Abstract
Background
Pancreatitis is a known risk factor for pancreatic cancer; however, an unknown fraction of the disease is thought to be a consequence of tumor-related duct obstruction.
Patients and methods
A pooled analysis of a history of pancreatitis and risk of pancreatic cancer was carried out considering the time interval between diagnoses and potential modification by covariates. Adjusted pooled odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from 10 case–control studies (5048 cases of ductal pancreatic adenocarcinoma and 10 947 controls) taking part in the International Pancreatic Cancer Case-Control Consortium (PanC4).
Results
The association between pancreatitis and pancreatic cancer was nearly three-fold at intervals of >2 years between diagnoses (OR: 2.71, 95% CI: 1.96–3.74) and much stronger at intervals of ≤2 years (OR: 13.56, 95% CI: 8.72–21.90) probably reflecting a combination of reverse causation and antecedent misdiagnosis of pancreas cancer as pancreatitis. The younger (<65 years) pancreatic cancer cases showed stronger associations with previous (>2 years) pancreatitis (OR: 3.91, 95% CI: 2.53–6.04) than the older (≥65 years) cases (OR: 1.68, 95% CI: 1.02–2.76; P value for interaction: 0.006).
Conclusions
Despite a moderately strong association between pancreatitis (diagnosed before >2 years) and pancreatic cancer, the population attributable fraction was estimated at 1.34% (95% CI: 0.612–2.07%), suggesting that a relatively small proportion of pancreatic cancer might be avoided if pancreatitis could be prevented.
Keywords: case–control studies, pancreatitis, pancreatic cancer, pooled analysis, risk factors
introduction
Pancreatic cancer is the fourth leading cause of cancer-related mortality in both sexes in the United States [1]. Cigarette smoking is one of the most important environmental risk factors for pancreatic cancer, accounting for at least 20% of the disease [2]. Other risk factors include long-standing history of diabetes, history of overweight and obesity, non-O blood group, Helicobacter pylori infection, and possibly heavy alcohol consumption [3–6]. Chronic pancreatitis is a relatively rare inflammatory condition of the pancreas and is also a known risk factor for pancreatic cancer; however, a significant proportion of pancreatitis is thought to be a consequence of pancreatic tumor-related ductal obstruction [7–10]. Thus, estimates of the relative risk (RR) of developing pancreatic cancer among patients with pancreatitis tend to drop off as the time interval between the diagnoses of pancreatitis and pancreatic cancer increases [9]. The time interval between when the RR begins to drop and when it levels off is not known with certainty. Furthermore, very little is known regarding the possible modifying effect of demographic factors (such as age, sex, and geographic region) and risk factors for pancreatic cancer (such as smoking, alcohol intake, body weight, and history of diabetes) on the relation between pancreatitis and pancreatic cancer.
We carried out an individual-level pooled analysis of pancreatitis and risk of pancreatic cancer in 5048 cases and 10 947 controls from 10 case–control studies that are part of the Pancreatic Cancer Case-Control Consortium (PanC4, http://www.panc4.org) [11–19]. We estimated pooled odds ratios (ORs), population attributable fraction (AFp) and 95% confidence intervals (CIs) for having a history of pancreatitis, as well as the time interval (years) between diagnosis of pancreatitis and pancreatic cancer, and OR estimates stratified on the basis of demographics and risk factors for pancreatic cancer, including age, sex, ethnicity, study region, education, body mass index (BMI, kg/m2), tobacco smoking, alcohol intake, and history of diabetes.
methods
We identified 10 case–control studies of pancreatic adenocarcinoma within the PanC4 consortium that collected data on history of pancreatitis [11–19]. The total number of participants available for these analyses was 5048 pancreatic cancer cases and 10 947 controls. A proxy respondent was interviewed for 155 cases and 150 controls from the Shanghai study, 63 cases from the Toronto study, and 474 cases and 332 controls from the SEARCH study (for a total of 692 or 13.7% of cases and 472 or 4.3% of controls). All the individual studies included in this pooled analysis had previously obtained ethical approvals. The original datasets were restructured either by the original study investigators or by the PanC4 central coordinators, using a uniform format for data harmonization. Individual data on sociodemographic characteristics, anthropometric measures, smoking and alcohol consumption, history of diabetes, and first-degree family history of pancreatic cancer (in the Toronto study, family history positive if present in any relative) were collected.
