Abstract
Objectives. We evaluated an intervention designed to identify patients at risk for hepatitis C virus (HCV) through a risk screener used by primary care providers.
Methods. A clinical reminder sticker prompted physicians at 3 urban clinics to screen patients for 12 risk factors and order HCV testing if any risks were present. Risk factor data were collected from the sticker; demographic and testing data were extracted from electronic medical records. We used the t test, χ2 test, and rank-sum test to compare patients who had and had not been screened and developed an analytic model to identify the incremental value of each element of the screener.
Results. Among screened patients, 27.8% (n = 902) were identified as having at least 1 risk factor. Of screened patients with risk factors, 55.4% (n = 500) were tested for HCV. Our analysis showed that 7 elements (injection drug use, intranasal drug use, elevated alanine aminotransferase, transfusions before 1992, ≥ 20 lifetime sex partners, maternal HCV, existing liver disease) accounted for all HCV infections identified.
Conclusions. A brief risk screener with a paper-based clinical reminder was effective in increasing HCV testing in a primary care setting.
Hepatitis C virus (HCV) is a significant public health problem. With 3.2 million Americans chronically infected,1 HCV is the leading cause of liver-related deaths,2 accounting for 15 000 deaths in 2007.3 Although earlier treatments were moderately effective in reducing the HCV disease burden,4 new treatments with greater promise have become available.5 Because treatment cannot be offered without diagnosis and 45% to 85% of patients with HCV are unaware of their infection,6,7 interventions designed to increase the number of HCV cases diagnosed are urgently needed.
Guidelines for HCV screening vary. The Centers for Disease Control and Prevention (CDC) recommends that patients who have injected drugs, who have long-term hemodialysis histories or persistently abnormal alanine aminotransferase (ALT) levels, who had blood transfusions or organ transplants before July 1992 (when HCV was eradicated from the nation’s blood supply), who have been exposed to HCV (e.g., their mothers were HCV positive or they have been exposed at their workplace), or who are HIV positive8 be assessed for HCV risk. Other authorities have expanded recommendations to include current sexual partners of individuals with HCV,9,10 people who have had multiple sex partners, intranasal cocaine users, people with tattoos or repeated body piercings, people with high levels of daily alcohol use over time, Vietnam-era veterans,11 and immigrants from countries with high HCV prevalence rates.12
In addition, with respect to research on HCV risk, various studies have shown that homelessness, incarceration,13 tattoos,14 barbershop shaving,15 body piercing,16 ear piercing among men,17 use of intranasal drugs and crack cocaine,18 and mental illness19 are associated with higher risk. Although not explicitly recommending testing, this literature suggests that these are potential HCV risk factors for which screening may be appropriate.
Multiple approaches can be used in HCV testing programs. Universal screening of people with identified risks appears to best meet CDC’s recommendations and to be the most efficient strategy, given that individuals with identified risk factors have been shown to have a much higher prevalence of HCV than the general population.1 As the front-line health care providers for most Americans, primary care settings offer an important opportunity to incorporate HCV risk assessments, although examination of this model has been limited.
In 2 studies conducted in primary care settings, patient self-administered questionnaires have been used to assess HCV risk screening. In one of these studies, set in an urban clinic, patients completed a 27-item risk assessment20; the other study, set in a Veterans Health Administration facility, involved a retrospective analysis of HCV testing among veterans who had reported HCV risk factors on a self-administered questionnaire.21 To date, no HCV screening tools have been validated, and no studies comparing different types of interventions have been conducted, including comparisons of patient-completed screening instruments and screeners implemented by primary care providers (PCPs).
We implemented a PCP-based risk screening intervention that successfully increased rates of HCV testing among patients at risk.22 Because existing guidelines do not concur on what factors should trigger HCV testing, we included a moderately large number of risk factors (12) in assessing the intervention. However, it was unknown which factors of the screening intervention were responsible for the screener’s success and whether an abbreviated set of risk factors would be equally successful. To inform both the development of a parsimonious screening intervention and the revision of risk-based HCV testing guidelines, we examined which factors were the strongest independent predictors of testing and diagnosis of HCV.
