Abstract
Objectives. We compared the theoretical performance of a 1-time, birth cohort strategy with the currently recommended risk strategy for screening for hepatitis C virus (HCV) infection, which is undetected in an estimated 75% of 4 million affected people in the United States.
Methods. We applied current American Association for the Study of Liver Disease risk screening guidelines and a targeted birth cohort strategy to National Health and Nutrition Examination Survey data from 2003 to 2006 to estimate their performance in identifying HCV cases.
Results. Risk guidelines would recommend testing 25% of the US population aged 20 years or older and, if fully implemented, identify 82% of the projected HCV-exposed population. A targeted birth cohort (1946–1964) strategy would test 45% of the same population and identify 76% of the projected HCV population.
Conclusions. In this ideal-world simulation, birth year and risk screening had similar theoretical performances for predicting HCV infection. However, actual implementation of risk screening has not achieved its theoretical performance, and birth cohort screening might increase HCV testing rates.
An epidemic of hepatitis C virus (HCV) acquisition occurred between the 1960s and the 1980s in the United States; at its peak, an estimated 250 000 persons per year were newly infected. Since 1990, new cases of HCV infection have declined by 90%, and it is estimated that fewer than 20 000 persons a year are becoming infected.1,2 Up to 4 million persons in the United States are estimated to be chronically infected with HCV, making it the most common blood-borne infection.1–3 Chronic HCV infection strikes a narrow age range: more than two thirds of those affected were born between 1946 and 1964.2,4,5 As of 2010, the majority of these persons have been living with HCV for 20 to 40 years.
The natural history of chronic HCV infection is characterized by a long period (usually > 20 years) in which individuals are relatively asymptomatic and often lack signs indicative of chronic liver disease.4,6 During this time, chronic liver inflammation and fibrosis progress,2,7 and severe fibrosis and cirrhosis can develop before liver disease is diagnosed.1,2,8,9 Only 15% of affected individuals will have persistently elevated liver enzymes during the asymptomatic period, and intermittently elevated liver enzyme levels may not be appreciated as a potential sign of chronic HCV infection.8
The majority of persons who have chronic HCV have been infected for more than 20 years; an estimated 25% of them (∼800 000 persons) have developed cirrhosis, and approximately 40% have developed moderate to severe fibrosis.2 These persons are at risk for decompensated liver disease (ascites, gastroesophageal variceal hemorrhage, or hepatic encephalopathy),8 hepatocellular carcinoma,2,10 liver transplantation,2,10 and liver-related death.2,4,5 Cases of liver decompensation and hepatocellular carcinoma are expected to increase dramatically over the next 10 to 13 years, and annual liver-related deaths are projected to increase by 74%, from 145 667 in 2010 to 254 550 in 2019.2 Total medical costs for HCV-infected patients are also expected to increase dramatically over the next 20 years, from $30 billion in 2009 to $85 billion in 2028.5
Current HCV screening practices are based on the assessment of risk factors. The 1998 Centers for Disease Control guidelines,11 2002 National Institutes of Health guidelines,12 and 2009 American Association for the Study of Liver Disease (AASLD) guidelines8 recommend screening individuals who have risk factors such as elevated liver enzymes; blood transfusion before 1992; injection drug use, even once; dialysis treatment, ever; and HIV infection. However, a managed care organization analysis of HCV testing found that only 0.7% of its members received anti-HCV screening over a 3-year period.13 Another managed care study found that over an 8-year period, only 4.3% of the study population was tested for HCV, and among this group, 5.2% had detectable HCV antibodies.14 Several groups, including the Institute of Medicine, have estimated that up to three quarters of persons with chronic HCV infection are unaware of their infection.4–6,15
Suboptimal diagnosis rates may be attributable to shortcomings in the application of screening guidelines in practice. Health care providers do not always ask about HCV risk factors,16,17 and patients may fail to disclose them because of a lack of knowledge or a fear of stigmatization.4,18 The 2010 Institute of Medicine report on viral hepatitis recommended large-scale educational campaigns directed at primary care providers, the general public, and those most at risk for HCV, which would raise disease awareness and address the knowledge gaps and stigma associated with HCV infection.4
More than half of persons with HCV infection remain undiagnosed despite 12 years of experience with risk factor screening guidelines.4–6,15 Because HCV infection affects certain birth cohorts disproportionately, we explored the potential effectiveness of 1-time HCV screening of a targeted birth cohort in increasing diagnosis rates in the United States. We compared the birth cohort screening strategy with the current risk strategy for the proportion of HCV-infected persons that would be detected and the total number that would be tested.