Pancreatitis variables were based upon the self-reported questionnaire information from each of the studies and referred to diagnoses of pancreatitis before the date of diagnosis of pancreatic cancer or interview. For participants who reported any pancreatitis, we estimated the time interval between the first diagnosis of pancreatitis and the diagnosis of pancreatic cancer for cases (or interview date for controls) as the difference in years between the age at the first diagnosis of pancreatitis and age at diagnosis of pancreatic cancer (or age at interview in controls). The type of pancreatitis was not determined in the majority of the studies in this analysis, and thus, we were unable to assess the effects of acute-versus-chronic pancreatitis or risk of specific types of chronic pancreatitis. Further, we did not have adequate information on the number and the duration of episodes of pancreatitis for meaningful analyses in the pooled dataset.
statistical analysis
We conducted an aggregate analysis with individual-level data from all the participating PanC4 studies pooled into a single dataset [20, 21]. The association between a history of pancreatitis and risk of pancreatic cancer was assessed by estimating the ORs and the corresponding 95% CI through unconditional multiple logistic regression models [11] adjusted for study center, age (<50, 50–54, 55–59, 60–64, 65–69, 70–74, ≥75 years), sex, education (≤8th grade, 9th–11th grade, 12th grade or high school graduates, some college or college graduates, ≥1 year of graduate or professional school), race/ethnicity (non-Hispanic White, Hispanic, non-Hispanic Black, others), BMI (<20, 20 to <25, 25 to <30, ≥30 kg/m2), tobacco smoking (never smokers, smokers of products other than cigarettes, current cigarette smokers of 1 to ≤20 cigarettes per day, current cigarette smokers of ≥20 cigarettes per day, ex-cigarette smokers with >10 years since quitting, ex-cigarette smokers with 1 to ≤10 years since quitting), alcohol intake (never drinkers or drinkers of <1 drink per day, 1–4 drinks per day, or ≥4 drinks per day), and history of diabetes. The MSKCC study did not have information on alcohol intake; so, for analyses of pancreatitis and pancreatic cancer including adjustment for alcohol, intakes were set to zero drinks per day for all the participants from that study. To evaluate the influence of individual study centers on the final results, we carried out influence analyses by eliminating each center from the pooled dataset and re-estimating the pooled OR for pancreatitis (diagnosed >2 years before) and pancreatic cancer. Study-specific ORs and the pooled OR with 95% CIs were plotted for visual comparison. Tests for linear trend of the ORs were based on ordinal coding of the categories and the corresponding χ2 statistic [11]. To investigate whether the effect of a history of pancreatitis was homogeneous across the strata of selected covariates, we estimated ORs for pancreatitis and pancreatic cancer stratified on the basis of sex, age (<65, ≥65 years), race/ethnicity (non-Hispanic white, other), education (high school graduate or less, some college or more), BMI (<25, ≥25 kg/m2), tobacco smoking (never, former, current ≤20 cig/day, current >20 cig/day), alcohol intake (0 to <1 drink/day, 1 to <4 drinks/day, ≥4 drinks/day), history of diabetes, and study area (North America, other). The categories with similar ORs were collapsed to ensure adequate power in stratified analyses. Heterogeneity across the strata was based on a likelihood ratio test and the resulting χ2 statistic [11]. Population attributable fractions for the association between pancreatitis and pancreatic cancer were estimated using adjusted ORs with standard errors, and represent the proportion of all pancreatic cancer cases in PanC4 that might have been avoided had pancreatitis been prevented [22].
results
Of the 10 case–control studies that were included in this pooled analysis, six studies were from the United States, one each from Canada, Europe, and China, and one multicenter study included participants from Canada, Europe, and Australia (supplementary Table S1, available at Annals of Oncology online). The earliest study period was 1983–1989 [13], while the most recent was 2005–2009 [17]. The median age of pancreatic cancer cases across the studies ranged from 60 to 68 (supplementary Table S1, available at Annals of Oncology online). Most of the studies used regional cancer registries for the case identification of pancreatic cancer, while three studies used hospital registries exclusively (supplementary Table S1, available at Annals of Oncology online). For control identification, three studies used population registries, three used hospital visitors or non-cancer patients, two used random digit dial methods, and two used a combination of random digit dial and sampling of lists from the Centers for Medicare and Medicaid Services (supplementary Table S1 available at Annals of Oncology online). A total of 5048 cases with adenocarcinoma of the exocrine pancreas (the majority of which were based upon clinical diagnosis; information on histology was available for 1442 or 28.6% of these cases) and 10 947 controls were included in these analyses. The numbers of cases and controls may differ slightly from those found in the published reports of the same studies because of missing data on the relevant variables used in these analyses and of possible continuing recruitment in ongoing studies.
