Abstract
Peroxidase activity was characterized in lettuce (Lactuca sativa L.) leaf tissue. Changes in the activity and distribution of the enzyme were examined during the development of a nonhost hypersensitive reaction (HR) induced by Pseudomonas syringae (P. s.) pv phaseolicola and in response to an hrp mutant of the bacterium. Assays of activity in tissue extracts revealed pH optima of 4.5, 6.0, 5.5 to 6.0, and 6.0 to 6.5 for the substrates tetramethylbenzidine, guaiacol, caffeic acid, and chlorogenic acid, respectively. Inoculation with water or with wild-type or hrp mutant strains of P. s. pv phaseolicola caused an initial decline in total peroxidase activity; subsequent increases depended on the hydrogen donor used in the assay. Guaiacol peroxidase recovered more rapidly in tissues undergoing the HR, whereas changes in tetramethylbenzidine peroxidase were generally similar in the two interactions. In contrast, increases in chlorogenic acid peroxidase were significantly higher in tissues inoculated with the hrp mutant. During the HR, increased levels of Mn2+/2,4-dichlorophenol-stimulated NADH and NADPH oxidase activities, characteristic of certain peroxidases, were found in intercellular fluids and closely matched the accumulation of H2O2 in the apoplast. Histochemical analysis of peroxidase distribution by electron microscopy revealed a striking, highly localized increase in activity within the endomembrane system and cell wall at the sites of bacterial attachment. However, no clear differences in peroxidase location were observed in tissue challenged by the wild-type strain or the hrp mutant. Our results highlight the significance of the subcellular control of oxidative reactions leading to the generation of reactive oxygen species, cell wall alterations, and the HR.
ROS, a collective term for radicals and other nonradical but reactive species derived from oxygen, have been implicated in numerous developmental and adaptive responses in both animal and plant cells (Dypbukt et al., 1994; de Marco and Roubelakis-Angelakis, 1996; Lamb and Dixon, 1997). In plants, the increased production of both the superoxide radical and H2O2 is a common feature of defense responses to challenge by microbial pathogens and elicitors (Lamb and Dixon, 1997). It has been proposed that a rapid increase in either intra- or extracellular H2O2 is involved in the induction and/or execution of the HR (Levine et al., 1994; Low and Merida, 1996; Bestwick et al., 1997).
The HR represents the rapid and localized death of plant cells in response to challenge with an avirulent pathogen and is observed in most examples of race-specific resistance and in many examples of nonhost resistance (Mansfield et al., 1997). ROS may induce lipid peroxidation, which has been detected as an early event in some HRs and may also damage DNA directly and modify or inactivate proteins, leading to a loss of cell viability (Ádám et al., 1989, 1995; Croft et al., 1990). Of potential importance is the reaction of H2O2 with Fe2+/Cu+ to form the highly reactive hydroxyl radical (Halliwell and Gutteridge, 1989). In addition to direct participation in the destruction of the host cell, ROS themselves or the direct products of their action may also induce more complex programmed cell death pathways in plants. Some investigators have described the HR as resembling the process of apoptosis, the principal manifestation of programmed cell death in many animal cell types (Dangl et al., 1996; Morel and Dangl, 1997).
There is considerable evidence that H2O2 has a wider role in resistance reactions, since it is required for cross-linking plant cell wall components as part of structural defense reactions and may also regulate gene expression associated with antioxidant defenses, phytoalexin biosynthesis, and the development of systemic acquired resistance (Lamb and Dixon, 1997). The efficacy and scope of H2O2 action is dependent on the intensity and longevity of its production. Plasma membrane-bound, neutrophil-like NADPH oxidase activity has been implicated in H2O2 production in many plant species (Low and Merida, 1996; Lamb and Dixon, 1997). However, peroxidase activity, which is an important component of plant stress responses, may also regulate the level of ROS (Wojtaszek, 1997).
Plant peroxidases are monomeric, heme-containing proteins that are usually glycosylated. Isoperoxidases, arising from the transcription of different genes or from posttranslational modification, are widely distributed within both the intra- and extracellular environment (Rathmell and Sequeira, 1974; Campa, 1991; Ievenish, 1992; Jackson and Ricardo, 1994). Peroxidases are active in the H2O2-dependent polymerization of hydroxycinnamyl alcohols (monolignols) during the final stages of lignin biosynthesis (Monties, 1989). Increases in peroxidase activity during incompatible plant-pathogen/elicitor interactions are often associated with a progressive incorporation of phenolic compounds within the cell wall (Fink et al., 1991; Graham and Graham, 1991; Reimers et al., 1992; Milosevic and Slusarenko, 1996). Peroxidase also catalyzes rapid, H2O2-dependent cross-linking of cell wall proteins such as the Hyp-rich glycoproteins and Pro-rich proteins, as well as cross-links between other wall components (Iiyama et al., 1994). The reinforcement of the wall reduces susceptibility to wall-degrading enzymes, possibly restricts diffusion of pathogen-derived toxins to the host, and, in the case of some fungal pathogens, acts as a mechanical barrier to physical penetration toward the protoplast (Aist and Gold, 1987; Brisson et al., 1994).
Peroxidase activity might be expected to reduce the level of ROS by metabolizing H2O2, but peroxidase is also capable of various “oxidase” reactions leading to H2O2 generation. For example, the oxidation of IAA, NADPH, NADH, certain phenols, and thiols in vitro has been shown to produce H2O2 (Pedreño et al., 1990; Vianello and Macri, 1991; Pichorner et al., 1992; Jiang and Miles, 1993). Both intra- and extracellular peroxidases may be involved in these reactions (Vianello and Macri, 1991). However, to our knowledge, the nature of the in vivo reductant for peroxidase-catalyzed generation of H2O2 has not yet been identified (Bolwell et al., 1995; Wojtaszek, 1997).
Here we examine changes in activity of peroxidase with reference to phenol oxidation and H2O2 generation in the apoplasm and symplasm of lettuce (Lactuca sativa L.) leaves challenged with wild-type and nonpathogenic hrp mutant strains of Pseudomonas syringae (P. s.) pv phaseolicola. Both strains caused localized wall alterations and the formation of paramural deposits or papillae that contain peroxidase substrates such as Hyp-rich glycoproteins and phenolic compounds, but only the wild type induced a confluent HR (Bestwick et al., 1995, 1997). By comparative studies it is therefore possible to identify responses specific to the HR. In addition to biochemical analyses, cytochemistry using DAB as a substrate was used to identify spatial changes in peroxidase activity and to relate such changes to the timing and location of H2O2 production, papilla formation, and the development of the HR.
MATERIALS AND METHODS
Plant Inoculation
Alternate panels in leaves of 4-week-old lettuce (Lactuca sativa L.) plants were inoculated with suspensions of 108 bacteria mL−1 in sterile distilled water as previously described (Bestwick et al., 1995). Inoculated areas were excised from leaves using new razor blades. In some experiments 10 leaf discs, 1.4 cm in diameter, were removed from the inoculation sites with a cork borer. The tissue pieces were weighed and placed in perforated aluminum foil bags, which were then plunged into liquid nitrogen and stored at −80°C for no longer than 24 h following excision.