METHODS
We extracted data from the 2003 to 2004 and 2005 to 2006 National Health and Nutrition Examination Survey (NHANES) database. NHANES is a multistage, stratified, and clustered survey of a representative sample of the civilian, noninstitutionalized US population. We weighted samples in our analysis to account for oversampling of certain demographic groups, such as older adults, Hispanics, non-Hispanic Blacks, and low-income persons. We defined ethnicity according to the data provided in the NHANES database. Categories available for respondent selection included Hispanic, non-Hispanic White, non-Hispanic Black, and other race (including multiracial).
Analytic Data Set
The initial sample size of NHANES respondents was 16 933. As part of the NHANES initiative, all respondents who provided a blood sample were tested for HCV regardless of previous diagnosis. We excluded from the analytic data set data respondents with missing information on HCV status or without assessment of at least 1 HCV risk factor, as stated in the current AASLD screening guidelines.8 NHANES did not query drug use in participants who were aged 60 years or older, so we excluded them from the analysis. The final analytic data set contained 5917 respondents, or 35% of the potential respondents included in the NHANES.
We applied the risk screening criteria according to AASLD guidelines for HCV screening. We mapped each risk factor in the guidelines to a corresponding variable in the NHANES data set. The factors that had corresponding variables in the data set were (1) elevated alanine aminotransferase (≥ 40 IU/L), (2) history of intravenous drug use, (3) blood transfusion prior to 1992, and (4) history of dialysis in the past 12 months. According to the AASLD guidelines, the latter 3 factors are the primary mode of HCV transmission in the United States. Those risk factors, along with elevated alanine aminotransferase, are strong predictors of HCV infection.8 We excluded the HIV indicator from our analysis because NHANES determined this only for respondents aged 20 through 49 years.
Implementation of Screening Guidelines
Regardless of whether individuals had a previous diagnosis of HCV listed in the NHANES data set, our analysis assumed that all individuals were not previously tested for, or diagnosed with, HCV. For the risk strategy, all individuals who were assessed to have at least 1 risk factor were hypothetically tested, under the following assumptions: (1) health care professionals had full access to accurate individual patient information (ideal circumstances scenario), (2) 100% of the individuals having at least 1 risk factor would be tested for HCV, and (3) individuals who did not have any of the risk factors would not be tested.
For the birth cohort strategy, we assigned a logical indicator of test or no test, based solely on respondent birth year. The experimental birth cohort guideline assumed that all persons in a specified age group were referred for testing, regardless of any other factor. We tested 2 birth cohorts: 1946 to 1964 (aged 46–64 years in 2010) and 1949 to 1958 (aged 52–61 years in 2010). Selection of these cohorts was guided by studies suggesting that 2 out of 3 HCV infections occurred in individuals born between 1946 and 19645 and that the highest prevalence of HCV infection was in individuals born between 1949 and 1958.1
For both screening strategies, we used the NHANES weighting variables to project actual population sizes according to 2009 US Census estimates for the noninstitutionalized population aged 20 years or older. The analysis of both birth cohort and risk strategies estimated the number of persons who would be tested for HCV, the number who would be subsequently diagnosed, and the proportion of all persons who carried anti-HCV antibodies who would be diagnosed. We did not account for false-positive or false-negative rates of testing procedures or the use of confirmatory HCV RNA testing to determine whether an individual was chronically infected with HCV. We conducted all statistical analyses with SAS version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
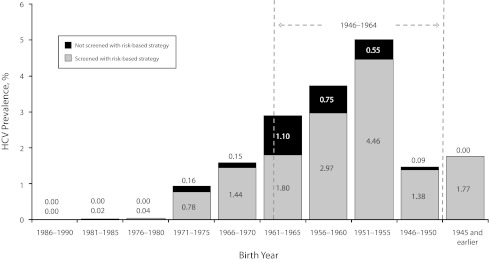
Demographic characteristics for the total NHANES data set, final analytic data set, and excluded respondents are listed in Table 1. We estimated that the overall prevalence of HCV in the United States among persons aged 20 years or older was 2.1% (4.5 million persons). Baby boomers—born between 1946 and 1964—accounted for approximately 75% of the HCV-exposed population (3.4 million persons; Figure 1); within this birth cohort the prevalence of HCV was 3.5%. We found that injection drug use was the risk factor most highly associated with HCV infection in the NHANES data set, with 49.4% of HCV-positive persons reporting a history of intravenous drug use. Having a high level of alanine aminotransferase (> 40 IU/L) and a history of dialysis in the past 12 months also predicted HCV infection in 7.1% and 16.1% of respondents, respectively. A history of blood transfusions prior to 1992 had the lowest association with HCV infection, with 3.4% of those infected reporting this history.