The pancreatic cancer cases and controls were generally well-balanced with regard to sex, age, and alcohol intake, with slight differences by race/ethnicity and education (Table 1). As expected, pancreatic cancer cases were generally more likely than controls to have smoked, to have had higher BMI, and to have had a history of diabetes (Table 1).
Table 1.
Distribution of 5048 cases of pancreatic cancer and 10 947 controls according to sex, age, smoking, and other covariates, International Pancreatic Cancer Case-Control Consortium (PanC4)
| Characteristics | Cases |
Controls |
||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Men | 2853 | 56.5 | 6388 | 58.3 |
| Women | 2195 | 43.5 | 4559 | 41.6 |
| Age (year) | ||||
| <50 | 507 | 10.0 | 1637 | 14.9 |
| 50–54 | 500 | 9.9 | 1234 | 11.3 |
| 55–59 | 726 | 14.4 | 1586 | 14.5 |
| 60–64 | 869 | 17.2 | 1710 | 15.6 |
| 65–69 | 900 | 17.8 | 1824 | 16.7 |
| 70–75 | 817 | 16.2 | 1667 | 15.2 |
| ≥75 | 729 | 14.4 | 1289 | 11.8 |
| Race/ethnicity | ||||
| Non-Hispanic White | 3981 | 78.9 | 7568 | 69.1 |
| Non-Hispanic Black | 346 | 6.8 | 1118 | 10.2 |
| Hispanic | 106 | 2.1 | 208 | 1.9 |
| Others | 610 | 12.1 | 1743 | 15.9 |
| Missing | 5 | 0.1 | 310 | 2.8 |
| Education | ||||
| 8th grade or less | 1055 | 20.9 | 3065 | 28.0 |
| 9th–11th grade | 722 | 14.3 | 1530 | 14.0 |
| 12th grade or high school graduate | 968 | 19.2 | 1818 | 16.6 |
| Some college or college graduate | 1471 | 29.1 | 2936 | 26.8 |
| ≥1 year of graduate school | 794 | 15.7 | 1523 | 13.9 |
| Missing | 38 | 0.7 | 75 | 0.7 |
| Body mass index (kg/m2) | ||||
| <20 | 422 | 8.4 | 1055 | 9.6 |
| 20 to <25 | 1950 | 36.6 | 4996 | 45.6 |
| 25 to <30 | 1787 | 35.4 | 3637 | 33.2 |
| ≥30 | 836 | 16.6 | 1149 | 10.5 |
| Missing | 53 | 1.0 | 110 | 1.0 |
| Tobacco smoking | ||||
| Never | 1813 | 35.9 | 4559 | 41.6 |
| Smokers other than cigarettes | 130 | 2.6 | 312 | 2.8 |
| Current (cigarettes/day) | ||||
| ≤20 | 888 | 17.6 | 1976 | 18.0 |
| >20 | 433 | 8.6 | 556 | 5.1 |
| Former (years since quitting) | ||||
| >10 | 1122 | 22.2 | 2358 | 21.5 |
| ≤10 | 590 | 11.7 | 1077 | 9.8 |
| Missing | 72 | 1.4 | 109 | 1.0 |
| Alcohol intake (drinks/day)a | ||||
| 0 to <1 | 2778 | 55.0 | 6277 | 57.3 |
| 1 to <4 | 1172 | 23.2 | 2996 | 27.4 |
| ≥4 | 575 | 11.4 | 1317 | 12.0 |
| Missing | 523 | 10.4 | 357 | 3.2 |
| History of diabetes | ||||
| No | 4042 | 80.1 | 9965 | 91.0 |
| Yes | 1002 | 19.8 | 976 | 8.9 |
| Missing | 4 | 0.1 | 6 | 0.1 |
aNo information on alcohol intake was available from the MSKCC study.