Extraction of Enzymes from Leaf Tissue
Frozen leaf tissue was added to a mortar containing liquid nitrogen. The nitrogen was allowed to evaporate and the tissue was ground to a fine powder, which was then transferred to a mortar chilled on ice containing 200 mg of coarse sand and extraction buffer (4°C) containing 50 mm phosphate buffer (Na2HPO4/NaH2PO4), pH 6.8, and 1% (w/v) sodium metabisulphite. Homogenization was for 1 min with a ratio of 6 mL of buffer:1g fresh weight leaf tissue, and then polyvinylpolypyrrolidone was gently mixed into the grindate (0.5 g polyvinylpolypyrrolidone:1 g fresh weight tissue). The grindate was filtered through a layer of Miracloth (Calbiochem), and the filtrate was centrifuged at 20,000g for 25 min at 4°C. Supernatants were desalted by loading onto spin columns of Sephadex G-15 (Pharmacia) or they were concentrated and desalted by ultrafiltration using 10,000 Mr cutoff microconcentrators (Amicon, Beverly, MA). Finally, supernatants were aliquoted, glycerol was added to give a final concentration of 10% (v/v), and the samples were snap-frozen in liquid nitrogen and stored at −80°C. Protein concentrations were determined by the method of Bradford (1976).
Extraction of Intercellular Fluid
Intercellular fluid was extracted essentially according to the method of Rathmell and Sequeira (1974). Ten discs, 1.4 cm in diameter, were removed from lettuce leaves using a cork borer. The leaves were gently washed in sterile water at 4°C for 3 min and were then blotted dry and placed in 3 mL of 10 mm phosphate buffer (Na2HPO4/NaH2PO4) at 4°C, pH 6.8, containing 0.1% (w/v) sodium metabisulphite. Discs were infiltrated under vacuum (−100 KPa) for a period of 3 min, with the vacuum broken and reinstated every 30 s to achieve effective infiltration of the tissue. The infiltrated leaf discs were then washed in sterile water, dried by blotting on filter paper, and transferred to the barrel of a 5-mL syringe that had been placed inside a centrifuge tube. The discs were centrifuged at 500g for 10 min at 4°C. The resulting fluid expressed (consistently 80 μL) was used as the source of extracellular enzymes.
Enzyme Assays
All assays were performed in 1-mL volumes at 25°C in a UV/visible light spectrophotometer (model SP8–100, Pye Unicam, Cambridge, UK). Increases in absorbance were recorded at selected wavelengths depending on the substrate. For measurement of guaiacol peroxidase activity (A470) the assay contained 800 μL of 10 mm guaiacol in 50 mm potassium phosphate buffer, pH 6.0, 10 to 80 μL of extracted supernatant or intercellular fluid, 20 to 90 μL of sterile water, and 100 μL of 35 mm H2O2. TMB peroxidase activity (A654) was measured with a minor modification to the method of Imberty et al. (1984). The assay contained 50 μL of 20 mm TMB dissolved in absolute ethanol, 800 μL of sodium phosphate-citric acid buffer (90 mm Na2HPO4 and 55 mm citric acid), pH 4.5, 0 to 45 μL of water, 5 to 50 μL of total soluble extract or intercellular fluid, and 100 μL of 35 mm H2O2. Assays of chlorogenic acid peroxidase (A410) and caffeic acid peroxidase (A450) were conducted essentially as described by Mäder et al. (1977). The chlorogenic acid peroxidase assays comprised 800 μL of 50 mm potassium phosphate buffer, pH 6.5, 50 μL of 80 mm chlorogenic acid, 100 μL of 35 mm H2O2, 10 to 50 μL of extracted protein, and 0 to 40 μL of water. The caffeic acid peroxidase assay contained 800 μL of McIlvaine buffer (0.043 m citric acid and 0.114 m Na2HPO4), pH 5.5, 50 μL of 80 mm caffeic acid, 100 μL of 35 mm H2O2, 10 to 50 μL of extract/intercellular fluid, and 0 to 40 μL of water. Peroxidase assays were initiated by the addition of H2O2.
NADH and NADPH oxidase activities in supernatants and intercellular fluid were measured by following the decrease in A340 nm according to the method of Mäder and Amberg-Fisher (1982). For time-course studies the assay contained 45 mm Mes, pH 6.0, 0.15 mm NADPH or NADH, 0.1 mm MnCl2, 1 mm 2,4-dichlorophenol, and a suitable quantity of supernatant or intercellular fluid.
Glc-6-P dehydrogenase activity was measured as described by Reimers et al. (1992). The reaction mixture in a volume of 1 mL contained 100 μm Tricine buffer (adjusted to pH 8.0 with 0.1 m NaOH), 2 μm MgCl2, 6 mm Glc-6-P, 0.6 mm NADP+, and 25 μL of diluted supernatant or intercellular fluid, and the increase in A340 was monitored.
The effect of protein concentration on activity was assessed for all enzymes studied. All peroxidase activities measured at their optimal pH showed linear increases in activity with increasing protein concentrations, suggesting that there were no effects of inhibitors/effectors within the supernatants. Where appropriate, the pH optima and the effect of substrate concentration on activity were also determined. Buffers were prepared as described by Dawson et al. (1989). To determine the efficiency of extraction of enzymes, the pellets resulting from the 20,000g centrifugation were re-extracted with buffer containing 0.1% (w/v) Triton X-100; no significant further release of peroxidase or NADH/NADPH oxidase activity was detected. Recovery experiments were conducted by adding 25 μL of a 10% (w/v) preparation of horseradish peroxidase (Sigma) to the extraction buffer. Approximately 91% of activity was recovered under the optimized extraction conditions.
Supernatants from sonicated bacteria were analyzed for peroxidase activity, but even in the presence of 80 μg of protein from the supernatants, no activity was detected.
Peroxidase Localization
The protocol used was a modification of that of Sexton and Hall (1978). Samples of lettuce leaf panels were cut under fixative into 1- to 3-mm2 pieces, which were then fixed in a mixture of 1% (v/v) glutaraldehyde/1% (w/v) paraformaldehyde at pH 7.0 in buffer A (50 mm sodium cacodylate) for 45 min at room temperature. Following fixation, samples were washed twice for 10 min in buffer A and transferred for 30 min into 50 mm potassium phosphate buffer at pH 7.8 (buffer B) or at pH 6.0 (buffer C). Following washing, samples were transferred to 0.5 mg mL−1 DAB and 5 mm H2O2 dissolved in either buffer B or C. To prevent autooxidation of DAB, the staining medium was freshly prepared and staining was undertaken in dim light. Optimal times for staining were determined by incubation for 10, 15, 30, 60, and 90 min.
Samples were washed twice for 10 min in buffer B or C, postfixed in 1% (w/v) osmium tetroxide in buffer A for 45 min, and washed twice for 10 min in buffer A and twice for 10 min in water. Subsequent dehydration was undertaken in a graded-ethanol series. Following two 10-min washes in 100% ethanol, samples were transferred to propylene oxide for two washes of 10 min each. Samples were then embedded in Epon-araldite resin following progressive incubation in propylene oxide/resin mixtures, with the ratio of propylene oxide to resin decreased as follows: 3:1 for 15 min, 2:1 for 12 h or overnight, 1:1 for 30 min, 1:2 for 30 min, and 1:3 for 30 min. Following incubation in resin alone for 12 h, samples were transferred to fresh resin for 4 h. Finally, samples were placed in molds containing fresh resin and polymerized at 60°C for 48 h. Blocks were sectioned (70–90 nm) using a diamond knife (Diatome, Bienne, Switzerland), mounted on uncoated copper grids (300-mesh, Agar Aids, Bishops Stortford, UK), and examined without further treatment or stained with uranyl acetate and lead citrate.