TABLE 1—
Demographic Characteristics of Respondents: National Health and Nutrition Examination Survey, United States, 2003–2006
| Characteristic | Total Populationa (n = 9515), % | Analytic Population (n = 5917), % | Excluded Population (n = 3958), % |
| Gender | |||
| Male | 48.0 | 48.9 | 45.8* |
| Female | 52.0 | 51.0 | 54.2* |
| Age, yb | |||
| 20–34 | 19.1 | 22.6 | 10.6* |
| 35–44 | 19.5 | 23.4 | 10.0* |
| 45–54 | 21.3 | 26.5 | 8.6* |
| 55–64 | 17.2 | 20.8 | 8.5* |
| ≥ 65c | 22.8 | 6.7 | 62.2* |
| Race/ethnicity | |||
| White | 72.0 | 71.6 | 72.8 |
| Non-Hispanic Black | 11.4 | 11.1 | 12.1 |
| Hispanic | 11.3 | 12.4 | 8.8* |
| Other | 5.4 | 5.0 | 6.3* |
Note. HCV = hepatitis C virus.
Aged ≥ 20 years.
Age as of 2010.
Majority were excluded from the analysis because HCV status was unknown or they lacked at least 1 risk factor.
Significant difference from analytic population (α < 0.05).
FIGURE 1—
Prevalence of HCV infection and percentage of HCV-infected persons in each birth cohort who would be screened under AASLD screening guidelines: National Health and Nutrition Examination Survey, United States, 2003–2006.
Note. AASLD = American Association for the Study of Liver Disease; HCV = hepatitis C virus; NHANES = National Health and Nutrition Examination Survey. The risk-based screening guidelines were applied to the final analytic NHANES data set and grouped by birth cohort (x-axis). Stacked bars indicate the proportion of HCV-infected individuals in the overall US population who would (gray) or would not (black) be tested under current AASLD guidelines in ideal circumstances. Percentages of testing prevalence for each birth cohort are displayed within or above their corresponding bar.
Our analysis suggested that if current risk guidelines were followed perfectly, 24.7% of the general population would be tested, and 1.7% of those tested would be positive for anti-HCV antibodies (Table 2). This approach would leave an estimated 17.5% of HCV-exposed patients untested, because they did not have at least 1 of the triggering risk factors. When we assessed the performance of the risk strategy screening for specific birth cohorts, we found it more accurate for young and elderly populations (birth years after 1970 and before 1955; Figure 1): 95% of HCV-infected individuals in these groups would be identified by the risk strategy, because most reported at least 1 risk factor. However, for the rest of the population (i.e., persons born between 1956 and 1969), the theoretical diagnosis rate was approximately 79%. Because of greater prevalence and poorer diagnosis rates in this birth cohort, undiagnosed HCV-infected persons born between 1956 and 1969 composed 75% of the overall undiagnosed population.
TABLE 2—
Comparison of Risk Factor and Birth Cohort Strategies for Hepatitis C Virus Screening: National Health and Nutrition Examination Survey, United States, 2003–2006
| Variable | Risk Strategy, % | Birth Cohort Strategya (1949–1958), % | Birth Cohort Strategyb (1946–1964), % |
| Overall population testedc | 24.7 | 24.2 | 44.8 |
| HCV positive | 1.7 | 1.2 | 1.6 |
| HCV negative | 23.0 | 23.0 | 43.2 |
| Overall population not tested | 75.3 | 75.8 | 55.2 |
| HCV positive | 0.4 | 0.9 | 0.5 |
| HCV negative | 74.9 | 74.9 | 54.7 |
| HCV-positive persons detected | 82 | 58 | 76 |
Note. HCV = hepatitis C virus.
Age as of 2010: 52–61 years.
Age as of 2010: 46–64 years.
Latest US Census estimate for noninstitutionalized population older than 20 years.
The 1949 to 1958 birth cohort had the greatest HCV prevalence among all age groups in our analysis. According to our model, a birth cohort strategy targeted to this cohort would screen 24.2% of the overall population, and 1.2% of the US population would test positive. However, 42% of HCV-positive persons (i.e., 0.9% of 2.1%) would not be tested because they were not members of this birth cohort. If the birth cohort screening was expanded to include all baby boomers, 44.8% of the overall population would be tested and 76% of all infected patients would be diagnosed (Table 2).