Having had a history of pancreatitis was associated with a nearly six-fold risk of pancreatic cancer (OR: 5.57, 95% CI: 4.39–7.07) (Table 2). The AFp for pancreatitis was estimated to be 5.15% (95% CI: 4.12–6.16%) (data not in table). When accounting for the time interval between the diagnosis of pancreatitis and pancreatic cancer, ORs ranged from 21 (for an interval ≤1 year) to 3.4 (for an interval >25 years) (Table 2). The association between pancreatitis and pancreatic cancer leveled off to a nearly three-fold increase for intervals between diagnoses of >2 years (see Table 2, for intervals >2 years, OR: 2.71, 95% CI: 1.96–3.74) (OR not in table). The AFp for pancreatitis diagnosed >2 years before pancreatic cancer was 1.34% (95% CI: 0.612–2.07%) (data not in table). Of the 313 pancreatic cancer cases who reported a history of pancreatitis, 49.8% reported that they had pancreatitis within 1 year of the diagnosis of their cancer, while a further 8.6% reported that they had pancreatitis within 2 years of the diagnosis of cancer, for a total of 58.4% (Table 2). In contrast, among the controls who reported a history of pancreatitis, 22.3% reported that they had pancreatitis within 2 years of the date of the interview (Table 2). Subtraction of 22.3% from 58.4% results in 36.1%, which can be considered an estimate of the minimum proportion of pancreatitis that might be a consequence of pancreatic cancer (or the result of an antecedent misdiagnosis) in this group of PanC4 case participants.
Table 2.
Distribution of 5048 cases of pancreatic cancer and 10 947 controls, pooled odds ratios (ORs) and 95% confidence intervals (CIs) for history of pancreatitis and risk of pancreatic cancer, International Pancreatic Cancer Case-Control Consortium (PanC4)
| Cases |
Controls |
ORa (95% CI) | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| History of pancreatitis | |||||
| No | 4674 | 92.6 | 10 703 | 97.8 | 1b |
| Yes | 313 | 6.2 | 112 | 1.0 | 5.57 (4.39–7.07) |
| Missing | 61 | 1.2 | 132 | 1.2 | |
| Years between diagnosis of pancreatitis and diagnosis of pancreatic cancer/interviewc | |||||
| ≤1 | 156 | 49.8 | 14 | 12.5 | 21.35 (12.03–37.86) |
| >1 to 2 | 27 | 8.6 | 11 | 9.8 | 4.08 (1.90–8.78) |
| >2 to 5 | 31 | 9.9 | 19 | 17.0 | 2.82 (1.47–5.39) |
| >5 to 10 | 30 | 9.6 | 17 | 15.2 | 3.33 (1.74–6.37) |
| >10 to 25 | 25 | 8.0 | 28 | 25.0 | 1.90 (1.06–3.39) |
| >25 | 20 | 6.4 | 15 | 13.4 | 3.45 (1.67–7.13) |
| Missing | 24 | 7.7 | 8 | 7.1 | |
| P value for trendc | <0.0001 | ||||
aPooled ORs were estimated using logistic regression models adjusting for study, age, sex, race/ethnicity, education, body mass index, tobacco smoking, alcohol intake and history of diabetes.
bReference category.
cAmong participants with pancreatitis.
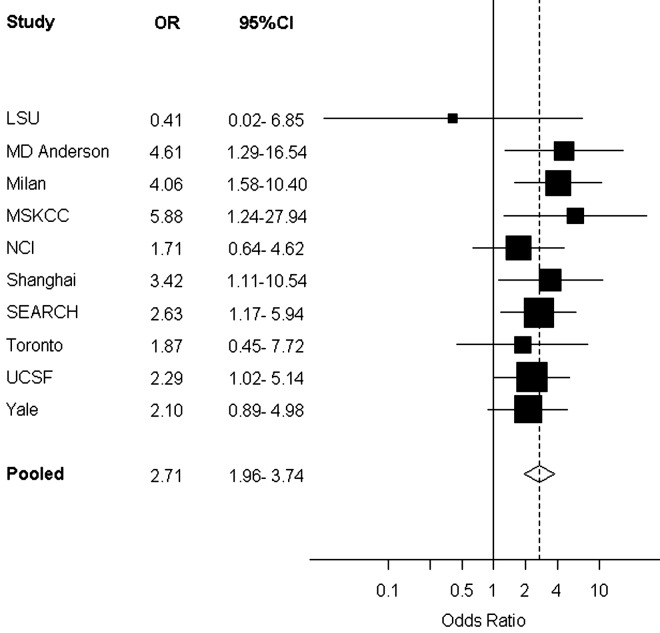
The results of the influence analyses (omitting each study and then re-estimating the OR for pancreatitis diagnosed >2 years before and pancreatic cancer) yielded ORs ranging from 2.53 (omitting the Milan study) to 2.82 (omitting the Yale study). Omission of the MSKCC study which did not have covariate data on alcohol intake resulted in a pooled OR estimate of 2.62 (95% CI: 1.88–3.66). Figure 1 gives a forest plot of the results of the individual PanC4 studies and the overall pooled estimate for pancreatitis (diagnosed >2 years before) and pancreatic cancer.