The Hepatitis C Assessment and Testing project (HepCAT), a prospective cross-sectional evaluation conducted in 3 urban primary care clinics, was designed to inform CDC’s revision of its HCV testing recommendations. HepCAT’s major goal was to evaluate an intervention designed to identify patients at risk for HCV with a PCP-implemented risk screener and test those identified as at risk. Another objective was to parse out a limited number of factors to include in a simple and effective screener. We hypothesized that using the risk screener would increase testing rates and that a brief screener incorporating fewer risk factors would perform as well as the full screener. We examined the performance of the screener overall as well as the extent to which each specific risk factor predicted HCV.
METHODS
The HepCAT project was funded by the CDC through the Agency for Healthcare Research and Quality’s Accelerating Change and Transformation in Organizations and Networks (ACTION) program. ACTION promotes “field-based research designed to promote innovation in health care delivery by accelerating the diffusion of research into practice”23 rather than funding only traditional research studies involving comparison and control groups.
The ACTION network includes 15 partnerships, each with a lead organization that serves as the prime contractor for projects. Boston University is the prime contractor for one partnership that includes the Montefiore Medical Center in the Bronx, New York, and HepCAT, a collaborative effort between these 2 organizations, was conducted in 3 clinics affiliated with the center. The 3 clinics provide approximately 150 000 primary care visits annually to more than 54 000 adults; the clinics are located in economically depressed areas of the Bronx and serve patients with high rates of poverty, substance use, and sexually transmitted diseases. The estimated prevalence of HCV infection in the adult population seen in the 3 clinics is 7.7%.24
Intervention
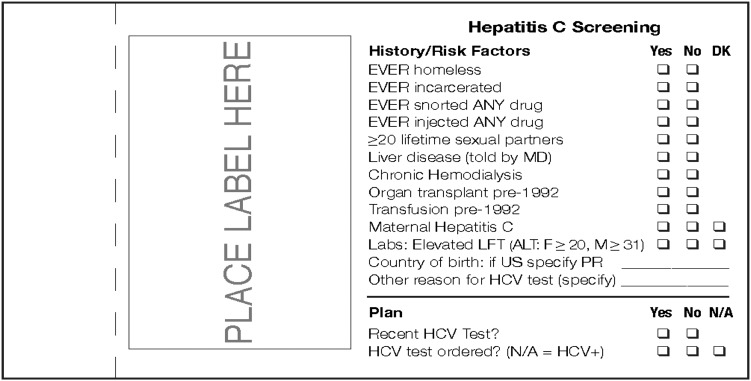
The risk-based screening intervention involved prompting physicians with a clinical reminder sticker to ask whether a patient had any of 12 specific HCV-related risks and to order HCV tests according to the presence of those risks. The sticker (Figure 1) incorporated a double-layer “carbon copy” design; once completed, the top copy of the sticker could be removed and placed in a secured box in the examination room for research purposes while the other copy remained affixed to the medical chart.
FIGURE 1—
Clinical reminder sticker used in the risk screening intervention.
Note. ALT = alanine aminotransferase; DK = don’t know; HCV = hepatitis C virus; LFT = liver function test; N/A = not applicable; PR = Puerto Rico.
In addition to the sticker, intervention training included the following: on-site educational sessions including a standardized presentation for all PCPs and clinic staff delivered prior to and once during the intervention period, regular communication between the research team and clinical leadership, electronic provision of a weekly scientific article on HCV to all PCPs, and environmental reminders (HepCAT buttons, pocket cards, and posters). Also, project staff visited each clinic twice per week to place stickers on all progress notes, encourage adherence to screening protocols, and elicit feedback from PCPs and other clinic staff. Furthermore, each clinic had a “physician champion,” a member of the research team who regularly visited the clinic to maintain PCPs’ engagement in the intervention. All PCPs were supplied with a script (in English and Spanish) to standardize and normalize the introduction of the screener.
Each PCP was asked to complete the sticker at every visit for patients who had not been tested for HCV in the preceding 12 months and to order an HCV antibody test if any risk factor was identified. The risk-based screening intervention was conducted over a 15-week period from November 2008 to March 2009.
Data Collection
Given the expectation that all adults seen in the clinics would be screened, demographic and HCV testing information on each adult (18 years old or older) seen during the intervention period was extracted from the electronic medical record. Risk factor data were collected from the risk screener sticker. These data sets were merged into a single database. We compared patients who had been screened with those who had not been screened. Among screened patients, we examined risk factors, HCV testing, and HCV positivity rates.