We also compared absolute numbers of individuals who would be tested for HCV infection with the 2 strategies. In ideal circumstances, approximately 54.2 million persons overall would be tested under AASLD guidelines. The birth cohort strategy would test 53.2 million persons born between 1949 and 1958 and 98.5 million persons born between 1946 and 1964 (Table 3). Among HCV-exposed persons, 82% (3.7 million of 4.5 million) would be tested with the risk strategy; 58% of the HCV-exposed members of the 1949 to 1958 birth cohort and 76% of the HCV-exposed members of the 1946 to 1964 cohort would be tested with the targeted birth cohort strategy.
TABLE 3—
Frequency and Success Rates of Risk Factor and Birth Cohort Strategies for Hepatitis C Virus Screening: National Health and Nutrition Examination Survey, United States, 2003–2006
| Variable | Risk Strategy, No. or % | Birth Cohort Strategya (1949–1958), No. or % | Birth Cohort Strategyb (1946–1964), No. or % |
| Overall populationc | 219 755 021 | 219 755 021 | 219 755 021 |
| In birth cohort | … | 53 180 715 | 98 494 200 |
| Outside birth cohort | … | 166 574 306 | 121 260 821 |
| HCV-positive population | 4 504 978 | 4 504 978 | 4 504 978 |
| Within age category | … | 2 593 109 | 3 428 178 |
| Outside age category | … | 1 911 869 | 1 076 800 |
| Population tested for HCV | 54 257 515 | 53 180 715 | 98 494 200 |
| Positive | 3 713 860 | 2 593 109 | 3 428 178 |
| Negative | 50 543 655 | 50 587 606 | 95 066 022 |
| HCV-positive population tested | 82 | 58 | 76 |
Note. HCV = hepatitis C virus. The sample size was n = 5917.
Age as of 2010: 52–61 years.
Age as of 2010: 46–64 years.
Latest US Census estimate for noninstitutionalized population older than 20 years.
DISCUSSION
The recommended risk factor screening strategy has resulted in the diagnosis of only an estimated 25% of HCV-infected individuals in the United States.4–6 Our data suggested that nearly 25% of the population aged 20 years or older had 1 or more risk factors that should have prompted HCV testing. The gulf between the theory and practice of HCV screening is illustrated by the finding that only 4.3% of the members of a managed care organization were tested over an 8-year period.14 Among persons with commercial health insurance and with access to appropriate medical care, as many as 78% were estimated to be unaware of their infection.5 Thus, the actual implementation of risk screening for HCV has not performed at its theoretical potential.
Our analysis suggested that 76% of persons with HCV infection were born between 1946 and 1964, so it is likely that the majority of HCV infections were acquired 20 to 40 years ago. Approximately half of these persons reported a history of injection drug use, so even if every provider asked about lifetime injection drug use, and every patient was willing to be honest about illicit activities that may have occurred 20 years ago, only half of all persons with HCV infection would be identified. In reality, risk factor history is often not assessed,16 so many persons with a history of injection drug use are not identified.
Identifying persons who have had HCV infections for 20 to 40 years is a matter of urgency in the next 5 to 10 years. One study estimated that in 1990, about 5% of persons with HCV infection had cirrhosis.2 Because the majority of infections occurred between 1960 and 1990,3 few persons had been infected with HCV infection for more than 20 years at that point. It is clear that 20 years makes a substantial difference in the progression of HCV-related liver disease.1,2 Currently, 40% of these persons have moderate to severe liver fibrosis and are at risk for advanced liver disease complications,1–3,7 and the prevalence of advanced liver disease is projected to continue to increase if current screening and treatment practices continue.2 Unfortunately, HCV may be asymptomatic until severe complications such as liver decompensation and hepatocellular carcinoma occur, after which liver transplantation may be the only option to prolong survival.4,6
We estimated that risk screening would be effective in identifying patients with HCV infection—in theory. Considering only the number of persons who would be tested with each strategy and what proportion of patients with HCV infection would be diagnosed in ideal circumstances, the risk factor strategy would be more effective than the birth cohort strategy. However, the discrepancy between the theoretical efficacy of risk factor screening and current estimates of the undiagnosed population indicates that implementation of this approach is impeded by such challenges as the stigma associated with disclosing risk factors and the failure of physicians to consistently ask about them.4,18
Birth cohort screening for HCV would use a demographic characteristic (year of birth) that is available in every patient’s medical record. It is easily amenable to electronic prompts, opt-out programs, and other automated reminder systems. Therefore, birth cohort screening might eliminate some of the challenges of risk strategy implementation and minimize the discrepancy between theory and application. A 1-time testing of 98.5 million persons born between 1946 and 1964 would, theoretically, identify 76% of the 3.4 million persons with HCV infection in the United States. This strategy would reach the approximately 20% of persons in this birth cohort who have no identifiable risk factors and would not otherwise be tested. Because HCV-positive members of this age group have generally been infected for 20 to 40 years and are at risk for severe sequelae of HCV infection,1,2 this is also the group with the most urgent need for diagnosis.