To inhibit all enzyme activity following washing in buffer B or C, selected leaf samples were heated at 95°C for 15 min. To determine the H2O2 dependency of staining, H2O2 was omitted from the staining medium. Furthermore, because H2O2 may be generated in planta, samples were preincubated in buffer B or C containing 20 μg mL−1 catalase (commercial preparation from bovine liver; Sigma) and then placed in a staining medium containing catalase from which H2O2 had been omitted. To inhibit endogenous catalase activity, samples were incubated for 30 min in 20 μm ATZ in buffer B or C prior to staining. During staining, 20 μm ATZ was also included in the staining medium. To inhibit peroxidase, catalase, and Cyt oxidase activity, samples were incubated in 5 mm KCN for 30 min prior to staining, and cyanide was included in the staining medium.
H2O2 Localization
H2O2 production was assessed cytochemically via determination of cerium perhydroxide formation after reaction of CeCl3 with endogenous H2O2 as previously described (Bestwick et al., 1997). Sites of positive staining, given categories 1 to 3 (lowest to highest intensity of staining) in the cell wall were recorded as described by Bestwick et al. (1997), and an index of cerium perhydroxide deposition was calculated using the formula: ([percentage of sites in category 1 × 1] + [percentage of sites in category 2 × 2] + [percentage of sites in category 3 × 3]) ÷ 3, giving a maximum possible score of 100.
RESULTS
Lettuce HR
The development of the HR induced in lettuce by P. s. pv phaseolicola has already been described in detail (Bestwick et al., 1995). Following an induction time of approximately 2.5 h, visible signs of cell collapse are observed by 5 h after inoculation, and the lesion is brown and dessicated by 16 h. Analysis of cell plasmolysis indicated that irreversible membrane damage is detectable in approximately 10% of cells as early as 3 h after inoculation (i.e. 0.5 h postinduction time). The progressive development of membrane damage is followed by increased electrolyte leakage, which coincides with increasing ultrastructural evidence of cell decompartmentation. The hrpD mutant induces only a transient glazing of the leaf surface, occurring between 5 and 12 h after inoculation, but ultrastructural and plasmolysis studies demonstrated that approximately 10% of cells collapsed in this interaction within the first 24 h after inoculation.
Peroxidase Activity in Noninoculated Tissue
Given the broad substrate (hydrogen donor) utilization by peroxidase, we assayed activity in the presence of both natural (caffeic and chlorogenic acid) and artificial (guaiacol and TMB) hydrogen donors. Both caffeic and chlorogenic acids have been described as abundant phenolic compounds in lettuce tissues (Bennett et al., 1996). NADH and NADPH were also added to supernatants, because they represent potential in vivo reductants for peroxidase-catalyzed H2O2 generation (Vianello and Macri, 1991).
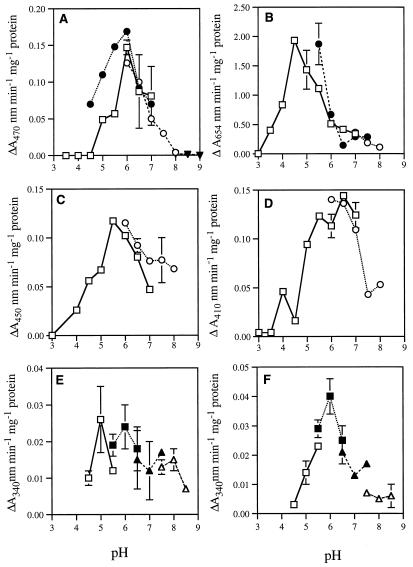
Different substrates were used, and significant differences in pH optima were found as follows: TMB, 4.5; guaiacol, 6.0; caffeic acid, 5.5 to 6.0; and chlorogenic acid, 6.0 to 6.5 (Fig. 1). The addition of the catalase inhibitor ATZ (20 μm) to prevent depletion of H2O2 from the assay medium did not influence pH profiles. No activity was detected following denaturation of extracts for 5 min in a boiling water bath. The omission of H2O2 or the inclusion of 20 μg mL−1 catalase abolished the oxidation of guaiacol, TMB, and caffeic acid. A low rate of heat-denaturable, catalase-insensitive oxidation of chlorogenic acid was observed and a correction was made for this rate. All peroxidase activities were totally inhibited by 20 μm KCN.
Figure 1.
pH profiles of soluble peroxidase-oxidase activities within 20,000g lettuce leaf supernatants. Effect of pH on peroxidase activity with different substrates: A, guaiacol; B, TMB; C, caffeic acid; D, chlorogenic acid. E and F, Effects of pH on the oxidation of NADH and NADPH. Buffers used were Mes (▪), citric acid-sodium phosphate (□), Mops (▴), N-2 hydroxyethylpiperazine-N-3-propane sulfuric acid (▵), borate-HCl (▾), Tris-maleate (•), and potassium phosphate (○). Data are the means ± se of a minimum of four experiments, each consisting of two replicates.
The rates of heat-denaturable NADPH and NADH oxidation by leaf extracts were determined (Fig. 1, E and F). Because numerous enzymes may be responsible for this oxidation, we attempted to stimulate peroxidases specifically by addition of Mn2+ and monophenols, as demonstrated by Vianello and Macri (1991). A combination of 0.1 mm MnCl2 and 1 mm 2,4-dichlorophenol produced maximal, heat-sensitive stimulation of both NADH and NADPH oxidation, both of which had optima between 5.5 and 6.5. Oxidation was accompanied by catalase-sensitive tetraguaiacol formation, indicating the production of H2O2.
Changes in Total Peroxidase, NADH, and NADPH Oxidase Activities after Inoculation
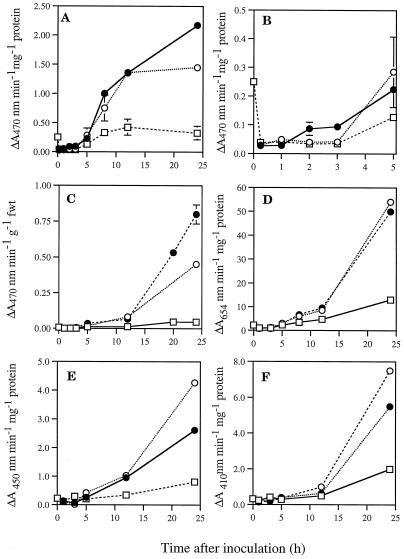
Time courses were conducted with both the “artificial” and the “natural” substrates and, as shown in Figure 2, certain common alterations to activity were observed at their pH optima. First, there was a rapid decline in activity following all inoculations. A detailed time course using guaiacol as the substrate (Fig. 2, A and B) revealed that this decline occurred by approximately 15 min after infiltration and represented a striking decrease to only 11% to 15% of the activity in uninoculated tissue. Activity gradually recovered to preinoculation levels but increased further only in response to bacterial challenge. The timing of recovery and the extent of the increase depended on the hydrogen donor used. Guaiacol peroxidase activity recovered more rapidly in tissues undergoing the HR and eventually reached levels considerably in excess of those in tissues responding to the hrp mutant. However, increases in TMB peroxidase were similar in response to both wild-type and mutant bacteria. Increases in chlorogenic acid and caffeic acid peroxidase activity were significantly higher in tissues inoculated with the hrp mutant relative to those undergoing the HR. Both caffeic acid and chlorogenic acid peroxidase activity in water-inoculated tissues recovered to preinoculation levels more rapidly than that of extracts from tissues inoculated with bacteria.