Measures
The main outcomes in our analysis were HCV testing and HCV positivity. A patient was considered to have been tested if an anti-HCV antibody test was performed within 90 days of the clinic visit date. HCV antibody positivity was defined as a positive anti-HCV test within the same time period. To determine the effects of the screener on HCV testing and positivity, we analyzed patients who had and had not been screened separately. A patient was considered screened if a sticker was submitted with any information about HCV risk factors recorded. A patient was considered not screened if no sticker was submitted or if the submitted sticker was blank. After examining the main outcomes, we examined the incremental value of each element of the screener to assess its performance with respect to promoting HCV testing and identifying cases of anti-HCV positivity; analyses focused on proportions of patients tested and results for patients with each risk factor.
Data Analysis
We used Stata software in conducting our analyses.25 We initially conducted the t test, the χ2 test, the rank sum test to compare screened and nonscreened patients with respect to demographic characteristics. Among screened patients, we examined the proportions with any risk identified (vs no risk identified), the proportions who had (vs had not) been tested, and rates of HCV positivity. We then investigated the rates of testing and HCV positivity associated with each risk factor.
We identified the incremental value of each screener element through an iterative process as follows. Initially we determined which risk factor was most strongly associated with positivity and counted the number of positive cases identified by asking about that risk factor. Next, we removed the HCV-positive cases identified by the first risk factor and determined which of the other risk factors identified the most remaining positive cases. Then the HCV-positive cases identified by the second risk factor were removed and a third risk factor that identified the most remaining cases was sought. The process was repeated until the remaining risk factors did not identify any new HCV positive cases.
RESULTS
Of the 13 371 patients with at least 1 primary care visit during the intervention period, 4339 had previously been tested and 51 were missing critical data; as a result, 8981 patients were included in our analysis. Table 1 shows comparisons of patients screened and not screened during the intervention period.
TABLE 1—
Characteristics of Patients Seen During the Intervention Period: Bronx, NY, 2008–2009
| Characteristic | Total (n = 8981), No. (%) | Screened (n = 3250), No. (%) | Not Screened (n = 5731), No. (%) | P |
| Gender | .002a | |||
| Male | 2330 (25.9) | 906 (27.9) | 1424 (24.8) | |
| Female | 6651 (74.1) | 2344 (72.1) | 4307 (75.2) | |
| Race/ethnicity | .01a | |||
| White | 389 (4.3) | 272 (4.7) | 117 (3.6) | .01a |
| Black | 2733 (30.4) | 1698 (29.6) | 1035 (31.8) | .03a |
| Latino | 4734 (52.7) | 3025 (52.8) | 1709 (52.6) | .86 |
| Other/unknown | 1125 (12.5) | 736 (12.8) | 389 (12.0) | .23 |
| Insurance coverage | .09 | |||
| Medicare | 1029 (11.5) | 645 (11.3) | 384 (11.8) | .42 |
| Medicaid | 4609 (51.3) | 2981 (52.0) | 1628 (50.1) | .08 |
| Commercial | 2062 (23.0) | 1283 (22.4) | 779 (24.0) | .09 |
| None | 1272 (14.2) | 819 (14.3) | 453 (13.9) | .64 |
Significant difference between groups at P < .05.
The mean age of the 8981 patients was 47.8 years, and one fourth were male. More than half were Latino, about 30% were Black, and fewer than 5% were White. About half were insured via Medicaid, just under a quarter had commercial insurance, about 14% were uninsured, and 12% had Medicare. A screener was completed for 3250 (36.2%) of the patients seen. Male and White patients were more likely to be screened, and Black patients were less likely to be screened; there were no differences in screening rates according to age or insurance status.
Table 2 presents proportions of testing and yield (rate) of anti-HCV positivity among those screened and not screened and among those with and without identified risks. During the intervention, 13.1% of all patients seen in the clinics were tested for HCV. However, this level of testing was driven primarily by screening: 25.3% of screened patients were tested, as opposed to only 6.2% of unscreened patients. The yields of anti-HCV positivity were 5.9% among those with no screening documentation and 5.0% among the screened population.