Unlike HIV or hepatitis B virus, HCV is curable,8 and treatment with pegylated interferon and ribavirin is associated with a 40% to 45% cure rate for HCV genotype 1 (the most common genotype in the United States) and a 70% to 80% cure rate for genotypes 2 and 3.19,20 Several direct-acting antiviral agents are in development for the treatment of HCV,21–23 and 2 orally administered protease inhibitors are approved for use in combination with pegylated interferon and ribavirin in adults with chronic genotype 1 HCV infection.24,25 Even in patients with severe fibrosis (who do not have decompensation), eradication of HCV with pegylated interferon alfa plus ribavirin is associated with an 80% reduction in liver decompensation, hepatocellular carcinoma, and liver-related death.26
Not everyone is a candidate for this treatment.8,27 However, a primary care provider who is aware of chronic HCV infection in a patient has additional care management options, such as vaccinating for hepatitis B or hepatitis A as appropriate, counseling about limiting alcohol use, ensuring that excessive doses of medications (such as acetaminophen) not be taken, and screening for hepatocellular carcinoma for patients who develop cirrhosis.
We made several assumptions in defining HCV screening strategies. For instance, for the risk strategy, we assumed that health care professionals had full access to accurate patient information and, without exception, persons with at least 1 risk factor were tested for HCV. Similarly, for the birth cohort strategy, we assumed that every individual in the selected birth cohort would be tested for HCV, without exception. For both strategies we assumed that all individuals had access to health care services, which may not be the case in the field. These assumptions likely resulted in an overestimation of the effectiveness of both strategies, which may explain the difference between the theoretical and actual effectiveness of the risk factor strategy. We could not assess the implications of these assumptions for the birth cohort strategy because we lacked data on its effectiveness in actual practice; however, the birth cohort strategy would likely not be affected as much as the risk strategy because it faces fewer implementation challenges than does risk factor screening.
We did not investigate the potential impact on patients who tested positive for anti-HCV antibodies but not for HCV RNA, representing the approximately 21% of patients in the database who had spontaneous clearance of infection. However, current practice typically includes a second confirmatory HCV RNA test for patients who have a positive anti-HCV antibody test.
More than half of all NHANES respondents aged 65 years or older were excluded from the analysis because their HCV status was unknown or they did not report at least 1 HCV risk factor. However, excluding these respondents likely did not significantly affect our results because the prevalence of HCV among the population older than 65 years was relatively low.1,3,5 The NHANES sampling frame excludes persons who are not civilians or are institutionalized, such as those who are incarcerated, homeless, hospitalized, serving in the military, and living in nursing homes.28 With the exception of active military personnel, these groups tend to have a greater prevalence of HCV infection than the overall population.1–3,28 Approximately 800 000 to 1.2 million of the approximately 6.5 million members of these groups have been infected with HCV, of which an estimated 61% to 91% are chronically infected.28 In addition, incarcerated persons, of whom an estimated 13% to 40% are HCV infected, may be more likely to be exposed to risk factors, making birth cohort a less obvious indicator for this population.29 The average age of HCV-infected prisoners is 31 to 41 years, placing them outside of the 1946 to 1964 birth cohort.29 Therefore, testing all incarcerated persons for HCV may be an appropriate complementary strategy.
We investigated the anticipated performances of the 2 strategies only according to the proportion of infected individuals who could be diagnosed. However, to be able to understand the entire risk–benefit profile of each strategy, a broader list of outcomes should be explored, such as the economic implications of adopting any specific strategy and future clinical outcomes associated with potential changes in future HCV-related mortality and morbidity.
