Abstract
Recent data from the AZURE, ABCSG-12, and ZO-FAST clinical trials have challenged our understanding of the potential anticancer activity of zoledronic acid (ZOL). Although the results of these studies may appear to be conflicting on the surface, a deeper look into commonalities among the patient populations suggest that some host factors (i.e. patient age and endocrine status) may contribute to the anticancer activity of ZOL. Indeed, data from these large clinical trials suggest that the potential anticancer activity of ZOL may be most robust in a low-estrogen environment. However, this may be only part of the story and many questions remain to be answered to fully explain the phenomenon. Does estrogen override the anticancer activity of ZOL seen in postmenopausal women? Are hormones other than estrogen involved that contribute to this effect? Does the role of bone turnover in breast cancer (BC) growth and progression differ in the presence of various estrogen levels? Here, we present a review of the multitude of factors affected by different endocrine environments in women with BC that may influence the potential anticancer activity of ZOL.
Keywords: anticancer, breast cancer, endocrine, estrogen, hormones, zoledronic acid
introduction
Recent clinical trial data have challenged our understanding of the potential anticancer activity of zoledronic acid (ZOL). Specifically, the AZURE, ABCSG-12, and ZO-FAST studies are large clinical trials that have reported on the potential anticancer activity of ZOL in women with breast cancer (BC). Although the results of these studies appear confounding at first glance, a deeper look into commonalities of the specific patient populations provides clues regarding host factors (i.e. patient age and endocrine status) that may contribute to the optimal anticancer activity of ZOL.
For example, the AZURE trial evaluated the potential anticancer effects of ZOL in combination with standard adjuvant therapy (i.e. chemotherapy and endocrine therapy) in pre- and postmenopausal women with early (stage II/III) BC (N = 3360). This heterogeneous group received standard adjuvant therapy (chemotherapy±endocrine therapy) plus a tapered dosing schedule of ZOL (4 mg every 3–4 weeks ×6; 4 mg every 3 months ×8; 4 mg every 6 months ×5). Although the primary end point of disease-free survival (DFS) was not met in the overall patient population, prospective subgroup analyses showed that ZOL significantly improved invasive DFS by 25% [hazard ratio (HR) = 0.75; P = 0.02] and overall survival (OS) by 26% (HR = 0.74; P = 0.04) in women who were more than 5 years postmenopausal at study entry (n = 1041) [1].
In contrast with the AZURE study, only premenopausal women (median age, 45 years) with endocrine-responsive early-stage BC undergoing ovarian suppression (N = 1803) were enrolled in ABCSG-12 [2]. However, the endocrine status of these women was likely similar to those in the postmenopausal subgroup in the AZURE trial. Indeed, in premenopausal women, ovarian suppression with a luteinizing hormone (LH)-releasing hormone agonist (e.g. goserelin) decreases circulating estrogen to postmenopausal levels for the duration of treatment and may have long-term efficacy [3]. In fact, all of the women in ABCSG-12 experienced endocrine therapy-induced artificial menopause due to goserelin therapy for 3 years. Moreover, half of these patients also received anastrozole [2], an aromatase inhibitor (AI) that might have depressed estrogen levels further.
Notably, the results from ABCSG-12 showed that the addition of ZOL to adjuvant endocrine therapy significantly improved DFS (HR = 0.72; P = 0.014) and OS (HR = 0.63; P = 0.049) at 84 months' median follow-up, and reduced recurrences both in and outside of bone [4]. In addition, preplanned subgroup analyses based on age (≤40 years or >40 years) showed that ZOL significantly improved DFS by 34% in women over 40 years of age (n = 1390; HR = 0.66; P = 0.013). However, ZOL did not improve the DFS in women who were 40 years of age or younger (n = 413) [4]. These data support previously published reports showing that women over 40 years of age may achieve more complete estrogen suppression [5–8]. Furthermore, chemotherapy-induced amenorrhea has been reported to occur less frequently in women less than 40 years of age compared with those over 40 [5, 6].
Similar results were observed in the ZO-FAST trial, in which postmenopausal women receiving adjuvant letrozole therapy for stage I–III BC (N = 1065) received ZOL (upfront versus delayed-start ZOL) to prevent AI-associated bone loss [9, 10]. After 60 months' median follow-up, the addition of upfront ZOL to AI therapy was associated with a 34% reduction in the risk of DFS events (disease recurrence or death) compared with delayed ZOL (HR = 0.66; P = 0.0375) [10]. It should be noted that this significant improvement in DFS also was observed in a low-estrogen environment. Indeed, AIs (i.e. anastrozole and letrozole) reduce circulating estrogens to levels substantially lower than those observed after natural menopause [11]. Moreover, letrozole, the AI used in the ZO-FAST trial, reduces estradiol levels to a greater extent than anastrozole in postmenopausal women [11, 12].
Taken together, these data provide supporting evidence that the possible anticancer activity of ZOL may be most robust in a low-estrogen environment. However, this may be only part of the story, and many questions remain to be answered before the phenomenon can be fully explained. Does estrogen override the anticancer activity of ZOL seen in postmenopausal women with BC? With 200–300 estrogen-response genes in the human genome [13, 14], can the responsible pathways be narrowed down? Do hormones other than estrogen modulate ZOL's potential anticancer effect in this patient population? Does the role of bone turnover in BC tumor growth and progression change in response to different estrogen levels?
Another issue that confounds interpretation of available data from clinical studies is defining endocrine status. There is no standard definition of ‘postmenopausal’ to enable the comparison of between-trial results, and estrogen levels were not repeatedly measured in the majority of BC clinical trials. Other important questions also remain unanswered. For example, how closely do animal models of a low-estrogen environment (e.g. ovariectomy and pharmacologic aromatase inhibition) mimic the postmenopausal status in women with BC? Endocrine systems differ between humans and animals, making the clinical relevance of preclinical results unknown. Although we cannot answer all questions at this time, an extensive review of the literature has provided insight into the complex interplay between the bone microenvironment, the endocrine system, and antiresorptive therapies that may help to clarify the clinical trial data reported to date.
nitrogen-containing bisphosphonates and bone
Nitrogen-containing bisphosphonates (N-BPs) affect a multitude of cellular pathways important for cell growth and differentiation. These pathways likely modulate the multifactorial mechanism of action of ZOL-mediated anticancer activity. Bisphosphonates (BPs) are cleared rapidly from the blood stream via avid binding to mineralized bone surfaces undergoing active remodeling and by renal filtration of unbound drug [15]. Furthermore, as these agents do not readily cross the plasma membrane, the intracellular concentration of BPs in most tissues is very low [16]. This combination of properties leads to BPs accumulating only in those cells that exhibit fluid-phase endocytosis, such as osteoclasts, macrophages [17, 18], and monocytes [19]. Thus, in addition to targeting bone remodeling, BPs can potentially affect other tissues through accumulation within other cells, especially those of the immune system. Notably, human cancer cell lines (e.g. BC, prostate cancer, and myeloma) also have been shown to internalize BPs by fluid-phase endocytosis [20].