Figure 2.
Changes in peroxidase activity within inoculated lettuce leaves. Effects of inoculation with wild-type (•) and hrp D mutant (○) strains of P. s. pv phaseolicola, or sterile water (□) were examined. Activities of guaiacol (A–C) and TMB peroxidase (D) are presented as a function of total soluble protein (A, B, and D) or leaf fresh weight (C). Changes in caffeic acid peroxidase activities (E) and chlorogenic acid (F) are presented as a function of protein concentration. Data are means ± se of activities from three replicate supernatants; similar trends were found in repeated experiments. Note that tissues desiccated during the HR. fwt, Fresh weight.
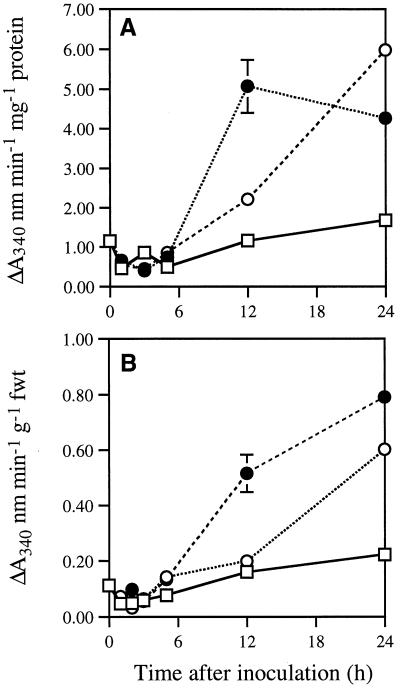
Changes in NADPH oxidase activity in the presence of 0.1 mm MnCl2 and 1 mm 2,4-dichlorophenol are presented in Figure 3. A profile similar to that of peroxidase activity was determined: an initial decline followed by recovery, which was far more rapid in tissues undergoing the HR.
Figure 3.
Changes in NADPH oxidase activity within inoculated lettuce leaves. Effect of inoculation with wild-type (•) and hrp D mutant (○) strains of P. s. pv phaseolicola or sterile water (□) on the rate of 2,4-dichlorophenol/Mn2+-stimulated NADPH oxidation by lettuce leaf extracts. A and B, Rates of NADPH oxidation mg−1 protein and g−1 fresh leaf weight, respectively. Data are means ± se of three replicates; duplicate experiments demonstrated a similar trend in both NADPH and NADH oxidation. Note that tissues desiccated during the HR. fwt, Fresh weight.
Changes in Extracellular Peroxidase, NADH, and NADPH Oxidase Activities following Inoculation
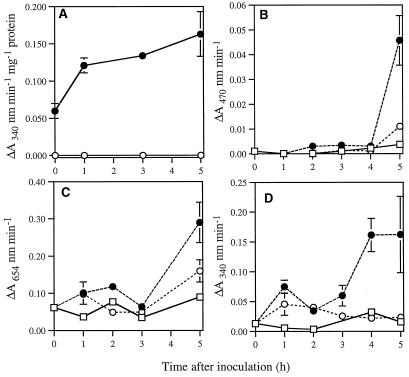
Within the first 5 h of the HR, no activity of the marker enzyme Glu-6-P dehydrogenase could be detected in the intercellular fractions, implying no appreciable symplastic contamination of the apoplast (Fig. 4A). However, after 5 h the extensive cell collapse occurring during the HR resulted in varying levels of contamination. Time-course studies of enzyme activities were therefore confined to the first 5 h after inoculation.
Figure 4.
Changes in peroxidase and oxidase activities determined in intercellular fluids. A, Activity of the cytoplasmic marker enzyme Glc-6-P dehydrogenase within homogenates (•) and intercellular fluids (○) extracted from lettuce leaves inoculated with wild-type P. s. pv phaseolicola. Changes in the activities of guaiacol and TMB peroxidase (B and C) NADH oxidase (D) following inoculation with wild-type (•) or hrp D mutant (○) of P. s. pv phaseolicola or sterile water (□) are shown. Analysis of NADPH oxidation by intercellular fluids revealed a trend similar to that of NADH oxidation. Data are based on the intercellular fluids recovered from three leaf discs. Means ± se of four replicates are given; the trends observed were confirmed in repeated experiments.
No peroxidase activity was detected using natural hydrogen donors, but considerable H2O2-dependent activity was found in the presence of guaiacol and TMB (Fig. 4, B and C). Following an initial decline, guaiacol peroxidase activity increased in tissue challenged with both the hrp mutant and the wild-type strain, the rate and magnitude of increase being far greater in tissues undergoing the HR. With TMB, no decline in peroxidase activity was observed after bacterial infiltration, but bimodal increases occurred in response to both strains, with a much greater increase occurring during the HR (Fig. 4D). The peroxidases recovered in intercellular fractions would be expected to represent a subset of isoforms contributing to total soluble peroxidase activity.
An increase in Mn2+/2,4-dichlorophenol-stimulated NADH and NADPH oxidase activity was observed by 1 h after inoculation with both bacterial strains. Between 3 and 5 h a second striking increase in activity occurred in response only to wild-type bacteria (Fig. 4D).
Localization of Peroxidase Activity
Noninoculated Leaves
Following fixation in paraformaldehyde/glutaraldehyde, peroxidase was detected by the formation of osmium black after treatment of sections with DAB/H2O2 at pH 6.0 or 7.8. Staining was first observed after 15 min of incubation in DAB/H2O2 at pH 6.0 and after 30 min at pH 7.8, and in both cases optimal stain intensity was reached after 60 min. Despite the short fixation times used (45 min), cell ultrastructure was remarkably well preserved. When present, peroxidase activity was usually observed in the middle lamella and on the extracellular face of the cell wall (Fig. 5).
Figure 5.
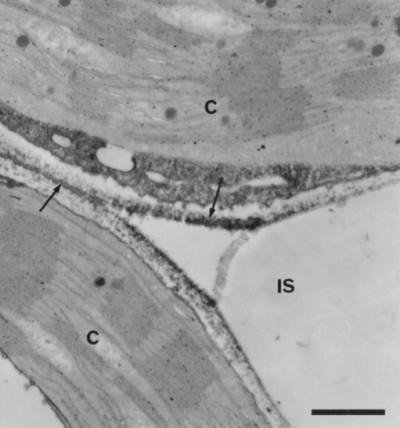
Cytochemical localization of peroxidase activity in uninoculated tissue. Note that staining, indicated by the formation of electron-dense deposits (arrows), is mainly located on the extracellular face of the plant cell wall and in the middle lamella. Bar = 0.5 μm. IS, Intercellular space; C, chloroplast.