TABLE 2—
Hepatitis C Virus (HCV) Screening, Testing, and Yield During the Intervention Period: Bronx, NY, 2008–2009
| Tested for HCV, No. (%) | Yield (Anti-HCV Positivity), No. (%) | |
| Total seen (n = 8981) | 1179 (13.1) | 62 (5.3) |
| Not screened (n = 5731) | 357 (6.2) | 21 (5.9) |
| Screened (n = 3250) | 822 (25.3) | 41 (5.0) |
| No risk (n = 2348) | 322 (13.7) | 7 (2.2) |
| Any risk (n = 902) | 500 (55.4) | 34 (6.8) |
Of the 3250 patients screened, 27.8% (n = 902) had at least 1 HCV risk factor. Of these patients, 55.4% (n = 500) were tested for HCV; 13.7% of tested patients had no identified risk. The yield of anti-HCV positivity among tested patients was higher for those with a risk factor (6.8%) than for those without a risk factor (2.2%).
Identification of Risk Factors
Table 3 provides details on the 902 screened patients identified as having at least 1 HCV risk factor. The most commonly identified risk factors, documented among more than 20% of screened patients, were history of multiple sex partners, intranasal drug use, elevated ALT, homelessness, and incarceration. Histories of liver disease and blood transfusions before 1992 were reported by 10% to 15% of those screened. A smaller percentage (6.2%) of patients reported injection drug use; just over 2% reported maternal HCV. Very few patients (< 1%) reported chronic hemodialysis or organ transplants before 1992.
TABLE 3—
Risk Factor Identification, Hepatitis C Virus (HCV) Testing, and Yield: Bronx, NY, 2008–2009
| Factor | Risk Factor Identified, No. (%) | Tested for HCV, No. (%) | Yield (Anti-HCV Positivity), No. (%) |
| Ever homeless | 234 (25.9) | 111 (47.2) | 10 (9.0) |
| Ever incarcerated | 214 (23.7) | 119 (55.6) | 13 (10.9) |
| Ever snorted drugs | 252 (27.9) | 134 (53.2) | 21 (15.7) |
| Ever injected drugs | 56 (6.2) | 27 (48.2) | 17 (63.0) |
| ≥ 20 lifetime sex partners | 270 (29.9) | 167 (61.9) | 14 (8.4) |
| Liver disease (physician diagnosis) | 115 (12.7) | 56 (48.7) | 15 (26.8) |
| Chronic hemodialysis | 9 (1.0) | 2 (22.2) | 0 (0.0) |
| Transplant before 1992 | 5 (0.5) | 2 (40.0) | 0 (0.0) |
| Transfusion before 1992 | 108 (12.0) | 72 (66.7) | 5 (6.9) |
| Maternal hepatitis C | 19 (2.1) | 12 (63.2) | 2 (16.7) |
| Elevated alanine aminotransferase (documented in electronic medical record) | 242 (26.8) | 137 (56.6) | 10 (7.3) |
Note. Totals sum to more than 902 because patients often had multiple factors identified. The sample size was n = 902.
Testing Rates for Each Risk Factor
All prevalent risk factors predicted testing relatively well, at close to or above 50% of the time. Testing was most often conducted (> 60% of the time) among those who reported histories of multiple sex partners, transfusions, and maternal hepatitis. In addition, more than half of those with histories of incarceration, intranasal drug use, and elevated ALT were tested, as were almost half of those with histories of homelessness, injection drug use, and liver disease.
Yield of Patients Tested
Overall, the yields of HCV testing were high. Sixty-three percent of patients with a history of injection drug use had positive anti-HCV test results. Positivity was also high among those reporting liver disease (26.8%), maternal HCV (16.7%), intranasal drug use (15.7%), incarceration (10.9%), homelessness (9.0%), multiple sex partners (8.4%), elevated ALT (7.3%), and transfusions (6.9%).
Incremental Value of Screener Elements
Table 4 illustrates the incremental predictive value of each risk factor starting with the factor with the highest yield: injection drug use. Injection drug use was the strongest predictor of anti-HCV positivity; sole inclusion of the “ever injected drugs” variable would have predicted 41.5% of identified cases. Intranasal drug use was the second strongest predictor; the incremental benefit of adding “ever snorted drugs” as a second factor would have led to the additional identification of 14.6% of cases, for a total yield from a 2-question screener of 56.1% of the identified cases of anti-HCV positivity. Ultimately, we determined that a 7-element screener that comprised injection drug use, intranasal drug use, elevated ALT, transfusions before 1992, maternal HCV, 20 or more lifetime sex partners, and existing liver disease would have accounted for all anti-HCV cases identified.