Figure 1.
Forest plot of individual PanC4 studies—odds ratios (ORs) and 95% confidence intervals (CIs) for pancreatitis (diagnosed >2 years before) and risk of pancreatic cancer. Study-specific ORs adjusted for age, sex, race/ethnicity, education, body mass index, tobacco smoking, alcohol intake and history of diabetes. Squares indicate study-specific OR; size of the square denotes weight given to this study (inverse of the variance of the log OR). Horizontal lines indicate study-specific CI; diamond indicates summary pooled OR, adjusted for age, sex, study, race/ethnicity, education, body mass index, tobacco smoking, alcohol intake and history of diabetes. Solid vertical line denotes OR of 1. Dashed vertical line denotes summary OR.
We investigated modification of the association between a history of pancreatitis (diagnosed either ≤2 years or >2 years before cancer diagnosis) and risk of pancreatic cancer by covariates such as sex, age, race/ethnicity, education, BMI, smoking, alcohol intake, history of diabetes, and study area (Table 3). Of all variables evaluated, only age at the diagnosis of pancreatic cancer was identified as a potential effect modifier of the association between pancreatitis (diagnosed >2 years before cancer) and risk of pancreatic cancer (the association between pancreatitis and pancreatic cancer was stronger in younger cancer patients; P value for interaction: 0.006) (Table 3).
Table 3.
Pooled odds ratiosa (ORs) and 95% confidence intervals (CIs) for the history of pancreatitis and risk of pancreatic cancer, by covariates, International Pancreatic Cancer Case-Control Consortium (PanC4)
| History of pancreatitis |
|||||
|---|---|---|---|---|---|
| No. | Years between diagnosis of pancreatitis and diagnosis of pancreatic cancer/interview |
||||
| ≤2 |
>2 |
||||
| N (cases : controls) | N (cases : controls) | OR (95% CI) | N (cases : controls) | OR (95% CI) | |
| Overall | 4674 : 10 703 | 183 : 25 | 13.56 (8.72–21.90) | 106 : 79 | 2.71 (1.96–3.74) |
| Sex | |||||
| Men | 2651 : 6215 | 102 : 15 | 11.73 (6.56–20.99) | 59 : 46 | 2.34 (1.50–3.63) |
| Women | 2023 : 4488 | 81 : 10 | 17.32 (8.68–34.54) | 47 : 33 | 3.12 (1.94–5.03) |
| P value for interaction | (0.59) | (0.44) | |||
| Age (years) | |||||
| <65 | 2369 : 6028 | 119 : 16 | 15.45 (8.89–26.85) | 69 : 40 | 3.91 (2.53–6.04) |
| ≥65 | 2305 : 4675 | 64 : 9 | 10.24 (4.96–21.14) | 37 : 39 | 1.68 (1.02–2.76) |
| P value for interaction | (0.30) | (0.006) | |||
| Race/ethnicity | |||||
| Non-Hispanic White | 3681 : 7410 | 151 : 19 | 13.41 (8.15–22.04) | 83 : 59 | 2.48 (1.72–3.58) |
| Other | 990 : 2991 | 31 : 6 | 11.83 (4.65–30.05) | 22 : 17 | 3.28 (1.62–6.66) |
| P value for interaction | (0.96) | (0.40) | |||
| Education | |||||
| High school graduate or less | 2576 : 6257 | 65 : 14 | 10.29 (5.52–19.18) | 52 : 50 | 2.21 (1.44–3.39) |
| College graduate or more | 2062 : 4377 | 118 : 11 | 18.09 (9.49–34.46) | 54 : 29 | 3.48 (2.09–5.79) |
| P value for interaction | (0.27) | (0.20) | |||
| Body mass index (kg/m2) | |||||
| <25 | 2209 : 5935 | 75 : 11 | 13.48 (6.91–26.26) | 49 : 37 | 3.12 (1.96–4.96) |
| ≥25 | 2418 : 4663 | 107 : 14 | 13.50 (7.46–24.46) | 55 : 41 | 2.36 (1.49–3.72) |
| P value for interaction | (0.89) | (0.84) | |||
| Tobacco smoking | |||||