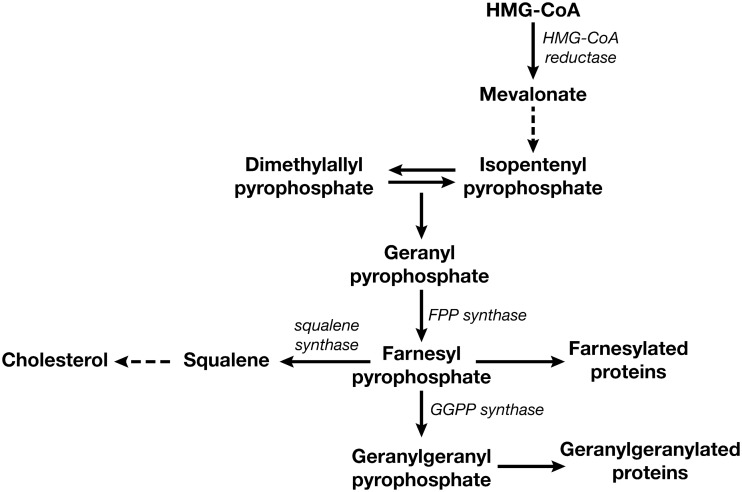
Once internalized, N-BPs influence multiple pathways and effectively inhibit osteoclast-mediated bone resorption through inhibition of the mevalonate pathway (Figure 1) [21]. This is an important biochemical pathway involved in the production of cholesterol and isoprenoids, which are required for maintaining cell-membrane integrity, producing steroids, and regulating cellular metabolism. Furthermore, isoprenyl precursors are crucial for the prenylation of regulatory proteins involved in the control of cell proliferation, tumor progression, and cell death induced by anticancer therapies. These factors suggest that inhibition of the mevalonate pathway may have an effect on cellular activities that goes beyond inhibition of bone resorption (i.e. anticancer activity).
Figure 1.
The mevalonate pathway is important in the synthesis of cholesterol, and of farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which provide the farnesyl and geranylgeranyl groups, respectively, for protein prenylation. Reproduced from Shipman et al. [21].
The major molecular target inhibited by all N-BPs is farnesyl pyrophosphate synthase (FPPS), an enzyme in the mevalonate pathway [22]. Geranylgeranyl pyrophosphate synthase (GGPPS), another downstream component of the mevalonate pathway, also has been shown to be inhibited, although less actively, by ZOL [23]. Inhibition of FPPS and GGPPS causes a reduction in the posttranslational prenylation of small guanosine triphosphatases (GTPases) (‘G proteins’; e.g. Ras, Rho, and Rac), which are key components of many intracellular signaling pathways and play key roles in cell proliferation and survival. As a result, inhibition of prenylation leads to loss of cellular functions and induces apoptosis.
The biochemical effects of N-BPs may not be limited to the mevalonate pathway, and modulation of other pathway components, such as inhibition of kinases and phosphatases, cannot be excluded. Although little direct evidence is available, these putative additional biochemical effects of N-BPs appear intuitive from their chemical structure. Moreover, the effects of N-BPs on osteoclast-mediated bone resorption and several anticancer effects (e.g. decreased cancer cell viability, proliferation, adhesion and invasion, modulation of endothelial cell function and angiogenesis, and decreased macrophage activity) are closely correlated with FPPS inhibition. Therefore, potential mechanisms of action for the likely anticancer activity of N-BPs, including ZOL, may act through modulation of several complex biochemical pathways downstream of FPPS inhibition. Indeed, numerous studies have demonstrated potential anticancer activity of BPs in preclinical BC model systems [24, 25] (supplementary Table S1, available at Annals of Oncology online).
In preclinical model systems, ZOL was the most potent inhibitor of FPPS activity among the N-BPs tested, and correlated with the greatest antiresorptive activity in vitro and in vivo [19, 22, 25]. In addition to inhibiting FPPS, N-BPs have been shown to induce the production of an intracellular adenosine triphosphate analogue (triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester [ApppI]) that can directly induce cellular apoptosis and modulate the immune response [20]. As a result, N-BPs interfere with multiple cellular functions required for the bone-resorbing activity and survival of osteoclasts. Moreover, the cellular functions affected by N-BPs may also be involved in cancer cell growth as well as osteoclast survival. Additionally, a multitude of other factors in and outside of the bone microenvironment may influence the relative activity of ZOL.
It should be noted that preclinical studies have shown that ZOL inhibits osteoclast activity in animal models of both benign and malignant disease regardless of gender or endocrine status (i.e. estrogen-deficient compared with normal females) [26–50]. It is well established that ZOL potently inhibits osteoclast-mediated bone resorption in female animals rendered estrogen-deficient via ovariectomy or aromatase inhibition [26–29], similar to the endocrine environment of postmenopausal women receiving ZOL to maintain bone health in the osteoporosis or adjuvant BC settings. However, preclinical studies also have shown ZOL to be equally effective in nonmalignant male and nonovariectomized female animal models [31–37], suggesting that ZOL-mediated osteoclast inhibition is independent of the hormone environment. Furthermore, the potential anticancer activity of ZOL has been demonstrated in malignant tumor models in both male and nonovariectomized female animals [38–50]. It should be noted that most experiments use young animals with inherently high rates of bone turnover, a markedly different bone environment from that found in premenopausal women. These data suggest that additional factors independent of osteoclast inhibition may contribute to the anticancer activity of ZOL observed in AZURE, ABCSG-12, and ZO-FAST.
In the clinical setting, ZOL has been shown to improve bone mineral density (BMD) in men and women with cystic fibrosis [51], women with postmenopausal osteoporosis [52, 53], and premenopausal women receiving adjuvant chemotherapy for BC [54–56]. Thus, ZOL-mediated osteoclast inhibition and subsequent bone resorption appear to be independent of estrogen levels. Combined with the results of the AZURE and ABCSG-12 trials, these preclinical and clinical data imply that ZOL may affect other cell types or pathways modulated by estrogen levels [57]. Because ZOL rapidly binds to bone and soft tissue exposure is low, these target cells may be residing in bone marrow (e.g. dormant tumor cells and endothelial precursor cells) or could be cells that can efficiently internalize ZOL (e.g. macrophages and monocytes).
bone microenvironment
Although the cellular and molecular mechanisms by which a cancer cell undergoes metastasis are largely unknown, studies show that bone marrow produces a number of growth factors and cytokines that attract cancer cells [58–60]. These factors are secreted by bone marrow-derived stem cells in the bone microenvironment, providing a supportive niche that facilitates cancer cell survival and proliferation [61, 62]. Furthermore, the molecular interactions between the bone marrow microenvironment and cancer cells may shield cancer cells from cytotoxic chemotherapy, allowing them to remain dormant for extended periods of time before becoming active and metastasizing to secondary sites [58–62]. As a result, the bone marrow acts as a sanctuary for cancer cells, which can contribute to subsequent relapse in bone and other sites [61, 62].