Inoculated Leaves
For the time-course studies, tissues were stained at pH 6.0. Between 3 and 8 h after inoculation, a localized increase in peroxidase activity, indicated by the presence of electron-dense deposits, was observed in cell walls at the sites of both wild-type and hrp mutant attachment and, therefore, to sites of papilla development (Fig. 6A). Coincident with increased wall activity, peroxidase was also detected within the nuclear envelope, the ER, the Golgi apparatus, and variously sized vesicles within the challenged cells (Fig. 6, B and C). High levels of peroxidase were also identified within the material surrounding and encapsulating both strains of bacteria on the epidermal and mesophyll cell walls (Fig. 6, B and C).
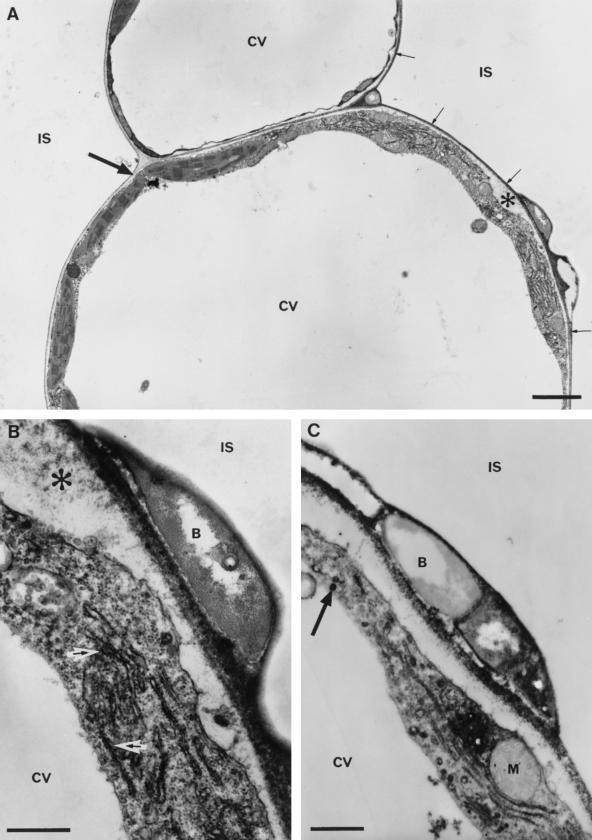
Figure 6.
Peroxidase activity in the cell wall and cytoplasm of mesophyll cells after inoculation with P. s. pv phaseolicola. A, Low magnification (bar = 2 μm) showing the extension of electron-dense staining (small arrows) from sites of bacterial attachment (wild-type strain) onto the plant cell wall 5 h after inoculation. Note that the cell wall away from the bacteria is unstained (large arrow). The large papilla (asterisk) has patchy staining that is more distinct at higher magnification in B. Bar = 0.5 μm. B, Staining for peroxidase can be seen to extend into the material surrounding bacteria, and activity is also associated with the rough ER (arrows) and small vesicles in the dense plant cell cytoplasm at reaction sites. C, Reaction to the hrpD mutant 8 h after inoculation includes increased peroxidase activity around attached bacteria, within the plant cell wall, and within small vesicles (arrow). B, Bacterium; IS, intercellular space; CV, central vacuole; M, mitochondrion.
In cells showing signs of collapse during the HR, peroxidase activity was not greater than that detected in cells with little evidence of disruption (Fig. 7, A and B). Condensation of the cytoplasm to form an electron-dense osmiophilic “corpse” during the terminal phases of the HR prevented further observations on cytoplasmic peroxidase in this interaction. The detection of peroxidase activity in the cell wall also became increasingly inconsistent and it was necessary to include ATZ to detect peroxidase reliably within the walls of collapsing cells. By contrast, high levels of peroxidase activity were maintained at sites of hrp mutant attachment (Fig. 6C).
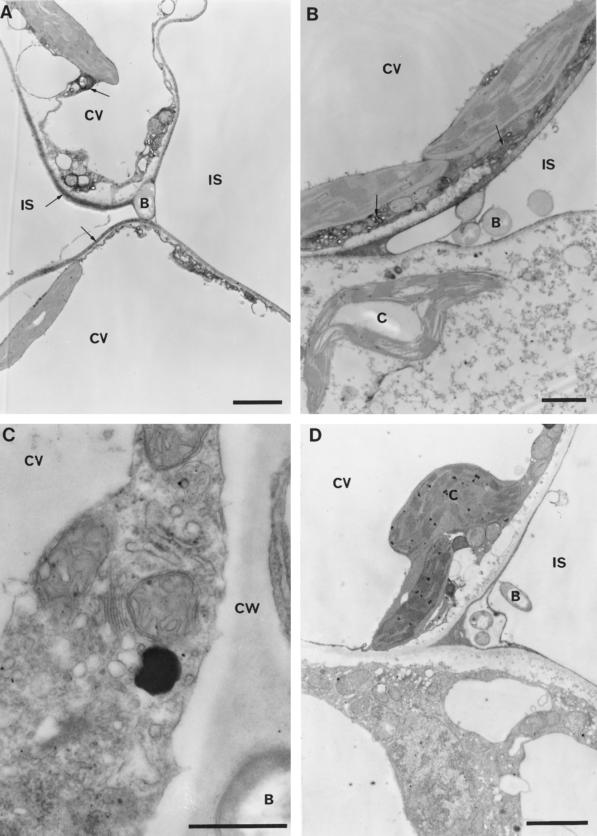
Figure 7.
Peroxidase localization in cells collapsing during the HR and controls showing the specificity of staining for enzyme activity. A and B, Sites undergoing the HR in response to the wild-type strain 5 and 8 h after inoculation, respectively. Note that activity (arrows) is detected in the cell wall, ER, and small vesicles. Staining within the wall is less intense than observed at earlier stages of the plant cell's response (Fig. 6). Bars = 1 and 2 μm in A and B, respectively. C and D, Sections from sites 3 and 8 h after inoculation with the wild-type strain used as controls in which 5 mm KCN was added to the staining medium to inhibit peroxidase activity. The sites examined were directly comparable to those shown in Figure 6, in which the similar electron density of organelles such as mitochondria, which lack peroxidase activity, contrasts with the strongly stained plant cell wall in the absence of KCN. Bars = 0.5 and 2 μm for C and D, respectively. B, Bacterium; IS, intercellular space; CW, cell wall; CV, central vacuole; C, chloroplast.
Controls
Catalase may be detected using DAB as a substrate because of its “peroxidative” activity, which is reportedly favored by fixation in glutaraldehyde (Lewis, 1977). Although considerable ATZ-sensitive staining of peroxisomes and the surrounding cytoplasm was observed following incubation at pH 7.8, no such staining was apparent at pH 6.0. In addition, at pH 6.0, ATZ did not affect the distribution of DAB oxide during the first 8 h after inoculation but was required to observe oxide formation within the cell walls of collapsed tissues during the later stages of the HR. There have also been reports that DAB may serve as a substrate for polyphenol oxidase, but no staining could be attributed to this enzyme, since thylakoid membranes were not stained and the polyphenol oxidase inhibitor diethyldithiocarbamate failed to prevent staining. All staining was heat labile and was verified as being associated with the activity of a heme-containing protein by its inhibition with cyanide (Fig. 7). The H2O2 dependence of DAB oxide formation was confirmed when the omission of H2O2 from the staining medium totally abolished activity in noninoculated tissues; however, during the HR the inclusion of catalase was required to prevent staining completely.