Risk and birth cohort strategies for HCV screening may be complementary. The Institute of Medicine report suggested testing persons who were more likely to have engaged in high-risk activities, such as prisoners, clients in drug rehabilitation programs, and patients of sexually transmitted infection clinics. This could reach some of the 24% of HCV-positive persons in our analysis who fell outside the 1946 to 1964 cohort, namely, those who were younger and those who were still engaging in high-risk behaviors. Further studies are needed to explore the risks and benefits of alternative screening strategies.
Acknowledgments
This work was funded by Vertex Pharmaceuticals Inc.
We gratefully acknowledge Tiffany Bonus, MS, an employee at KJT Group, and Patricia Bauch, PhD, MPH, an employee at KJT Group when the study was conducted, for their research support and help with preparation of the article. We also thank Susan Wu, PhD, and Kristin Stephan, PhD, both employees and stockholders of Vertex Pharmaceuticals Inc, for editorial coordination and support.
Note. Baris Deniz and Camilla S. Graham are stockholders of Vertex Pharmaceuticals Inc.
Human Participant Protection
No protocol approval was required because no human participants were involved.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714 [DOI] [PubMed] [Google Scholar]
- 2.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–521 [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OVet al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556–562 [DOI] [PubMed] [Google Scholar]
- 4.Colvin HM, Mitchell AE. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: National Academies Press; 2010 [PubMed] [Google Scholar]
- 5.Pyenson BS, Fitch K, Iwasaki K. Consequences of Hepatitis C Virus (HCV): Costs of a Baby Boomer Epidemic of Liver Disease. New York, NY; 2009 [Google Scholar]
- 6.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50(6):1750–1755 [DOI] [PubMed] [Google Scholar]
- 7.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–431 [DOI] [PubMed] [Google Scholar]
- 8.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345(1):41–52 [DOI] [PubMed] [Google Scholar]
- 10.Sangiovanni A, Prati GM, Fasani Pet al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43(6):1303–1310 [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39 [PubMed] [Google Scholar]
- 12.NIH consensus statement on management of hepatitis C. NIH Consens State Sci Statements. 2002;19(3):1–46 [PubMed] [Google Scholar]
- 13.Shatin D, Schech SD, Patel K, McHutchison JG. Population-based hepatitis C surveillance and treatment in a national managed care organization. Am J Manag Care. 2004;10(4):250–256 [PubMed] [Google Scholar]
- 14.Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000–2007. Am J Manag Care. 2011;17(8):548–555 [PubMed] [Google Scholar]
- 15.McHutchison JG, Bacon BR. Chronic hepatitis C: an age wave of disease burden. Am J Manag Care. 2005;11(10 suppl):S286–S295; quiz S307–S311 [PubMed] [Google Scholar]
- 16.Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol. 2003;98(3):639–644 [DOI] [PubMed] [Google Scholar]
- 17.Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat. 2001;8(5):377–383 [DOI] [PubMed] [Google Scholar]
- 18.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36(5 suppl 1):S30–S34 [DOI] [PubMed] [Google Scholar]
- 19.Fried MW, Shiffman ML, Reddy KRet al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982 [DOI] [PubMed] [Google Scholar]
- 20.Manns MP, McHutchison JG, Gordon SCet al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965 [DOI] [PubMed] [Google Scholar]
- 21.Kronenberger B, Welsch C, Forestier N, Zeuzem S. Novel hepatitis C drugs in current trials. Clin Liver Dis. 2008;12(3):529–555, viii. [DOI] [PubMed] [Google Scholar]
- 22.Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43(2 suppl 1):S207–S220 [DOI] [PubMed] [Google Scholar]
- 23.Sulkowski MS. Specific targeted antiviral therapy for hepatitis C. Curr Gastroenterol Rep. 2007;9(1):5–13 [DOI] [PubMed] [Google Scholar]
- 24.INCIVEK [US Prescribing Information]. Cambridge, MA: Vertex Pharmaceuticals Inc; 2012 [Google Scholar]
- 25.VICTRELIS [US Prescribing Information]. Whitehouse Station, NJ: Merck; 2011 [Google Scholar]
- 26.Morgan TR, Ghany MG, Kim HYet al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52(3):833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51(3):729–733 [DOI] [PubMed] [Google Scholar]
- 28.Edlin BR. Five million Americans infected with the hepatitis C virus: a corrected estimate. Hepatology. 2005;42(4 suppl 1):213 [Google Scholar]
- 29.Velez F, Deniz B, Aggarwal S. Review of chronic hepatitis C management in correctional facilities in the United States. Paper presented at: National Conference on Correctional Health Care; Las Vegas, NV; October 10, 2010 [Google Scholar]