The potential anticancer activity of ZOL may be mediated through its effects on the bone marrow microenvironment, macrophages, and myeloid-derived suppressor cells, and may be independent of its osteoclast-inhibition activity [40, 43, 49]. Specifically, ZOL may impede the development of bone metastases by rendering the bone microenvironment less conducive to cancer cell survival and proliferation [19, 22, 25]. Furthermore, the role of bone marrow-derived stem cells in the development of extraskeletal metastases might be influenced by a patient's endocrine status, and the ability of ZOL to help maintain cancer cell dormancy in bone marrow may be counteracted by various hormones in premenopausal women. This hypothesis is supported by the subset analyses in postmenopausal women in the AZURE study, which showed that the potential anticancer activity of ZOL observed in this patient population occur outside of bone. Only a weak, nonsignificant effect on bone recurrence was observed irrespective of menopausal status, with all of the anticancer activity observed in postmenopausal women occurring outside of bone [1].
impact of hormones on the bone microenvironment
A number of hormones in addition to estrogens (estradiol, estriol, and estrone) and progesterone are involved in regulating ovarian function in women. These include follicle-stimulating hormone (FSH), LH, activins, inhibins, follistatin, anti-Müllerian hormone (AMH), bone morphogenetic protein (BMP), and androgens [testosterone, androstenedione, and dehydroepiandrosterone sulfate (DHEAS)]. Some of these hormones, such as activins, inhibins, BMPs, and androgens, also have important effects on bone. Indeed, DHEAS has been shown to suppress secretion of the bone resorption-promoting cytokine, interleukin (IL)-6, by human bone marrow cells obtained from postmenopausal women [63]. Furthermore, activins, inhibins, and BMPs belong to the transforming growth factor-β (TGF-β) superfamily of growth and differentiation factors, which are known to influence bone physiology [64].
Several TGF-β family members (e.g. BMPs, TGF-β1, TGF-β2, and TGF-β3) are involved in bone remodeling [65, 66] and have been shown to induce osteoblast replication in culture [65]. Although, however, AMH belongs to the TGF-β superfamily, it is unknown if this hormone is involved in bone remodeling [67]. Studies have shown that TGF-β1-3 can enhance collagen and noncollagen protein synthesis in bone, increase production of proteoglycans, enhance differentiation of mesenchymal cells, and increase replication of chondroblasts [65, 66]. In contrast with its effects in undifferentiated cells, TGF-β is known to decrease cell proliferation and function in differentiated cells [65]. Studies also have shown that TGF-β stimulates IL-6 and IL-11 secretion from bone marrow stromal cells in culture [68]. This is of interest because IL-6 has been shown to enhance estrogen receptor-α-positive (ERα+) BC cell line (MCF-7) growth [69]. Furthermore, bone marrow-derived cytokines (e.g. IL-6) and related factors have been shown to affect BC metastasis, and preclinical evidence suggests that bone marrow-derived cytokines may enhance tumor cell proliferation, survival, and invasiveness [69–71]. Together, these data suggest that TGF-β can stimulate BC cell growth via IL-6.
Examining the pathways affected by members of the TGF-β superfamily can be complex, as family members often regulate the activity of one another. For example, betaglycan, a TGF-β type III receptor, is a membrane-anchored proteoglycan receptor that binds TGF-β1, TGF-β2, inhibin A, and BMP-2, -4, and -7, thereby modulating their actions [72–74]. Moreover, inhibins, follistatin, and BMP-3 are receptor antagonists that can block BMP signaling in bone, which modulates osteoblast and osteoclast development [75–77]. Notably, inhibin A in particular has been shown to influence bone health in women regardless of menopausal status [78].
Serum inhibin levels inversely correlate with bone-formation and bone-resorption markers in pre- and perimenopausal women and across the menopause transition, with bone turnover increasing as inhibin levels fall [75, 78]. Inhibins bind to cells during osteoblastogenesis and osteoclastogenesis and can block BMP-stimulated osteoblast and osteoclast development [75]. Furthermore, continuous in vivo exposure of gonadectomized mice to inhibin A is anabolic, and cycling inhibin exposure suppresses bone turnover, suggesting a bimodal mechanism of action for endocrine regulation of bone metabolism [75]. Moreover, an inducible human inhibin A transgenic mouse model showed that this hormone increased BMD and bone volume, and prevented gonadectomy-associated loss of BMD and bone volume [79].
Estrogen, a primary regulator of ovarian function, is also known to affect bone health in women [68, 75, 80–84]. One mechanism by which the estrogen estradiol may act on bone is through a pathway involving the receptor activator of nuclear factor-kappa B (RANK), RANK ligand (RANKL), and osteoprotegerin (OPG) in which RANKL induces osteoclast activity and OPG is a physiologic inhibitor of RANKL [85–88]. Studies in mouse models have shown that inactivation of ERα causes increased expression of RANKL and decreased expression of OPG [86]. These results have been confirmed in other mouse studies, which showed that withdrawal of estradiol reduced OPG levels while addition of estradiol increased OPG levels [87, 88]. Therefore, the loss of estradiol leads to increased osteoclast-mediated bone resorption subsequent to elevated levels of RANKL and decreased levels of OPG.
These data highlight the effect of estrogen deficiency on bone turnover and add to the hypothesis that estrogen deprivation enhances all aspects of bone turnover. Moreover, in a small study of patients with cancer (N = 49) ZOL therapy decreased RANKL expression by 22% and increased OPG expression by 96%, thereby altering the RANKL/OPG ratio in favor of bone formation [89]. Creating a less hospitable microenvironment in bone for cancer cell growth through bone turnover suppression, in addition to possible direct anticancer effects of BPs, may be a potential mechanism for the improved DFS observed with ZOL in the AZURE, ABCSG-12, and ZO-FAST trials.
hormonal transitions during early and late menopause
It is evident from the literature that hormones and cytokines involved in ovarian function also contribute to the dynamic bone microenvironment. However, the extent to which each of these factors contributes to various processes differs based on menopausal status. Natural menopause is the permanent end of menstruation due to cessation of ovarian follicular activity (i.e. the absence of oocytes in the ovaries) [90]. Cancer therapies (i.e. chemotherapy and endocrine therapy) can induce artificial menopause, which may be temporary. Notably, a confounding factor in using data from several of the large BC clinical trials is that each study used different, and often vague, definitions of ‘postmenopausal’ (Table 1) [1, 2, 9, 91–93]. Indeed, the term ‘postmenopausal’ is imprecise and can lead to confusion. To clarify, the events leading to and immediately following natural menopause can be divided into several stages: perimenopause, the menopausal transition, and menopause. Perimenopause refers to the time when the endocrine, biologic, and clinical features of menopause begin, which can be any time from several years before menopause to 1 year after the final menses [90]. The menopausal transition refers to the portion of perimenopause up to the final menses, whereas postmenopause refers to the time 12 months after the final menses. Levels of hormones involved in ovarian function vary greatly during each of these stages.
