breast, prognostic importance of characteristic histopathological features evaluated in a multivariate Cox analysis. Eur J Cancer. 1994;30A:1792–1797. doi: 10.1016/0959-8049(94)00251-y. [DOI] [PubMed] [Google Scholar]
- 17.Gamel JW, Meyer JS, Feuer E, et al. The impact of stage and histology on the long-term clinical course of 163.808 patients with breast carcinoma. Cancer. 1996;77:1459–1464. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1459::AID-CNCR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Vu-Nishino H, Tavassoli FA, Ahrens WA, et al. Clinicopathologic features and long-term outcome of patients with medullary breast carcinoma managed with breast-conserving therapy (BCT) Int J Radiat Oncol Biol Phys. 2005;62:1040–1047. doi: 10.1016/j.ijrobp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Vo T, Xing Y, Meric-Bernstam F, et al. Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg. 2007;194:527–531. doi: 10.1016/j.amjsurg.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Fisher ER, Kenny JP, Sass R, et al. Medullary cancer of the breast revisited. Breast Cancer Res Treat. 1990;16:215–229. doi: 10.1007/BF01806330. [DOI] [PubMed] [Google Scholar]
- 21.Cook DL, Weaver DL. Comparison of DNA content, s-phase fraction and survival between medullary and ductal carcinoma of the breast. Am J Clin Pathol. 1995;104:17–22. doi: 10.1093/ajcp/104.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Fisher ER, Anderson S, Redmond C, et al. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06. Cancer. 1993;71:2507–2514. doi: 10.1002/1097-0142(19930415)71:8<2507::aid-cncr2820710813>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Vincent-Salomon A, Gruel N, Lucchesi C, et al. Identification of typical medullary breast carcinoma as a genomic sub-group of basal-like carcinomas, a heterogeneous new molecular entity. Breast Cancer Res. 2007;9:R24. doi: 10.1186/bcr1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakha EA, Aleskandarany M, El-Sayed ME, et al. The prognostic significance of inflammation and medullary histological type in invasive carcinoma of the breast. Eur J Cancer. 2009;45:1780–1787. doi: 10.1016/j.ejca.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Rakha EA, El-Sayed E, Green RA, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor-positive phenotype. J Clin Oncol. 2007;25:4772–4778. doi: 10.1200/JCO.2007.12.2747. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig Breast Cancer Study Group. A randomized trial of adjuvant combination chemotherapy with or without prednisone in premenopausal breast cancer patients with metastases in one to three axillary lymph nodes. Cancer Res. 1985;45:4454–4459. [PubMed] [Google Scholar]
- 27.Ludwig Breast Cancer Study Group. Chemotherapy with or without oophorectomy in high-risk premenopausal patients with operable breast cancer. J Clin Oncol. 1985;3:1059–1067. doi: 10.1200/JCO.1985.3.8.1059. [DOI] [PubMed] [Google Scholar]
- 28.Pagani O, Price KN, Gelber RD, et al. Patterns of recurrence of early breast cancer according to estrogen receptor status: A therapeutic target for a quarter of a century. Breast Cancer Res Treat. 2009;117:319–324. doi: 10.1007/s10549-008-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colleoni M, Gelber S, Coates A, et al. Influence of endocrine-related factors on response to perioperative chemotherapy for patients with node-negative breast cancer. J Clin Oncol. 2001;19:4141–4149. doi: 10.1200/JCO.2001.19.21.4141. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig Breast Cancer Study Group. Combination adjuvant chemotherapy for node-positive breast cancer. Inadequacy of a single perioperative cycle. N Engl J Med. 1988;319:677–683. doi: 10.1056/NEJM198809153191104. [DOI] [PubMed] [Google Scholar]
- 31.The International Breast Cancer Study Group. Duration and reintroduction of adjuvant chemotherapy for node-positive premenopausal breast cancer patients. J Clin Oncol. 1996;14:1885–1894. doi: 10.1200/JCO.1996.14.6.1885. [DOI] [PubMed] [Google Scholar]
- 32.The International Breast Cancer Study Group. Effectiveness of adjuvant chemotherapy in combination with tamoxifen for node-positive postmenopausal breast cancer patients. J Clin Oncol. 1997;15:1385–1393. doi: 10.1200/JCO.1997.15.4.1385. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson P, Sun Z, Braun D, et al. Long term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer. Ann Oncol. 2011;22:2216–2226. doi: 10.1093/annonc/mdq735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aebi S, Sun Z, Braun D, et al. Differential efficacy of three cycles of CMF followed by tamoxifen in patients with ER-positive and ER-negative tumors: long-term follow up on IBCSG Trial IX. Ann Oncol. 2011;22:1981–1987. doi: 10.1093/annonc/mdq754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thürlimann B, Price KN, Gelber RD, et al. Is chemotherapy necessary for premenopausal women with lower-risk node-positive, endocrine responsive breast cancer? 10-year update of International Breast Cancer Study Group Trial 11-93. Breast Cancer Res Treat. 2009;113:137–144. doi: 10.1007/s10549-008-9912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.International Breast Cancer Study Group. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12-93 and 14-93. Ann Oncol. 2004;15:1749–1759. doi: 10.1093/annonc/mdh463. [DOI] [PubMed] [Google Scholar]
- 37.International Breast Cancer Study Group. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: IBCSG Trial 13-93. J Clin Oncol. 2006;24:1332–1341. doi: 10.1200/JCO.2005.03.0783. [DOI] [PubMed] [Google Scholar]
- 38.International Breast Cancer Study Group. Effects of a treatment gap during adjuvant chemotherapy in node-positive breast cancer: results of International Breast Cancer Study Group (IBCSG) trials 13-93 and 14-93. Ann Oncol. 2007;18:1177–1184. doi: 10.1093/annonc/mdm091. [DOI] [PubMed] [Google Scholar]
- 39.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Amer Stat Assoc. 1999;94:496–509. [Google Scholar]
- 40.Saphner T, Tomey T, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 41.Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 42.Jacquemier J, Padovani L, Rabayrol L, et al. Typical medullary breast carcinomas have a basal/myoepithelial phenotype. J Pathol. 2005;207:260–268. doi: 10.1002/path.1845. [DOI] [PubMed] [Google Scholar]
- 43.Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4:192–208. doi: 10.1016/j.molonc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huober J, von Minckwitz G, Denkert C, et al. Effect of neoadjuvant anthracycline-taxane based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124:133–140. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- 45.Liedtke C, Mazuoni C, Hess KR, et al. Response to neoadjuvant chemotherapy and long-term survival in patients with triple negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 46.National Comprehensive Cancer Network (NCCN). NCCN Guidelines Version 2.2011. Breast Cancer. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. (22 December 2011, date last accessed) [Google Scholar]
- 47.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.