| Never | 1695 : 4470 | 61 : 9 | 17.72 (8.51–36.91) | 29 : 33 | 2.18 (1.26–3.79) |
| Former | 1207 : 2464 | 57 : 9 | 7.16 (3.38–15.18) | 31 : 20 | 2.59 (1.37–4.89) |
| Current | |||||
| ≤20 cigarettes/day | 1038 : 2304 | 39 : 4 | 21.15 (6.73–66.49) | 28 : 15 | 3.97 (1.98–7.96) |
| >20 cigarettes/day | 574 : 1054 | 20 : 3 | 12.41 (3.51–43.85) | 12 : 9 | 1.94 (0.73–5.16) |
| P value for interaction | (0.56) | (0.61) | |||
| Alcohol intake (drinks/day)b | |||||
| 0 to <1 | 2562 : 6153 | 114 : 16 | 14.11 (8.11–26.55) | 54 : 43 | 2.73 (1.75–4.25) |
| 1 to <4 | 1109 : 2920 | 30 : 6 | 10.10 (4.19–27.30) | 21 : 17 | 3.18 (1.57–6.45) |
| ≥4 | 521 : 1276 | 24 : 3 | 12.62 (3.57–44.54) | 16 : 17 | 1.78 (0.81–3.91) |
| P value for interaction | (0.80) | (0.74) | |||
| History of diabetes | |||||
| No | 3767 : 9756 | 142 : 19 | 15.74 (9.57–25.89) | 69 : 60 | 2.75 (1.89–4.01) |
| Yes | 906 : 942 | 41 : 6 | 6.69 (2.57–17.37) | 37 : 19 | 2.42 (1.27–4.60) |
| P value for interaction | (0.17) | (0.87) | |||
| Study area | |||||
| North America | 3536 : 6709 | 158 : 16 | 15.88 (9.25–27.27) | 82 : 54 | 2.53 (1.72–3.72) |
| Other | 1138 : 3994 | 25 : 9 | 9.68 (4.37–21.45) | 24 : 25 | 3.05 (1.69–5.52) |
| P value for interaction | (0.20) | (0.59) | |||
aPooled ORs were computed using logistic regression models adjusted for study, age, sex, race/ethnicity, education, body mass index, tobacco smoking, alcohol intake and history of diabetes.
bNo information on alcohol intake was available in the MSKCC study.
discussion
In this pooled data analysis involving 10 case–control studies and 5048 pancreatic cancer cases and 10 947 controls, we observed a nearly three-fold increased risk between history of pancreatitis (first diagnosed >2 years before cancer diagnosis) and diagnosis of pancreatic cancer. The strength of this association was consistent for intervals between diagnoses of pancreatitis and pancreatic cancer of greater than 2 years, including intervals of >25 years. This implies a role for chronic inflammation in the development of some pancreatic cancers [23]. The proportion of pancreatic cancer cases with a positive history of pancreatitis who reported pancreatitis within the 2 years of the diagnosis of their cancer was 58% (the corresponding proportion in controls before interview was 22%). Thus, a substantial proportion (at least 36%) of pancreatitis diagnosed among the patients with pancreatic cancer in PanC4 may have been a result of the tumor itself (e.g. tumor-associated ductal obstruction), or may have been the result of initial misdiagnosis or misreporting of some pancreatic cancer cases as pancreatitis [10]. Our final pooled analysis results were not greatly influenced by any one study that took part.
Pancreatitis has been investigated as a risk factor for pancreatic cancer in at least 25 studies (reviewed in REF. 9); however, only eight of these considered the time interval between diagnoses of pancreatitis and pancreatic cancer, and of these, only four were able to adjust for potential confounders such as cigarette smoking and alcohol intake [9]. Despite this, all of these studies suggested an increased risk of pancreatic cancer even for pancreatitis diagnosed many years before the diagnosis of pancreatic cancer.