Table 1.
Definitions of menopause at study entry in adjuvant breast cancer clinical trials
| Study (N) | Not menopausal (n) | Postmenopausal (n) |
|---|---|---|
| AZURE [1] (N = 3360) | Premenopausal, ≤5 years after final menses, or of unknown menopausal status (n = 2318) | >5 years after final menses (n = 1041) |
| ABCSG-12 [2] (N = 1803) | Premenopausal: All patients developed amenorrhea due to ovarian suppression with goserelin | Not defined (none) |
| ZO-FAST [9] (N = 1065) | Premenopausal (none) | Established = Age ≥55 years and natural cessation of mensesa |
| Recent = Age <55 years and cessation of menses induced by chemotherapy or LHRH suppression: Amenorrheic <1 year and LH and FSH >40 iu/L or estradiol <5 ng/dL; or amenorrheic ≥1 yeara | ||
| ATAC [91] (N = 9366) | Premenopausal (none) | Bilateral oophorectomy, OR >60 years of age, OR 45–59 years of age with an intact uterus and ≥12 months of amenorrhea |
| For <12 months of amenorrhea (including history of hysterectomy or HRT), FSH required to be within ‘postmenopausal range’ | ||
| BIG 1–98 [92] (N = 8010) | Premenopausal (n = 23, ineligible) | Cessation of menses: Before chemotherapy (n = 7692) After chemotherapy (n = 192) Uncertain or unknown menstrual status (n = 103) |
| TEAM [93] (N = 9766) | Premenopausal (none) | Cessation of menses: <50 years (n = 331) 50–59 years (n = 3017) >60 years (n = 6418) |
FSH, follicle-stimulating hormone; HRT, hormone-replacement therapy; LH, luteinizing hormone; LHRH, luteinizing hormone-releasing hormone.
aDefinitions are based on Novartis Pharmaceuticals Corporation data on file.
Hormones that vary during perimenopause include AMH, estradiol, FSH, LH, and inhibins A and B. Serum levels of AMH, which are detectable from before birth through menopause, decline continuously with age in healthy women and become undetectable after menopause [67]. Low and rapidly declining AMH serum levels directly correlate with ovarian follicle reserves, making it a useful marker for ovarian function [67]. Notably, serum levels of AMH are elevated in women with polycystic ovary syndrome but are very low in women with ovarian failure. Thus, AMH can act as a marker of ovarian function in women approaching natural menopause and in women with cancer therapy-induced artificial menopause.
Other hormones (i.e. estradiol, FSH, and LH) regulating ovarian function have more complicated patterns during perimenopause because their levels also fluctuate during the menstrual cycle. However, overall, serum FSH and LH levels increase as inhibin B levels decrease during the menopausal transition [90, 94]. Changes in these hormone levels are linked to age, as progressive increases in serum FSH levels have been observed with increasing age in regularly cycling women over 40 years of age. Furthermore, it appears that inhibin B levels drop when the remaining ovarian follicles have reached a critically low number. During peri- and postmenopause, FSH and LH levels rise. In contrast, estradiol levels remain relatively constant until late perimenopause [90]. However, after the final menses, during late perimenopause and early postmenopause, estradiol levels decline rapidly, only stabilizing 2–3 years after menopause [95, 96].
Complicating matters further, after menopause and the subsequent loss of ovarian estrogen production, the enzyme aromatase converts circulating adrenal androgens (testosterone and androstenedione) to estrogens in peripheral tissues (e.g. breast, adipose, brain, and muscle) [97]. However, androgen levels (i.e. testosterone, androstenedione, and DHEAS) remain stable throughout perimenopause, although they do decline with age [90, 97]. Despite these overall trends, serum estradiol and inhibin (A and B) concentrations may fluctuate widely in individual women during the menopausal transition. The general overall changes in FSH, inhibins A and B, and estradiol levels during pre-, peri-, and postmenopause are depicted in Figure 2 [90, 98].
Figure 2.
Mean levels (with lower 95% confidence intervals) of (A) follicle-stimulating hormone (FSH), (B) immunoreactive inhibin (IR-INH), (C) inhibin A, (D) inhibin B, and (E) estradiol as a function of menopausal status. Group 1: premenopausal, without any change in menstrual cycle pattern; group 2: early perimenopausal, with a reported change in cycle frequency but experiencing menses in the preceding 3 months; group 3: late perimenopausal, with no menses in the preceding 3–11 months; group 4: postmenopausal, with no menses for more than 12 months. Values with the same superscript (* or †) are not statistically different; values with differing superscripts are significantly different, P < 0.05 [90]. Reproduced from Burger et al. [98]. Copyright Blackwell Science Ltd.
We already know that there is a constant interplay between the bone microenvironment, the endocrine system, and menopausal status. For example, high-serum FSH is associated with postmenopausal bone loss, and these effects are likely independent of estrogen [99]. Haploinsufficient FSHβ+/− mice with normal ovarian function have increased bone mass and decreased osteoclastic resorption, suggesting that FSH-mediated effects on bone remodeling are estrogen independent [99]. Thus, osteoclast-mediated bone resorption increases with rising FSH levels during peri- and postmenopause. Another study showed that, in women over 60 years of age, estradiol is associated with the conversion of thick trabeculae into thinner, but more numerous, trabeculae, thereby altering the microstructure of trabecular bone [100]. Furthermore, age and estrogen status, two other mediators of bone metabolism, correlate with cytokine secretion by the bone marrow [68]. As a result, bone health in women is tightly linked to ovarian function and endocrine status.
The effects of the endocrine system on bone health may extend to influences of the bone microenvironment on BC growth and progression. Menopause and related hormonal changes influence cytokine and growth factor production by bone marrow cells. These factors may play important direct or indirect roles in modulating the survival, invasion, and adhesion of disseminated tumor cells. Indeed, several studies show that cytokine (e.g. IL-6 and TGF-β) secretion by bone marrow stromal cells is influenced by menopausal status [63, 68, 80, 83, 101]. These studies provide insight into how hormonal changes during the menopausal transition might alter the bone marrow microenvironment for BC recurrence. Because of this, it is conceivable that many of these factors have undefined roles that may influence the efficacy of ZOL in various patient populations.
discussion
The findings of the AZURE, ABCSG-12, and ZO-FAST subset analyses suggest that hormonal effects on the bone microenvironment may play a substantial role in determining who may benefit most from adjuvant ZOL therapy. Current clinical data suggest that both hormone suppression and reduction of bone-turnover–derived growth factors are needed for sufficient suppression of dormant micrometastases in patients with early-stage BC. In retrospect, given the number of cellular pathways affected by the mevalonate pathway, TGF-β family members, and hormones affected by menopause, it is not surprising that the likely anticancer effects of ZOL, in and outside of bone, may be modulated by the patient's endocrine environment. Because some of the hormones involved in ovarian function also modulate bone health (e.g. activins, inhibins, and BMPs), these hormones also may influence the observed anticancer activity of ZOL in patients with BC. Clinical studies examining the endocrine profiles of women receiving ZOL during adjuvant BC therapy may help elucidate the role of the patient's endocrine environment.