TABLE 4—
Incremental Value of Hepatitis C Virus (HCV) Screening Items: Bronx, NY, 2008–2009
| Factor | No. of Patients With Identified HCV Risk | No. of Patients With Positive Test Results (% of Positive Cases Overall) | Cumulative % |
| Ever injected drugs | 56 | 17 (41.5) | 41.5 |
| Ever snorted drugs | 200 | 6 (14.6) | 56.1 |
| Elevated alanine aminotransferase (documented in electronic medical record) | 185 | 4 (9.8) | 65.9 |
| Transfusion before 1992 | 59 | 3 (8.0) | 73.1 |
| ≥ 20 lifetime sex partners | 115 | 2 (4.9) | 78.0 |
| Maternal hepatitis C | 10 | 1 (2.4) | 80.5 |
| Liver disease (physician diagnosis) | 23 | 1 (2.4) | 82.9 |
| Ever homeless | 66 | 0 (0.0) | 82.9 |
| Ever incarcerated | 67 | 0 (0.0) | 82.9 |
| Chronic hemodialysis | 0 | 0 (0.0) | 82.9 |
| Transplant before 1992 | 0 | 0 (0.0) | 82.9 |
| Total | 34/41 | 82.9 |
Note. The cumulative percentage does not reach 100% because 7 of the 41 patients with positive anti-HCV results had no risk factors identified on the risk screener.
DISCUSSION
We found that anti-HCV testing increased in a primary care setting when a 12-item risk screener was implemented and that 10 elements identified patients at risk. However, 6 of the elements would have identified the same number of cases of anti-HCV positivity, suggesting that a briefer screening instrument would be equally effective.
Our findings demonstrate the utility of risk-based testing in identifying individuals positive for anti-HCV, confirming the findings of previous studies.20,21 Earlier research showed that the 3 study clinics had already tested 39.7% of their patient populations and had estimated that 59.7% of these patients were anti-HCV positive before the risk screening intervention.24 Although it could be argued that the individuals identified before the intervention would have presented with the most obvious risks, the risk screener still identified an additional 13.1% for screening in the previously untested group, and of these patients 62 were anti-HCV positive. This increase in testing was primarily because of the screened population; 25.3% of screened patients were subsequently tested for HCV, whereas the testing rate among unscreened patients was 6.2%. More than half of patients who had at least 1 risk factor identified on the screener were tested. Thus, the intervention was effective in increasing testing rates both overall and, particularly, among patients with identified risk factors.
It is notable that the testing rate among patients identified as having HCV risk factors was not closer to 100%, particularly in the case of risk factors such as injection drug use that are widely known to be associated with HCV. There may be several reasons for this finding, including the steps required by both patient and PCP to complete the anti-HCV testing process. For patients, testing required going to a separate area in the clinic and waiting to have blood drawn; for PCPs, ordering tests required completing a lab slip. These may be important barriers to be mindful of in attempts to increase testing in primary care settings, particularly given the myriad screening and preventive care activities that are already recommended.
In addition, there was no requirement in the HepCAT protocol for patients to be tested at the visit when the sticker was placed in the chart, and the PCP may have planned to test the patient at a later visit. Only the Veterans Health Administration, via a federal mandate and real-time electronic clinical reminders, has developed a mechanism to fully implement risk-based HCV screening and testing in primary care settings23; an electronic reminder to screen might be the only method to achieve full implementation.
A key question in the development and implementation of the HepCAT intervention was which items to include in the screener. Although screened patients reported high rates of intranasal and injection drug use, as well as multiple sex partners, homelessness, incarceration, liver disease, and transfusions, our analysis illustrated that a screener with fewer risk factors could be as effective as the 12-item screener. Our results showed that two thirds of patients with positive anti-HCV test results were identified with a screener that included just 3 factors (injection drug use, intranasal drug use, elevated ALT); 4 additional factors (transfusions, maternal hepatitis C, 20 or more lifetime sex partners, liver disease) identified an additional 17% of cases.
It appears that some factors, such as incarceration and homelessness, may actually be proxies for the other risk factors and will not produce additional benefits in terms of identifying cases of anti-HCV positivity. However, because 17% of the patients with positive anti-HCV test results had no risk factors identified on the screener, it will be important to understand the characteristics of patients without risk factors who were tested and the reasons they were tested, including assessing how demographic differences may or may not relate to other risk factor differences. For example, it is important to examine reasons why male patients were more likely to be screened than were female patients and White patients were more likely to be screened than were Black patients.