In PanC4, the AFp for pancreatitis diagnosed >2 years before pancreatic cancer was estimated at 1.34% (95% CI: 0.61–2.07%). This suggests that 0.6–2% of all pancreatic cancer cases might be avoided if previous pancreatitis (diagnosed >2 years before) was eliminated or prevented. Our pooled OR estimate (of 2.71) for pancreatitis diagnosed >2 years before the diagnosis of cancer is lower than the RR of 5.8 estimated by Raimondi et al. in their recent meta-analysis of the association between pancreatitis (diagnosed >2 years before) and pancreatic cancer [8]. In their analysis the association was even stronger for hereditary pancreatitis (pooled RR: 69), but this estimate was based only on three studies [8].
When we investigated potential effect modification of the association between pancreatitis and pancreatic cancer, we found that younger patients with pancreatic cancer (<65 years) with a history of pancreatitis showed a substantially stronger association. This could imply that the type of pancreatitis more strongly associated with risk of pancreatic cancer is relatively more common in younger patients, and that there may be shared genetic influences. Indeed, hereditary pancreatitis has been shown to be associated with mutations in the cationic trypsinogen gene (PRSS1) and the serine protease inhibitor, Kazal type 1 gene (SPINK1) [24]. In some patients, these mutations may increase risk of pancreatitis diagnosis at younger ages, and in some of these patients, younger ages at diagnosis of pancreatic cancer [8, 24, 25]. Further, patients with hereditary pancreatitis may be at higher risk of pancreatic cancer than patients with other forms of pancreatitis [8, 24, 26–28].
In these pooled data analyses, the following were the drawbacks: the histories of pancreatitis were based upon self-report and we were unable to differentiate between acute and chronic pancreatitis or the various forms of chronic pancreatitis (alcoholic, hereditary, autoimmune, and idiopathic). However, the etiology of most (>70%) chronic pancreatitis in Western countries is thought to be related to chronic, excess alcohol intake with contributions from tobacco smoking and genetic factors [29]. Further, in some patients alcohol and smoking may be risk factors for progression from acute to chronic pancreatitis [30]. In addition, recurrent acute pancreatitis and chronic pancreatitis can be difficult to distinguish clinically, and the diagnostic criteria for chronic pancreatitis vary from center to center, creating a challenge for pooled analyses such as ours [8]. As a result, in our analysis we considered a history of any pancreatitis. It is also possible that most forms of pancreatitis, via inflammatory mechanisms, may contribute to an elevated risk of developing pancreatic cancer [8, 23]. Another drawback of our analyses is that we could not evaluate the timing, frequency, and duration of pancreatitis. The merits of our analysis include large sample sizes afforded due to consortial collaboration and individual-level data pooling and harmonization that allowed statistical adjustment for smoking, alcohol intake, BMI, history of diabetes, and other potential confounding or effect-modifying covariates. In conclusion, in this pooled data analysis of 5 048 pancreatic adenocarcinoma cases and 10 947 controls, we showed a nearly three-fold association between previous pancreatitis and pancreatic cancer, even for pancreatitis first diagnosed >25 years before the diagnosis of pancreatic cancer.
funding
The Pancreatic Cancer Family Registry at MSKCC was supported by the Prevention, Control and Population Research Goldstein Award; the society of MSKCC; and the Geoffrey Beene Cancer Research Fund. The Yale study was supported in part by National Institutes of Health grant 5R01CA098870; the cooperation of 30 Connecticut hospitals, including Stamford Hospital, in allowing patient access, is gratefully acknowledged. The Yale study was approved by the State of Connecticut Department of Public Health Human Investigation Committee. Certain data used in the Yale study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health. The NCI study was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The UCSF study was supported in part by National Institutes of Health grants (CA59706, CA108370, CA109767, CA89726, and CA121846) and by the Rombauer Pancreatic Cancer Research Fund. The collection of cancer incidence data for the UCSF study was supported by the California Department of Public Health as part of the statewide cancer reporting program; the National Cancer Institute's Surveillance, Epidemiology, and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The Milan study was supported by the Italian Association for Cancer Research (AIRC, Project no. 10068). The SEARCH study and the investigators were partially supported by The Ministry of Health, Welfare, and Sports (formerly Ministry of Welfare, Health, and Culture) of the Netherlands and the ‘ Europe against Cancer’ program of the Commission of the European Communities; and Fonds de recerche du Québec – Santé (FRSQ). The MD Anderson study was supported by the U.S. National Institutes of Health (CA98380). Financial support for ABL from the CD Smithers Foundation. EJD supported by the Spanish Ministry of Health (ISCIII RETICC RD06/0020). The authors assume sole responsibility for analyses and interpretation of these data.