The multitude of biochemical pathways that may be influenced by estrogen is an additional complication. Indeed, ∼200–300 estrogen-responsive genes have been identified in the human genome, suggesting a vast number of potential functions for estrogen [13, 14]. Studies examining estrogen-regulated pathways that may affect potential ZOL-mediated anticancer activity in women with BC could lead to a better understanding of its mechanism of action in this setting. Furthermore, the growth and differentiation factor TGF-β and small GTPases, the prenylation of which is controlled by the mevalonate pathway, are involved in regulating each other in various pathways [102–105]. This suggests that inhibition of the mevalonate pathway with ZOL also may influence TGF-β–mediated pathways. Finally, it is also possible that the potential anticancer effects of ZOL are masked in a high-estrogen environment because the increased estrogen levels enhance tumor growth in patients with estrogen receptor-positive BC. Preclinical and clinical studies may help elucidate the underlying mechanisms of the potential ZOL-mediated anticancer activity observed in women with a low-estrogen environment and thus help define the subpopulation of patients mostly likely to benefit from adjuvant ZOL therapy.
funding
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. The ZO-FAST study was sponsored by Novartis, whereas ABCSG-12 and AZURE are academic studies that received no support toward research costs from Novartis. No payments were made to the authors for developing this manuscript, and Novartis did not influence the content or views expressed, except for Jonathan Green, PhD (consultant for Novartis Pharma AG).
disclosure
Dr Hadji has received honoraria, unrestricted educational grants, and research funding from the following companies: Amgen, Astra Zeneca, Glaxo Smith Kline, Elli Lilly, Novartis, Pfizer, and Roche. Dr Coleman has received honoraria and research funding to his institution from Novartis and given expert testimony on their behalf. Dr Gnant has received consultancy fees (Novartis, Merrion), speaker's fees (Roche, Amgen, Novartis, Astra Zeneca, Glaxo Smith Kline), and unrestricted grant/research funding (Roche, sanofi-aventis, GlaxoSmithKline, Novartis, AstraZeneca). Dr Green is a paid external consultant for Novartis, the makers of Zometa, and holds stock in the company.
Supplementary Material
acknowledgements
We thank Duprane Pedaci Young, PhD, ProEd Communications, Inc., for her medical editorial assistance with this manuscript.
references
- 1.Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. doi:10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 2.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. doi:10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 3.Hackshaw A, Baum M, Fornander T, et al. Long-term effectiveness of adjuvant goserelin in premenopausal women with early breast cancer. J Natl Cancer Inst. 2009;101:341–349. doi: 10.1093/jnci/djn498. doi:10.1093/jnci/djn498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Long-term follow-up in ABCSG-12: significantly improved overall survival with adjuvant zoledronic acid in premenopausal patients with hormone-receptor-positive early breast cancer. In 34th Annual San Antonio Breast Cancer Symposium (Abstr S1–2); San Antonio, TX. 2011. [Google Scholar]
- 5.Del Mastro L, Venturini M, Sertoli MR, et al. Amenorrhea induced by adjuvant chemotherapy in early breast cancer patients: prognostic role and clinical implications. Breast Cancer Res Treat. 1997;43:183–190. doi: 10.1023/a:1005792830054. doi:10.1023/A:1005792830054. [DOI] [PubMed] [Google Scholar]
- 6.Gnant M. Bisphosphonates in the prevention of disease recurrence: current results and ongoing trials. Curr Cancer Drug Targets. 2009;9:824–833. doi: 10.2174/156800909789760267. doi:10.2174/156800909789760267. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez-Gordo AM, de las Heras B, Zamora P, et al. Failure of goserelin ovarian ablation in premenopausal women with breast cancer: two case reports. Gynecol Oncol. 2000;76:126–127. doi: 10.1006/gyno.1999.5641. doi:10.1006/gyno.1999.5641. [DOI] [PubMed] [Google Scholar]
- 8.Uncu G, Benderli S, Esmer A. Pregnancy during gonadotrophin-releasing hormone agonist therapy. Aust N Z J Obstet Gynaecol. 1996;36:484–485. doi: 10.1111/j.1479-828x.1996.tb02200.x. doi:10.1111/j.1479-828X.1996.tb02200.x. [DOI] [PubMed] [Google Scholar]
- 9.Bundred NJ, Campbell ID, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST study results. Cancer. 2008;112:1001–1010. doi: 10.1002/cncr.23259. doi:10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 10.de Boer RH, Bundred N, Eidtmann H, et al. Long-term survival outcomes among postmenopausal women with hormone receptor-positive early breast cancer receiving adjuvant letrozole and zoledronic acid: 5-year follow-up of ZO-FAST. In 34th Annual San Antonio Breast Cancer Symposium (Abstr S1–3); San Antonio, TX. 2011. [Google Scholar]
- 11.Dixon JM, Renshaw L, Young O, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol. 2008;26:1671–1676. doi: 10.1200/JCO.2007.13.9279. doi:10.1200/JCO.2007.13.9279. [DOI] [PubMed] [Google Scholar]
- 12.Geisler J, Haynes B, Anker G, et al. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–757. doi: 10.1200/JCO.2002.20.3.751. doi:10.1200/JCO.20.3.751. [DOI] [PubMed] [Google Scholar]
- 13.Bourdeau V, Deschenes J, Metivier R, et al. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. doi:10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- 14.Tang S, Tan SL, Ramadoss SK, et al. Computational method for discovery of estrogen responsive genes. Nucleic Acids Res. 2004;32:6212–6217. doi: 10.1093/nar/gkh943. doi:10.1093/nar/gkh943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skerjanec A, Berenson J, Hsu C, et al. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J Clin Pharmacol. 2003;43:154–162. doi: 10.1177/0091270002239824. doi:10.1177/0091270002239824. [DOI] [PubMed] [Google Scholar]
- 16.Weiss HM, Pfaar U, Schweitzer A, et al. Biodistribution and plasma protein binding of zoledronic acid. Drug Metab Dispos. 2008;36:2043–2049. doi: 10.1124/dmd.108.021071. doi:10.1124/dmd.108.021071. [DOI] [PubMed] [Google Scholar]
- 17.Coxon FP, Thompson K, Roelofs AJ, et al. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone. 2008;42:848–860. doi: 10.1016/j.bone.2007.12.225. doi:10.1016/j.bone.2007.12.225. [DOI] [PubMed] [Google Scholar]
- 18.Thompson K, Rogers MJ, Coxon FP, et al. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol Pharmacol. 2006;69:1624–1632. doi: 10.1124/mol.105.020776. doi:10.1124/mol.105.020776. [DOI] [PubMed] [Google Scholar]