H2O2
The distribution of peroxidase activity was closely compared with the accumulation of H2O2 detected by the catalase-sensitive formation of cerium perhydroxides at reaction sites after incubation with CeCl3. As previously reported by Bestwick et al. (1997), the main site of H2O2 accumulation was apoplastic, with staining present in both the cell wall and the papillae (Fig. 8A). We also observed a previously unreported, less frequent, and nonlocalized accumulation of cerium perhydroxides within the symplasm of cells undergoing the HR (Fig. 8B). The more widespread accumulation of cerium perhydroxides was first apparent 8 h after inoculation and was not necessarily associated with visible cellular disruption.
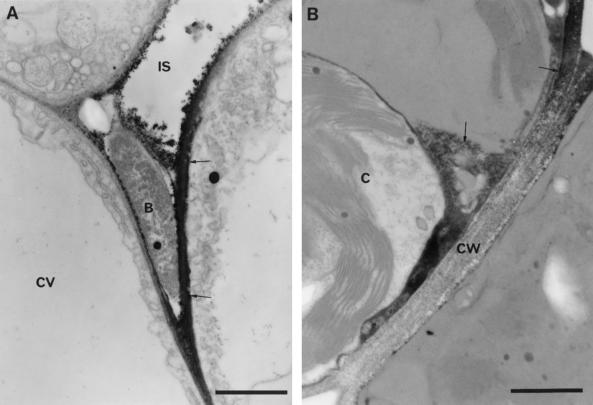
Figure 8.
Localization of H2O2 accumulation in lettuce cells adjacent to wild-type P. s. pv phaseolicola 5 h after inoculation. A, Electron-dense deposits of cerium perhydroxides extending from the site of attachment of bacteria into the surrounding plant cell wall (arrows). Bar = 1 μm. B, Detection of H2O2 in cells undergoing the HR. Deposits of cerium perhydroxide (arrows) are present in both the cell wall and in the degenerating cytoplasm. Bar = 0.5 μm. B, Bacterium; IS, intercellular space; CW, cell wall; CV, central vacuole; C, chloroplast.
An index based on the frequency and intensity of perhydroxide formation in the apoplasm was devised based on the scoring system reported by Bestwick et al. (1997). Time-course studies using the index to summarize staining (Fig. 9) revealed that high levels of H2O2 were maintained within the apoplast during the HR, with peak H2O2 production coinciding with the onset of membrane damage (Bestwick et al., 1995). Treatment with ATZ led to the maintenance of a high cerium perhydroxide index throughout the time course of the HR, indicating that H2O2 continued to be produced but was scavenged by catalase, probably as it was released from degrading peroxisomes. Unfortunately, it was not possible to devise an index for cytoplasmic H2O2 production/accumulation because the increased electron density associated with the condensation of the cytoplasm at later times, representing the terminal phase of cell death, often obscured perhydroxide formation.
Figure 9.
H2O2 accumulation during the HR. Tissues were stained with CeCl3 and processed for electron microscopy, and an intensity index for H2O2 production was compiled as described in Methods (maximum score, 100). Tissues inoculated with wild-type (circles) or hrpD mutant (squares) of P. s. pv phaseolicola were analyzed in the presence (open symbols) or absence (solid symbols) of the catalase inhibitor ATZ. Data are from analysis of a minimum of 30 interaction sites at each time after inoculation. Key stages of cellular responses are marked by numbers: 1, completion of the induction time; 2, 30% of mesophyll cells fail to plasmolyze; 3, 10% of cells fail to plasmolyze in response to the hrp mutant; 4, 70% of cells fail to plasmolyze and 50% have collapsed during the HR.
DISCUSSION
Both the activity and distribution of peroxidase were altered within lettuce cells following the inoculation of leaves with wild-type and hrp− bacteria. The changes observed indicate three levels of response: (a) nonspecific changes associated with inoculation; (b) changes associated with general defense responses; and (c) responses that have relevance to the development of the HR. Such complexity is perhaps predictable given the multifunctional role that isoforms of peroxidase may play in plant stress responses.
The initial decline in peroxidase activity is a response to inoculation because it was induced by water infiltration and bacterial treatment. Such decreases may arise from an inhibition of enzyme synthesis or activity or from relocalization to an environment such as the cell wall, which might be recalcitrant to buffer extraction. Subsequent increases in both apoplastic and symplastic peroxidase activities to levels above those found in noninoculated leaves were, however, found only in tissues challenged by bacteria. In general, increases in total and apoplastic peroxidase activities both correlated well with the microscopic detection of DAB oxide formation in cells challenged by bacteria; however, there were significant differences in the changes in activity recorded using various hydrogen donors as substrates. There appear to be differences in the substrate specificities of the isoenzymes that make up the total activities measured, which may reflect the different nature of the resistant responses induced by wild-type and hrp− strains.
Cytochemical localization and assays of intercellular fluids demonstrated that early increases in activity were mainly confined to the apoplasm and, in particular, to sites of bacterial attachment to lettuce cell walls. A rapid synthesis and site-directed secretion of the enzyme is probable, because the increase in apoplastic peroxidase activity coincided with or occurred following the appearance of DAB oxide within the endomembrane system. The addition of heme to the apoprotein moiety may occur within the ER; therefore, the synthesis of isoperoxidases may first become detectable by cytochemistry during this stage of processing (Birecka et al., 1975; Mendgen, 1975; Ebermann and Stich, 1986). The heavily stained vesicles in the cytoplasm of cells challenged by bacteria may be involved in peroxidase secretion, as suggested by van Huystee (1987). Pre-existing symplastic isoperoxidases may also be relocated to the wall, and it is also possible that a component of the increased wall activity represents the activation of pre-existing wall-bound peroxidase.
The sites of increased peroxidase activity in the cell wall are also regions of papilla development. Ca2+, which has been implicated in the deposition of callose (a major component of papillae), is known to regulate peroxidase secretion and may therefore act as a local signal for site-specific structural alterations (Sticher et al., 1981; Aist and Gold, 1987). It seems likely that peroxidase is responsible for cross-linking the phenolic compounds and Hyp-rich glycoproteins found within papillae and also for the modification of the cell wall at reaction sites (Bestwick et al., 1995). Such reactions are H2O2 dependent, and increased concentrations of H2O2, as revealed by catalase-sensitive cerium perhydroxide precipitation, were also localized to sites of papilla formation.
Clearly, there is no precise relationship between peroxidase levels and H2O2 accumulation. The markedly lower levels of H2O2 in the interaction with the hrp mutant contrasts sharply with the generally high level of peroxidase in this interaction, as determined both cytochemically and in assays using “natural” phenolic substrates. Nevertheless, our data demonstrate the production of H2O2 in response to the hrp mutant, with the ROS available (although at low levels) for cross-linking reactions mediated by peroxidase. It remains to be determined whether cross-linking reactions are effectively restrained by the lower level of H2O2 prevalent within the cell wall and papillae adjacent to the hrp mutant, or whether an excess of H2O2 is produced during the interaction with the wild-type strain (Bestwick et al., 1997). The extent to which cross-linking occurs may be resolved by examining the protoplasting efficiency of tissues in both interactions and by the susceptibility of wall-bound phenolic compounds to removal by mild saponification (Brisson et al., 1994; Bennett et al., 1996).