disclosures
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The PanC4 consortium gratefully acknowledges the National Cancer Institute (NCI) for partial support for its annual meetings.
references
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393(4):535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 3.Bracci PM. Obesity and pancreatic cancer: Overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog. 2012;51(1):53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duell EJ. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol Carcinog. 2012;51(1):40–52. doi: 10.1002/mc.20786. [DOI] [PubMed] [Google Scholar]
- 5.Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51(1):64–74. doi: 10.1002/mc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risch HA. Pancreatic cancer: Helicobacter pylori colonization, N-Nitrosamine exposures, and ABO blood group. Mol Carcinog. 2012;51(1):109–118. doi: 10.1002/mc.20826. [DOI] [PubMed] [Google Scholar]
- 7.Bracci PM, Wang F, Hassan MM, et al. Pancreatitis and pancreatic cancer in two large pooled case-control studies. Cancer Causes Control. 2009;20(9):1723–1731. doi: 10.1007/s10552-009-9424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24(3):349–358. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Olson SH. Selected medical conditions and risk of pancreatic cancer. Mol Carcinog. 2012;51(1):75–97. doi: 10.1002/mc.20816. [DOI] [PubMed] [Google Scholar]
- 10.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328(20):1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 11.Anderson LN, Cotterchio M, Gallinger S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer Causes Control. 2009;20(6):825–834. doi: 10.1007/s10552-009-9303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holly EA, Eberle CA, Bracci PM. Prior history of allergies and pancreatic cancer in the San Francisco Bay area. Am J Epidemiol. 2003;158(5):432–441. doi: 10.1093/aje/kwg174. [DOI] [PubMed] [Google Scholar]
- 13.Howe GR, Ghadirian P, Bueno de Mesquita HB, et al. A collaborative case-control study of nutrient intake and pancreatic cancer within the search programme. Int J Cancer. 1992;51(3):365–372. doi: 10.1002/ijc.2910510306. [DOI] [PubMed] [Google Scholar]
- 14.Ji BT, Chow WH, Dai Q, et al. Cigarette smoking and alcohol consumption and the risk of pancreatic cancer: a case-control study in Shanghai, China. Cancer Causes Control. 1995;6(4):369–376. doi: 10.1007/BF00051413. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301(24):2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson SH, Orlow I, Simon J, et al. Allergies, variants in IL-4 and IL-4R alpha genes, and risk of pancreatic cancer. Cancer Detect Prev. 2007;31(5):345–351. doi: 10.1016/j.cdp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Risch HA, Yu H, Lu L, Kidd MS. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J Natl Cancer Inst. 2010;102(7):502–505. doi: 10.1093/jnci/djq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman DT. Risk factors for pancreatic cancer: a case-control study based on direct interviews. Teratog Carcinog Mutagen. 2001;21(1):7–25. doi: 10.1002/1520-6866(2001)21:1<7::aid-tcm3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Talamini R, Polesel J, Gallus S, et al. Tobacco smoking, alcohol consumption and pancreatic cancer risk: a case-control study in Italy. Eur J Cancer. 2010;46(2):370–376. doi: 10.1016/j.ejca.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Bertuccio P, La VC, Silverman DT, et al. Cigar and pipe smoking, smokeless tobacco use and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2011;22(6):1420–1426. doi: 10.1093/annonc/mdq613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucenteforte E, La VC, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23(2):374–382. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd edition. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 23.McKay CJ, Glen P, McMillan DC. Chronic inflammation and pancreatic cancer. Best Pract Res Clin Gastroenterol. 2008;22(1):65–73. doi: 10.1016/j.bpg.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287(2):G315–G319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 25.Raimondi S, Maisonneuve P, Lohr JM, Lowenfels AB. Early Onset Pancreatic Cancer: Evidence of a Major Role for Smoking and Genetic Factors. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1894–1897. doi: 10.1158/1055-9965.EPI-07-0341. [DOI] [PubMed] [Google Scholar]
- 26.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89(6):442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 27.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286(2):169–170. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 28.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2(3):252–261. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 29.Uomo G, Manes G. Risk factors of chronic pancreatitis. Dig Dis. 2007;25(3):282–284. doi: 10.1159/000103903. [DOI] [PubMed] [Google Scholar]
- 30.Lankisch PG, Breuer N, Bruns A, et al. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104(11):2797–2805. doi: 10.1038/ajg.2009.405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.