- 19.Roelofs AJ, Thompson K, Ebetino FH, et al. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. doi:10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 20.Monkkonen H, Kuokkanen J, Holen I, et al. Bisphosphonate-induced ATP analog formation and its effect on inhibition of cancer cell growth. Anticancer Drugs. 2008;19:391–399. doi: 10.1097/CAD.0b013e3282f632bf. doi:10.1097/CAD.0b013e3282f632bf. [DOI] [PubMed] [Google Scholar]
- 21.Shipman CM, Rogers MJ, Vanderkerken K, et al. Bisphosphonates–mechanisms of action in multiple myeloma. Acta Oncol. 2000;39:829–835. doi: 10.1080/028418600750063587. doi:10.1080/028418600750063587. [DOI] [PubMed] [Google Scholar]
- 22.Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. doi:10.1002/1097-0142(20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Guo RT, Cao R, Liang PH, et al. Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases. Proc Natl Acad Sci USA. 2007;104:10022–10027. doi: 10.1073/pnas.0702254104. doi:10.1073/pnas.0702254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton E, Clay TM, Blackwell KL. New perspectives on zoledronic acid in breast cancer: potential augmentation of anticancer immune response. Cancer Invest. 2011;29:533–541. doi: 10.3109/07357907.2011.605413. doi:10.3109/07357907.2011.605413. [DOI] [PubMed] [Google Scholar]
- 25.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34:453–475. doi: 10.1016/j.ctrv.2008.02.004. doi:10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Binkley N, Kimmel D, Bruner J, et al. Zoledronate prevents the development of absolute osteopenia following ovariectomy in adult rhesus monkeys. J Bone Miner Res. 1998;13:1775–1782. doi: 10.1359/jbmr.1998.13.11.1775. doi:10.1359/jbmr.1998.13.11.1775. [DOI] [PubMed] [Google Scholar]
- 27.Gasser JA, Ingold P, Venturiere A, et al. Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat. J Bone Miner Res. 2008;23:544–551. doi: 10.1359/jbmr.071207. doi:10.1359/jbmr.071207. [DOI] [PubMed] [Google Scholar]
- 28.Hornby SB, Evans GP, Hornby SL, et al. Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int. 2003;72:519–527. doi: 10.1007/s00223-002-2015-4. doi:10.1007/s00223-002-2015-4. [DOI] [PubMed] [Google Scholar]
- 29.Gasser JA, Green JR, Shen V, et al. A single intravenous administration of zoledronic acid prevents the bone loss and mechanical compromise induced by aromatase inhibition in rats. Bone. 2006;39:787–795. doi: 10.1016/j.bone.2006.04.035. doi:10.1016/j.bone.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Pataki A, Muller K, Green JR, et al. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat Rec. 1997;249:458–468. doi: 10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N. doi:10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Le Goff B, Soltner E, Charrier C, et al. A combination of methotrexate and zoledronic acid prevents bone erosions and systemic bone mass loss in collagen induced arthritis. Arthritis Res Ther. 2009;11:R185. doi: 10.1186/ar2877. doi:10.1186/ar2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sims NA, Green JR, Glatt M, et al. Targeting osteoclasts with zoledronic acid prevents bone destruction in collagen-induced arthritis. Arthritis Rheum. 2004;50:2338–2346. doi: 10.1002/art.20382. doi:10.1002/art.20382. [DOI] [PubMed] [Google Scholar]
- 33.Lee JI, Kim HW, Rhee WI, et al. The beneficial effect of intravenous zoledronic acid therapy following an acute stroke in rats. Bone. 2006;39:377–382. doi: 10.1016/j.bone.2006.02.055. doi:10.1016/j.bone.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 34.Podworny NV, Kandel RA, Renlund RC, et al. Partial chondroprotective effect of zoledronate in a rabbit model of inflammatory arthritis. J Rheumatol. 1999;26:1972–1982. [PubMed] [Google Scholar]
- 35.Little DG, Peat RA, McEvoy A, et al. Zoledronic acid treatment results in retention of femoral head structure after traumatic osteonecrosis in young Wistar rats. J Bone Miner Res. 2003;18:2016–2022. doi: 10.1359/jbmr.2003.18.11.2016. doi:10.1359/jbmr.2003.18.11.2016. [DOI] [PubMed] [Google Scholar]
- 36.Muller K, Wiesenberg I, Jaeggi K, et al. Effects of the bisphosphonate zoledronate on bone loss in the ovariectomized and in the adjuvant arthritic rat. Arzneimittelforschung. 1998;48:81–86. [PubMed] [Google Scholar]
- 37.Little DG, Smith NC, Williams PR, et al. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res. 2003;18:1300–1307. doi: 10.1359/jbmr.2003.18.7.1300. doi:10.1359/jbmr.2003.18.7.1300. [DOI] [PubMed] [Google Scholar]
- 38.Peyruchaud O, Winding B, Pecheur I, et al. Early detection of bone metastases in a murine model using fluorescent human breast cancer cells: application to the use of the bisphosphonate zoledronic acid in the treatment of osteolytic lesions. J Bone Miner Res. 2001;16:2027–2034. doi: 10.1359/jbmr.2001.16.11.2027. doi:10.1359/jbmr.2001.16.11.2027. [DOI] [PubMed] [Google Scholar]
- 39.Hiraga T, Williams PJ, Ueda A, et al. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004;10:4559–4567. doi: 10.1158/1078-0432.CCR-03-0325. doi:10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- 40.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avcu F, Ural AU, Yilmaz MI, et al. The bisphosphonate zoledronic acid inhibits the development of plasmacytoma induced in BALB/c mice by intraperitoneal injection of pristane. Eur J Haematol. 2005;74:496–500. doi: 10.1111/j.1600-0609.2005.00427.x. doi:10.1111/j.1600-0609.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- 42.Duivenvoorden WC, Vukmirovic-Popovic S, Kalina M, et al. Effect of zoledronic acid on the doxycycline-induced decrease in tumour burden in a bone metastasis model of human breast cancer. Br J Cancer. 2007;96:1526–1531. doi: 10.1038/sj.bjc.6603740. doi:10.1038/sj.bjc.6603740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melani C, Sangaletti S, Barazzetta FM, et al. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. doi:10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arrington SA, Damron TA, Mann KA, et al. Concurrent administration of zoledronic acid and irradiation leads to improved bone density, biomechanical strength, and microarchitecture in a mouse model of tumor-induced osteolysis. J Surg Oncol. 2008;97:284–290. doi: 10.1002/jso.20949. doi:10.1002/jso.20949. [DOI] [PubMed] [Google Scholar]
- 45.Ottewell PD, Deux B, Monkkonen H, et al. Differential effect of doxorubicin and zoledronic acid on intraosseous versus extraosseous breast tumor growth in vivo. Clin Cancer Res. 2008;14:4658–4666. doi: 10.1158/1078-0432.CCR-07-1545. doi:10.1158/1078-0432.CCR-07-1545. [DOI] [PubMed] [Google Scholar]