The origin of the H2O2 produced during defense reactions in lettuce is intriguing. The largely apoplastic location of cerium perhydroxide formation suggests that either the plasma membrane or the cell wall is the primary site of the superoxide/H2O2 generator. Isolated plant protoplasts are capable of generating elictor-induced oxidative bursts, and a membrane-associated, neutrophil-like NADPH oxidase complex is thought to be responsible for superoxide/H2O2 generation within elicitor/microbe-stimulated soybean, potato, parsley, and rose cells (Lamb and Dixon, 1997). However, in cotton challenged with Xanthomonas, superoxide generation has been linked to the apoplastic NADH oxidase activity of a peroxidase-like enzyme, and a cell wall peroxidase has also been implicated in the generation of an elicitor-induced oxidative burst in bean cells (Bolwell et al., 1995; Martinez et al., 1997). The potential for ROS production by systems other than membrane-bound NADPH oxidase is indicated from experiments in which cell wall preparations from a number of species have been shown to generate oxidative bursts when challenged with elicitors or digested with cell wall-degrading enzymes (Ishii, 1987; Kiba et al., 1997). In tobacco there is evidence that multiple oxidase systems may operate and that individual contributions to H2O2 production may vary between interactions/elicitors (Allan and Fluhr, 1997).
In lettuce low concentrations of the neutrophil NADPH oxidase inhibitor diphenylene iodonium significantly reduced H2O2 levels detected by staining with CeCl3, and the in vivo production of H2O2 was also cyanide and azide sensitive, suggesting the involvement of both a neutrophil-like NADPH oxidase and peroxidase in ROS generation (Bestwick et al., 1997). Recently, Frahry and Schopfer (1998) demonstrated that the oxidase activity of horseradish peroxidase is also reduced by diphenylene iodonium, indicating that the inhibitor should no longer be considered a reliable marker for neutrophil-like NADPH oxidase in plants. We have now shown that heightened apoplastic Mn2+/2,4-dichlorophenol stimulated NADH and NADPH oxidase activity, which is characteristic of certain peroxidases (Vianello and Macri, 1991), coincides with peak H2O2 production during the HR.
Overall, our results suggest that certain isoforms of peroxidase with NAD(P)H oxidase activity are a major source of ROS production in lettuce. We have used NADPH and NADH as substrates for peroxidase-based H2O2 generation, and although the former may not be physiologically relevant in the apoplast, NADH may be supplied to the cell wall via a malate oxaloacetate shuttle across the plasma membrane involving a wall-bound malate dehydrogenase (Gross et al., 1977). Additional isoforms of peroxidase that may be strongly bound to the cell wall (either ionically or covalently) and may not have been extracted may also contribute to H2O2 generation in the apoplast. The isolation of peroxidase isoforms and the characterization of their enzymic activities is needed to confirm their proposed role in the HR.
Electron microscopy has demonstrated that the incorporation of new material into cell walls and papilla formation often occur in the absence of the HR (Bestwick et al., 1995; Brown et al., 1995). The extent to which oxidative processes are necessary for and controlled in these reactions may determine the continued viability of lettuce cells. It is possible that an imbalance in peroxidase-oxidase activity during the HR contributes to an increase in H2O2 and, therefore, the cerium perhydroxide precipitation observed in our experiments. For example, although increases in soluble peroxidase activity within tissues challenged by wild-type and hrp mutant strains are initially similar, the Mn2+/2,4-dichlorophenol-stimulated NADH/NADPH oxidase activity is primarily enhanced in response to challenge with wild-type bacteria. Significantly, during the HR, increases in extracellular, peroxidase-like NADH/NADPH oxidase activity preceded major increases in extracellular peroxidase activity detected in intercellular fluids. Peroxidase activity driven by H2O2 generated either by peroxidase isoforms or other sources (such as a neutrophil-like NADPH oxidase complex) may subsequently reduce the level of free H2O2.
In conclusion, our experiments demonstrate that resistance responses, including ROS production, are highly localized and tightly controlled within sites in contact with the invading microorganism. The localization observed highlights the potential importance of specific isoforms of peroxidase and reactions occurring in the apoplasm in mediating both the HR and other processes of resistance.
ACKNOWLEDGMENTS
We wish to thank Shelagh Reardon for assistance with electron microscopy and Atilla Ádám for valuable discussion about the role of peroxidase in the HR.
Abbreviations:
- AZT
3-amino-1,2,4-triazole
- DAB
3′3′-diaminobenzidine
- HR
hypersensitive reaction
- ROS
reactive oxygen species
- TMB
tetramethylbenzidine
Footnotes
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (UK) and the European Union Biotechnology Framework IV program.
LITERATURE CITED
- Ádám A, Farkas T, Somlyai G, Hevesi M, Király Z. Consequence of O2− generation during bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipids. Physiol Mol Plant Pathol. 1989;34:13–26. [Google Scholar]
- Ádám AL, Bestwick CS, Barna B, Mansfield JW. Enzymes regulating the accumulation of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringae pv phaseolicola. Planta. 1995;197:240–249. [Google Scholar]
- Aist JR, Gold RE (1987) Prevention of fungal ingress: the role of papillae and calcium. In S Nishimura, CP Vance, N Doke, eds, Molecular Determinants of Plant Disease. Japan Scientific Societies Press, Tokyo, pp 47–59
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M, Gallagher M, Fagg J, Bestwick C, Paule T, Beale M, Mansfield J. The hypersensitive reaction, membrane damage, and accumulation of autofluorescent phenolics in lettuce cells challenged by Bremia lactucae. Plant J. 1996;9:851–865. [Google Scholar]
- Bestwick CS, Bennett MH, Mansfield JW. Hrp mutant of Pseudomonas syringae pv phaseolicola induces cell wall alterations but not membrane damage leading to the hypersensitive reaction in lettuce (Lactuca sativa) Plant Physiol. 1995;108:503–516. doi: 10.1104/pp.108.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MH, Mansfield JW. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H, Catalfamo JL, Garraway MO. Cell wall and protoplast isoperoxidases of corn leaves in relation to cut injury and infection with Helminthosporium maydis. Plant Physiol. 1975;55:607–610. doi: 10.1104/pp.55.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Butt VS, Davies DR, Zimmerlin A. The origin of the oxidative burst in plants. Free Radical Res. 1995;23:517–532. doi: 10.3109/10715769509065273. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein using the principles of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb CJ. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I, Mansfield J, Bonas U. Hrp genes in Xanthomonas campestris pv vesicatoria determine ability to suppress papilla deposition in pepper mesophyll cells. Mol Plant-Microbe Interact. 1995;8:825–836. [Google Scholar]
- Campa A. Biological roles of plant peroxidases: known and potential function. In: Everse J, Grisham MB, editors. Peroxidases in Chemistry and Biology, Vol II. Boca Raton, FL: CRC Press; 1991. pp. 25–50. [Google Scholar]