Our findings mirror recent work showing that a parsimonious screener can be effective1,21 and practical in the context of routine care. Consistent with our results, Zuniga et al.,21 using retrospective data on veterans, found that screening only for injection drug use would have identified 41% of cases of anti-HCV positivity in that population; in addition, they found that a risk screener including only 5 factors (injection drug use, blood transfusion before 1992, service during the Vietnam era, tattoos, and history of abnormal liver function tests) and a risk screener incorporating the 5 factors independently associated with anti-HCV positivity would have identified 97% of cases with 20% fewer individuals being tested. Our results also are consistent with an analysis of National Health and Nutrition Examination Study data conducted by Armstrong et al.,1 who found that injection drug use, elevated ALT, and transfusions before 1992 identified 85% of cases of anti-HCV positivity.
An important difference between our work and previous studies is our inclusion of intranasal drug use as a screening item. We found that although intranasal drug use was not independently associated with anti-HCV positivity (probably as a result of sample size limitations), it identified almost 15% of cases of positivity. Given our findings and the possibility that patients will be more likely to acknowledge intranasal than injection drug use owing to the stigma often associated with injection drug use, we propose that intranasal drug use be considered for inclusion in brief screeners.
Overall, it is important to note that our study, conducted in the context of routine primary care in a high-risk population, is congruent with the results obtained by Armstrong et al. in their US population-based sample, and it appears clear that a brief screener including injection drug use, elevated ALT, and transfusions before 1992 will be effective in identifying HCV with or without the inclusion of intranasal drug use. Our results also concur with research by McGinn et al.20 supporting risk-based testing; however, their study involved a 27-item questionnaire, whereas our focus on using a brief screener is more practical for implementation.
With the current policy focus on the medical home model and the role of PCPs in coordinating and taking responsibility for all aspects of a patient’s care, the expectation will remain for PCPs to do more with less time. Thus, the challenge of integrating a new intervention to identify HCV in primary care settings remains. To minimize the impact on already-overtaxed PCPs, it is crucial to identify those elements most predictive of HCV positivity. We found that PCPs can perform effective HCV screening with a screener that includes many fewer risk factors than previously reported in the literature. Moreover, screening could be performed by ancillary providers, such as nursing staff, and this type of intervention could be easily generalized to other settings or other clinical conditions given its relative simplicity and the lack of technology required for implementation.
Limitations
Our study involved some limitations. First, it was difficult to sustain a high level of PCP adherence to the intervention, and PCPs were unable to do more than check a box on a screener (e.g., identify country of birth if outside the United States). Thus, it is important to consider whether screening could be conducted by other clinical staff. Second, because we were unable to track unused laboratory slips, we cannot determine whether untested patients with risk factors were referred for but did not undergo testing or whether these patients were not referred.
Third, this study was observational; without a comparison group, we cannot establish a causal link between the intervention and the increase in testing. Finally, the small number of cases of identified anti-HCV positivity limited our examination of the incremental value of each of the risk factors in creating a briefer risk screener.
Conclusions
We found that a brief risk screener with a paper-based clinical reminder was effective in increasing HCV testing in a primary care setting. With more effective treatments now available, it is critical that the process of identification of HCV be improved, given that care and treatment cannot be offered without diagnosis. Primary care is the front line of health care for most patients and the optimal location for simple risk screening.
Given the many challenges facing PCPs and the numerous preventive care activities expected of them, future efforts should focus on testing a more parsimonious risk screener and determining whether ancillary staff could conduct screening activities. In addition, there is a need for future research testing the use of HCV screening by different types of PCPs, including studies involving experimental designs. Studies are also needed to explore the actual utility of a brief screener, followed by validation of that screener. Finally, we recommend that the cost-effectiveness of our intervention be assessed; if it is cost-effective for HCV screening, it might serve as a model for primary care screening of other undiagnosed clinical conditions.
Acknowledgments
This study was funded by the Agency for Health Care Research and Quality (AHRQ) and the Centers for Disease Control and Prevention via the AHRQ Accelerating Change and Transformation in Organizations and Networks (ACTION) initiative (contract HHSA2902006000012 T0#4 to Boston University).
Note. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ.