- 46.Ottewell PD, Lefley DV, Cross SS, et al. Sustained inhibition of tumor growth and prolonged survival following sequential administration of doxorubicin and zoledronic acid in a breast cancer model. Int J Cancer. 2010;126:522–532. doi: 10.1002/ijc.24756. doi:10.1002/ijc.24756. [DOI] [PubMed] [Google Scholar]
- 47.Ottewell PD, Monkkonen H, Jones M, et al. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100:1167–1178. doi: 10.1093/jnci/djn240. doi:10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 48.Labrinidis A, Hay S, Liapis V, et al. Zoledronic acid inhibits both the osteolytic and osteoblastic components of osteosarcoma lesions in a mouse model. Clin Cancer Res. 2009;15:3451–3461. doi: 10.1158/1078-0432.CCR-08-1616. doi:10.1158/1078-0432.CCR-08-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coscia M, Quaglino E, Iezzi M, et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14:2803–2815. doi: 10.1111/j.1582-4934.2009.00926.x. doi:10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croucher PI, De Hendrik R, Perry MJ, et al. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18:482–492. doi: 10.1359/jbmr.2003.18.3.482. doi:10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- 51.Chapman I, Greville H, Ebeling PR, et al. Intravenous zoledronate improves bone density in adults with cystic fibrosis (CF) Clin Endocrinol (Oxf) 2009;70:838–846. doi: 10.1111/j.1365-2265.2008.03434.x. doi:10.1111/j.1365-2265.2008.03434.x. [DOI] [PubMed] [Google Scholar]
- 52.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. doi:10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 53.Brown JE, Ellis SP, Lester JE, et al. Prolonged efficacy of a single dose of the bisphosphonate zoledronic acid. Clin Cancer Res. 2007;13:5406–5410. doi: 10.1158/1078-0432.CCR-07-0247. doi:10.1158/1078-0432.CCR-07-0247. [DOI] [PubMed] [Google Scholar]
- 54.Hershman DL, McMahon DJ, Crew KD, et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26:4739–4745. doi: 10.1200/JCO.2008.16.4707. doi:10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hershman DL, McMahon DJ, Crew KD, et al. Prevention of bone loss by zoledronic acid in premenopausal women undergoing adjuvant chemotherapy persist up to one year following discontinuing treatment. J Clin Endocrinol Metab. 2010;95:559–566. doi: 10.1210/jc.2009-1366. doi:10.1210/jc.2009-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hines SL, Sloan JA, Atherton PJ, et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy. Breast. 2010;19:92–96. doi: 10.1016/j.breast.2009.12.001. doi:10.1016/j.breast.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gnant M. Targeted therapies: adjuvant bisphosphonates-an option with low estrogen. Nat Rev Clin Oncol. 2011;8:698–699. doi: 10.1038/nrclinonc.2011.170. doi:10.1038/nrclinonc.2011.170. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan RN, Rafii S, Lyden D. Preparing the "soil": the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. doi:10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wels J, Kaplan RN, Rafii S, et al. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22:559–574. doi: 10.1101/gad.1636908. doi:10.1101/gad.1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. doi:10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. doi:10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 62.Shiozawa Y, Havens AM, Pienta KJ, et al. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941–950. doi: 10.1038/leu.2008.48. doi:10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon CM, LeBoff MS, Glowacki J. Adrenal and gonadal steroids inhibit IL-6 secretion by human marrow cells. Cytokine. 2001;16:178–186. doi: 10.1006/cyto.2001.0962. doi:10.1006/cyto.2001.0962. [DOI] [PubMed] [Google Scholar]
- 64.Bilezikjian LM, Blount AL, Leal AM, et al. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol Cell Endocrinol. 2004;225:29–36. doi: 10.1016/j.mce.2004.02.010. doi:10.1016/j.mce.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Centrella M, McCarthy TL, Canalis E. Transforming growth factor-beta and remodeling of bone. J Bone Joint Surg Am. 1991;73:1418–1428. doi: [PubMed] [Google Scholar]
- 66.Juarez P, Guise TA. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone. 2011;48:23–29. doi: 10.1016/j.bone.2010.08.004. doi:10.1016/j.bone.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Hampl R, Snajderova M, Mardesic T. Antimullerian hormone (AMH) not only a marker for prediction of ovarian reserve. Physiol Res. 2011;60:217–223. doi: 10.33549/physiolres.932076. [DOI] [PubMed] [Google Scholar]
- 68.Cheleuitte D, Mizuno S, Glowacki J. In vitro secretion of cytokines by human bone marrow: effects of age and estrogen status. J Clin Endocrinol Metab. 1998;83:2043–2051. doi: 10.1210/jcem.83.6.4848. doi:10.1210/jc.83.6.2043. [DOI] [PubMed] [Google Scholar]
- 69.Sasser AK, Sullivan NJ, Studebaker AW, et al. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. doi:10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 70.Sasser AK, Mundy BL, Smith KM, et al. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett. 2007;254:255–264. doi: 10.1016/j.canlet.2007.03.012. doi:10.1016/j.canlet.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 71.Studebaker AW, Storci G, Werbeck JL, et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008;68:9087–9095. doi: 10.1158/0008-5472.CAN-08-0400. doi:10.1158/0008-5472.CAN-08-0400. [DOI] [PubMed] [Google Scholar]
- 72.Esparza-Lopez J, Montiel JL, Vilchis-Landeros MM, et al. Ligand binding and functional properties of betaglycan, a co-receptor of the transforming growth factor-beta superfamily. Specialized binding regions for transforming growth factor-beta and inhibin A. J Biol Chem. 2001;276:14588–14596. doi: 10.1074/jbc.M008866200. doi:10.1074/jbc.M008866200. [DOI] [PubMed] [Google Scholar]
- 73.Kirkbride KC, Townsend TA, Bruinsma MW, et al. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. doi:10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- 74.Lewis KA, Gray PC, Blount AL, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. doi:10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- 75.Nicks KM, Fowler TW, Akel NS, et al. Bone turnover across the menopause transition: the role of gonadal inhibins. Ann N Y Acad Sci. 2010;1192:153–160. doi: 10.1111/j.1749-6632.2009.05349.x. doi:10.1111/j.1749-6632.2009.05349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci. 2006;1068:19–25. doi: 10.1196/annals.1346.005. doi:10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- 77.Wiater E, Vale W. Inhibin is an antagonist of bone morphogenetic protein signaling. J Biol Chem. 2003;278:7934–7941. doi: 10.1074/jbc.M209710200. doi:10.1074/jbc.M209710200. [DOI] [PubMed] [Google Scholar]