- Croft KPC, Voisey CR, Slusarenko AJ. Physiol Mol Plant Pathol. 1990;36:49–62. [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy—cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson RMC, Elliott DC, Elliot WH, Jones KM. Data for Biochemical Research. Oxford, UK: Oxford University Press; 1989. [Google Scholar]
- de Marco A, Roubelakis-Angelakis KA. The complexity of enzymatic control of hydrogen peroxide concentration may affect the regeneration potential of plant protoplasts. Plant Physiol. 1996;110:137–145. doi: 10.1104/pp.110.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dypbukt JM, Ankarcrona M, Burkitt M, Sjoholm A, Strom K, Orrenius S, Nicotera P. Different pro-oxidant levels stimulate growth, trigger apoptosis or produce necrosis of insulin secreting RINm5F cells. J Biol Chem. 1994;269:30553–30560. [PubMed] [Google Scholar]
- Ebermann R, Stich K (1986) Evidence for the sole responsibility of heme for the function of peroxidase and polyphenoloxidase activity showing isoenzymes in Quercus robur. In H Grepin, C Penel, T Gaspar, eds, Molecular and Physiological Aspects of Plant Peroxidases. University of Geneva, Geneva, Switzerland, pp 287–290
- Fink W, Deising H, Mendgen K. Early defense responses of cowpea (Vigna sinensis L.) induced by non-pathogenic rust fungi. Planta. 1991;185:246–254. doi: 10.1007/BF00194067. [DOI] [PubMed] [Google Scholar]
- Frahry G, Schopfer P. Inhibition of O2-reducing activity of horseradish peroxidase by diphenyleneiodonium. Phytochemistry. 1998;48:223–227. doi: 10.1016/s0031-9422(98)00004-1. [DOI] [PubMed] [Google Scholar]
- Graham MY, Graham TL. Rapid accumulation of anionic peroxidases and phenolic polymers in soybean cotyledon tissues following treatment with Phytophthora megasperma f. sp. glycinea wall glucan. Plant Physiol. 1991;97:1445–1455. doi: 10.1104/pp.97.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GG, Janse C, Elstner EF. ) Planta. 1977;136:271–276. doi: 10.1007/BF00385995. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (1989) Free Radicals in Biology and Medicine. Oxford University Press Oxford, UK
- Ievinsh G. Characterization of the peroxidase system in winter rye seedlings: compartmentation and dependance on leaf development and hydrogen donors used. J Plant Physiol. 1992;140:257–263. [Google Scholar]
- Iiyama K, Lam TB-T, Stone BA. Covalent cross-links in the cell wall. Plant Physiol. 1994;104:315–320. doi: 10.1104/pp.104.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imberty A, Goldberg R, Catesson A-M. Tetramethylbenzidine and p-phenylenediamine-pyrocatechol for peroxidase histochemistry and biochemistry: two new, non-carcinogenic chromogens for investigating lignification process. Plant Sci Lett. 1984;35:103–108. [Google Scholar]
- Ishii S. Generation of active oxygen species during enzymic isolation of protoplasts from oat leaves. In Vitro Cell Dev Biol. 1987;23:653–658. [Google Scholar]
- Jackson P, Ricardo CPP. An examination of the peroxidases from Lupinus albus L. hypocotyls. Planta. 1994;194:311–317. [Google Scholar]
- Jiang Y, Miles PW. Generation of H2O2 during enzymic oxidation of catechin. Phytochemistry. 1993;33:29–34. [Google Scholar]
- Kiba A, Miyake C, Toyoda K, Ichinose Y, Yanada T, Shiraishi T. Superoxide generation in extracts from isolated plant cell walls is regulated by fungal signal molecules. Phytopathology. 1997;87:846–852. doi: 10.1094/PHYTO.1997.87.8.846. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon R. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Lewis PR (1977) Other cytochemical methods for enzymes. In PR Lewis, DP Knight eds, Staining Methods for Sectioned Material. North Holland Publishing, Amsterdam, The Netherlands, pp 225–288
- Low PS, Merida JR. The oxidative burst in plant defense: function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- Mäder M, Amberg-Fisher V. Role of peroxidase in the lignification of tobacco cells. I. Oxidation of nicotinamide adenine dinucleotide and formation of hydrogen peroxide by cell wall peroxidases. Plant Physiol. 1982;70:1128–1131. doi: 10.1104/pp.70.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäder M, Nessel A, Boff M. On the physiological significance of the isozyme groups of peroxidase from tobacco demonstrated by biochemical properties. Z Pflanzenphysiol. 1977;82:247–260. [Google Scholar]
- Mansfield JW, Bennett MH, Bestwick CS, Woods-Tor AM (1997) Phenotypic expression of gene-for-gene interaction: variation from recognition to response. In IR Crute, JJ Burden, EB Holub, eds, The Gene-for-Gene Relationship in Host-Parasite Interactions. CAB International, London, UK, pp 265–292
- Martinez C, Bresson E, Montellet JL, Angel JP, Daniel JF, Geiger JP, Nicole M (1997) The oxidative burst in Xanthomonas-infected cotton cells is associated with hypersensitive cell death. In Proceedings of the 10th Congress of the Mediterranean Phytopathological Union, Société Francaise de Phytopathologie, C.S.R. Saint Gély du Fesc, pp 413–417
- Mendgen K. Ultrastructural demonstration of different peroxidase activities during the bean rust infection process. Physiol Plant Pathol. 1975;6:275–282. [Google Scholar]
- Milosevic N, Slusarenko AJ. Active oxygen metabolism and lignification in the hypersensitive response in bean. Physiol Plant Pathol. 1996;49:143–158. [Google Scholar]
- Monties B. Lignins. In: Dey PM, Harborne JB, editors. Methods Plant Biochem Vol I. London: Academic Press; 1989. pp. 113–157. [Google Scholar]
- Morel JB, Dangl JL. The hypersensitive response and the induction of cell death in plants. Cell Death Differ. 1997;4:671–683. doi: 10.1038/sj.cdd.4400309. [DOI] [PubMed] [Google Scholar]
- Pedreño MA, Barceló AR, Garcia-Carmona F, Muñoz R. Oxidation of dihydroxyfumaric acid in the absence of H2O2 by cell-wall bound peroxidase from lupin: a possible general model. Plant Physiol Biochem. 1990;28:37–42. [Google Scholar]
- Pichorner H, Couperus A, Korori SAA, Ebermann R. Plant peroxidase has thiol oxidase activity. Phytochemistry. 1992;31:3371–3376. [Google Scholar]
- Rathmell WG, Sequeira L. Soluble peroxidase in fluid from the intercellular spaces of tobacco leaves. Plant Physiol. 1974;53:317–318. doi: 10.1104/pp.53.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers PJ, Guo A, Leach JE. Increased activity of a cationic peroxidase associated with an incompatible interaction between Xanthomonas oryzae pv oryzae and rice (Oryza sativa) Plant Physiol. 1992;99:1044–1050. doi: 10.1104/pp.99.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton R, Hall JL (1978) Enzyme cytochemistry. In JL Hall, ed, Electron Microscopy and Cytochemistry of Plant Cells. North Holland Publishing, London, pp 63–148
- Sticher L, Penel C, Greppin H. Calcium requirement for the secretion of peroxidases by plant cell suspensions. J Cell Sci. 1981;48:345–353. doi: 10.1242/jcs.48.1.345. [DOI] [PubMed] [Google Scholar]
- van Huystee RB. Some molecular aspects of plant peroxidase biosynthetic studies. Annu Rev Plant Physiol. 1987;38:205–219. [Google Scholar]
- Vianello A, Macri F. Generation of superoxide anion and hydrogen peroxide at the surface of plant cells. J Bioenerg Biomembr. 1991;23:409–423. doi: 10.1007/BF00771012. [DOI] [PubMed] [Google Scholar]
- Wojtaszek P. The oxidative burst: a plant's early response against infection. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]