Human Participant Protection
This study was approved by the institutional review boards of the Centers for Disease Control and Prevention, the Boston University Medical Center, and the Montefiore Medical Center. A waiver of informed consent was granted for all study activities.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WK, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714 [DOI] [PubMed] [Google Scholar]
- 2.Kanwal F, Schnitzler MS, Bacon BR, Hoang T, Buchanan PM, Asch SM. Quality of care in patients with chronic hepatitis C virus infection. Ann Intern Med. 2010;153(4):231–239 [DOI] [PubMed] [Google Scholar]
- 3.Ly K, Xing J, Klevens M, Jiles R, Ward J, Holmberg S. The growing burden of mortality from viral hepatitis in the US, 1999–2007. Paper presented at: annual meeting of the American Association for the Study of Liver Diseases, November 2011, San Francisco, CA.
- 4.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50(6):1750–1755 [DOI] [PubMed] [Google Scholar]
- 5.Kwo PY, Lawitz EJ, McCone Jet al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naïve patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705–716 [DOI] [PubMed] [Google Scholar]
- 6.Roblin DW, Smith BD, Weinbaum CM, Sabin M. Hepatitis C virus screening practices and prevalence in an MCO, 2000–2007. Am J Manag Care. 2011;17(8):548–555 [PubMed] [Google Scholar]
- 7.Wasley A, Finelli L, Bell BP, Alter MJ. The knowledge and behavior of HCV-infected persons identified in a national seroprevalence survey, United States, 2001–2004. Paper presented at: 12th International Symposium on Viral Hepatitis and Liver Disease, July 2006, Paris, France.
- 8.Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1–39 [PubMed] [Google Scholar]
- 9.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147–1171 [DOI] [PubMed] [Google Scholar]
- 11.US Dept of Veterans Affairs. Hepatitis C testing and prevention counseling guidelines for VA health care practitioners. Available at: http://www.hepatitis.va.gov/provider/guidelines/testing-prevention-counseling.asp. Accessed July 24, 2012.
- 12.Dienstag JL, McHutchinson JG. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology. 2006;130(1):225–230 [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health. National Institutes of Health Consensus Development Conference statement: management of hepatitis C: 2002. Hepatology. 2002;36(suppl 1):S3–S20.
- 14.Ko YC, Ho MS, Chiang TA, Chang SJ, Chang PY. Tattooing as a risk of hepatitis C virus infection. J Med Virol. 1992;38(4):288–291 [DOI] [PubMed] [Google Scholar]
- 15.Tumminelli F, Marcellin P, Rizzo Set al. Shaving as potential source of hepatitis C virus infection. Lancet. 1995;345(8950):658. [DOI] [PubMed] [Google Scholar]
- 16.Hayes MO, Harkness GA. Body piercing as a risk factor for viral hepatitis: an integrative research review. Am J Infect Control. 2001;29(4):271–274 [DOI] [PubMed] [Google Scholar]
- 17.Conry-Cantilena C, VanRaden M, Gibble Jet al. Routes of infection, viremia and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med. 1996;334(26):1691–1696 [DOI] [PubMed] [Google Scholar]
- 18.Tortu S, Neaigus A, McMahon J, Hagen D. Hepatitis C among non-injecting drug users: a report. Subst Use Misuse. 2001;36(4):523–534 [DOI] [PubMed] [Google Scholar]
- 19.Zalumas J, Rose C. Hepatitis C and HIV in incarcerated populations: fights, bites, searches, and syringes! J Assoc Nurses AIDS Care. 2003;14(suppl 5):108S–115S [DOI] [PubMed] [Google Scholar]
- 20.McGinn T, O’Connor-Moore N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. Arch Intern Med. 2008;168(18):2009–2013 [DOI] [PubMed] [Google Scholar]
- 21.Zuniga IA, Chen CC, Lane DS, Allmer J, Jimenez-Lucho VE. Analysis of a hepatitis C screening programme for US veterans. Epidemiol Infect. 2006;134(2):249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litwin AH, Smith BD, Drainoni MLet al. Primary care-based interventions are associated with increases in hepatitis c virus testing for patients at risk. Dig Liver Dis. 2012;44(6):497–503 [DOI] [PubMed] [Google Scholar]
- 23.Agency for Health Care Research and Quality. Accelerating Change and Transformation in Organizations and Networks (ACTION): field partnerships for applied research. Available at: http://www.ahrq.gov/research/action.htm. Accessed July 24, 2012.
- 24.Southern WN, Drainoni M, Smith BDet al. Hepatitis C testing practices and prevalence in a high-risk urban ambulatory care setting. J Viral Hepat. 2011;18(7):474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stata, Version 10.0. College Station, TX: StataCorp LP; 2007.