- 78.Perrien DS, Achenbach SJ, Bledsoe SE, et al. Bone turnover across the menopause transition: correlations with inhibins and follicle-stimulating hormone. J Clin Endocrinol Metab. 2006;91:1848–1854. doi: 10.1210/jc.2005-2423. doi:10.1210/jc.2005-2423. [DOI] [PubMed] [Google Scholar]
- 79.Perrien DS, Akel NS, Edwards PK, et al. Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology. 2007;148:1654–1665. doi: 10.1210/en.2006-0848. doi:10.1210/en.2006-0848. [DOI] [PubMed] [Google Scholar]
- 80.Bismar H, Diel I, Ziegler R, et al. Increased cytokine secretion by human bone marrow cells after menopause or discontinuation of estrogen replacement. J Clin Endocrinol Metab. 1995;80:3351–3355. doi: 10.1210/jcem.80.11.7593450. doi:10.1210/jc.80.11.3351. [DOI] [PubMed] [Google Scholar]
- 81.Chiu KM, Ju J, Mayes D, et al. Changes in bone resorption during the menstrual cycle. J Bone Miner Res. 1999;14:609–615. doi: 10.1359/jbmr.1999.14.4.609. doi:10.1359/jbmr.1999.14.4.609. [DOI] [PubMed] [Google Scholar]
- 82.Clowes JA, Eghbali-Fatourechi GZ, McCready L, et al. Estrogen action on bone marrow osteoclast lineage cells of postmenopausal women in vivo. Osteoporos Int. 2009;20:761–769. doi: 10.1007/s00198-008-0731-y. doi:10.1007/s00198-008-0731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen CJ, Verault D, Steffens C, et al. Effects of age and estrogen status on the skeletal IGF regulatory system. Studies with human marrow. Endocrine. 1997;7:77–80. doi: 10.1007/BF02778068. doi:10.1007/BF02778068. [DOI] [PubMed] [Google Scholar]
- 84.Sirola J, Kroger H, Honkanen R, et al. Factors affecting bone loss around menopause in women without HRT: a prospective study. Maturitas. 2003;45:159–167. doi: 10.1016/s0378-5122(03)00150-6. doi:10.1016/S0378-5122(03)00150-6. [DOI] [PubMed] [Google Scholar]
- 85.Hofbauer LC, Khosla S, Dunstan CR, et al. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. doi:10.1210/en.140.9.4367. [DOI] [PubMed] [Google Scholar]
- 86.Lindberg MK, Erlandsson M, Alatalo SL, et al. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the OPG/RANKL (osteoprotegerin/receptor activator of NF-kappa B ligand) ratio and serum interleukin-6 in male mice. J Endocrinol. 2001;171:425–433. doi: 10.1677/joe.0.1710425. doi:10.1677/joe.0.1710425. [DOI] [PubMed] [Google Scholar]
- 87.Rachner TD, Schoppet M, Niebergall U, et al. 17beta-Estradiol inhibits osteoprotegerin production by the estrogen receptor-alpha-positive human breast cancer cell line MCF-7. Biochem Biophys Res Commun. 2008;368:736–741. doi: 10.1016/j.bbrc.2008.01.118. doi:10.1016/j.bbrc.2008.01.118. [DOI] [PubMed] [Google Scholar]
- 88.Saika M, Inoue D, Kido S, et al. 17beta-estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-alpha. Endocrinology. 2001;142:2205–2212. doi: 10.1210/endo.142.6.8220. doi:10.1210/en.142.6.2205. [DOI] [PubMed] [Google Scholar]
- 89.Ibrahim T, Mercatali L, Sacanna E, et al. Circulating levels of RANK/RANKL and OPG in patients with bone metastases treated with zoledronic acid: a prospective study. In 2011 ASCO Annual Meeting (Abstr 10611); Chicago, IL,. 2011. [Google Scholar]
- 90.Burger HG, Dudley EC, Robertson DM, et al. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. doi:10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 91.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. doi:10.1016/S0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 92.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. doi:10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 93.van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377:321–331. doi: 10.1016/S0140-6736(10)62312-4. doi:10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 94.Welt CK, McNicholl DJ, Taylor AE, et al. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105–111. doi: 10.1210/jcem.84.1.5381. doi:10.1210/jc.84.1.105. [DOI] [PubMed] [Google Scholar]
- 95.Rannevik G, Jeppsson S, Johnell O, et al. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 2008;61:67–77. doi: 10.1016/j.maturitas.2008.09.010. doi:10.1016/j.maturitas.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 96.Sowers MR, Zheng H, McConnell D, et al. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab. 2008;93:3847–3852. doi: 10.1210/jc.2008-1056. doi:10.1210/jc.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Folkerd EJ, Dowsett M. Influence of sex hormones on cancer progression. J Clin Oncol. 2010;28:4038–4044. doi: 10.1200/JCO.2009.27.4290. doi:10.1200/JCO.2009.27.4290. [DOI] [PubMed] [Google Scholar]
- 98.Burger HG, Cahir N, Robertson DM, et al. Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clin Endocrinol (Oxf) 1998;48:809–813. doi: 10.1046/j.1365-2265.1998.00482.x. doi:10.1046/j.1365-2265.1998.00482.x. [DOI] [PubMed] [Google Scholar]
- 99.Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. doi:10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 100.Khosla S, Melton LJ, III, Achenbach SJ, et al. Hormonal and biochemical determinants of trabecular microstructure at the ultradistal radius in women and men. J Clin Endocrinol Metab. 2006;91:885–891. doi: 10.1210/jc.2005-2065. doi:10.1210/jc.2005-2065. [DOI] [PubMed] [Google Scholar]
- 101.Pfeilschifter J, Diel I, Scheppach B, et al. Concentration of transforming growth factor beta in human bone tissue: relationship to age, menopause, bone turnover, and bone volume. J Bone Miner Res. 1998;13:716–730. doi: 10.1359/jbmr.1998.13.4.716. doi:10.1359/jbmr.1998.13.4.716. [DOI] [PubMed] [Google Scholar]
- 102.Edlund S, Landstrom M, Heldin CH, et al. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. doi:10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edlund S, Landstrom M, Heldin CH, et al. Smad7 is required for TGF-beta-induced activation of the small GTPase Cdc42. J Cell Sci. 2004;117:1835–1847. doi: 10.1242/jcs.01036. doi:10.1242/jcs.01036. [DOI] [PubMed] [Google Scholar]
- 104.Kardassis D, Murphy C, Fotsis T, et al. Control of transforming growth factor beta signal transduction by small GTPases. FEBS J. 2009;276:2947–2965. doi: 10.1111/j.1742-4658.2009.07031.x. doi:10.1111/j.1742-4658.2009.07031.x. [DOI] [PubMed] [Google Scholar]
- 105.Ogata S, Morokuma J, Hayata T, et al. TGF-beta signaling-mediated morphogenesis: modulation of cell adhesion via cadherin endocytosis. Genes Dev. 2007;21:1817–1831. doi: 10.1101/gad.1541807. doi:10.1101/gad.1541807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.