Abstract
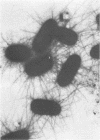
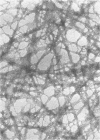
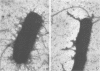
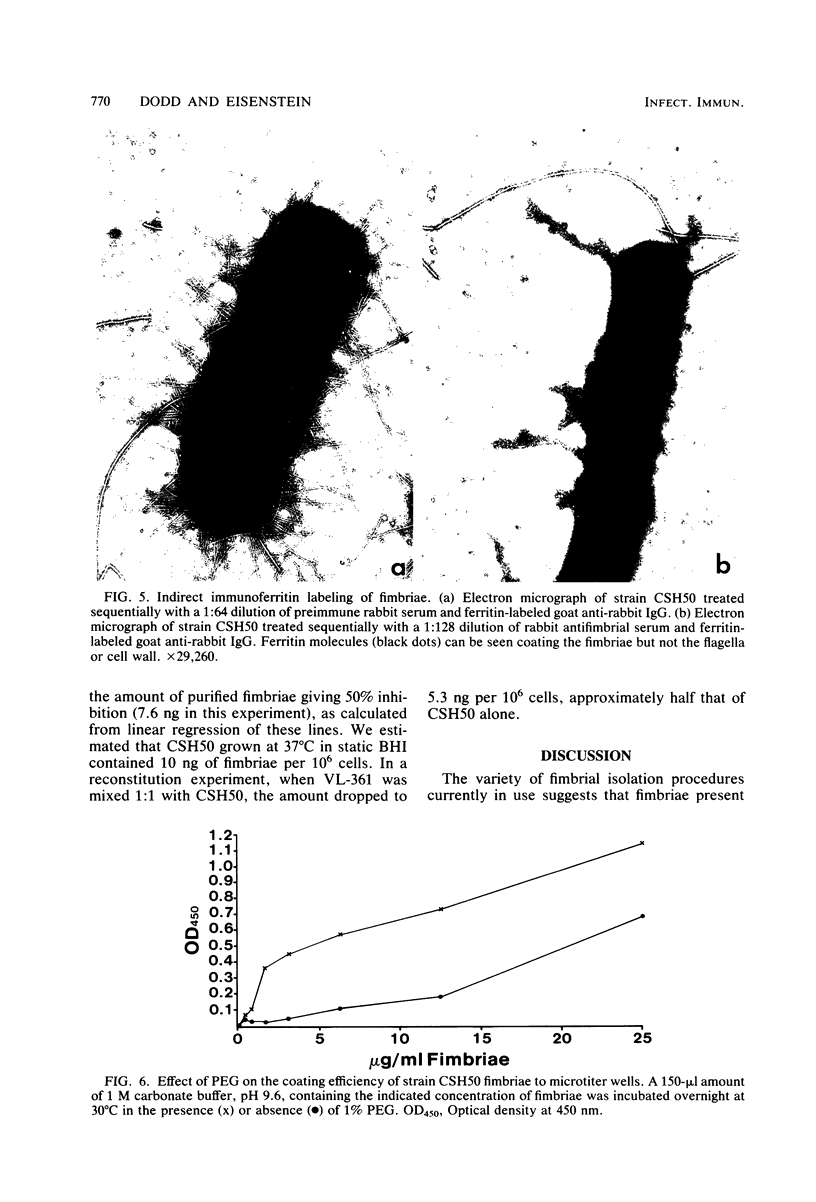
Type 1 fimbriae from two strains of Escherichia coli, K-12-derived CSH50 and a clinical isolate VL-2, were purified by a simplified procedure, which should be applicable to a variety of bacterial strains. After mechanical removal from the cells, the fimbriae were sedimented in the ultracentrifuge and resuspended in 5 M urea to disaggregate cell membranes and flagella, leaving the urea-resistant fimbriae intact. After several hours at 37 degrees C, this crude fimbrial suspension was diluted to 1 M urea, and the intact fimbriae were sedimented through a 1 M urea-1 M sucrose cushion. The pellet was found to be pure fimbriae by sodium docecyl sulfate-polyacrylamide gel electrophoresis, with apparent subunit molecular weights of 17,000 for the fimbriae from K-12 strain CSH50 and 19,000 for those from the clinical isolate VL-2. High-titer rabbit antiserum raised against CSH50 fimbriae was specific for fimbriae by indirect ferritin labeling and immunoprecipitation and was used to develop an enzyme-linked immunosorbent assay. Competitive inhibition of antifimbrial antiserum in the enzyme-linked immunosorbent assay by a known amount of either purified fimbriae or fimbriae-bearing bacteria permitted precise quantitation of fimbrial antigen in cultures of strain CSH50, thereby providing a simple means of determining the effects of environmental conditions on the synthesis of type 1 fimbriae.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Goldman R., Ofek I., Sharon N., Mirelman D. Mannose-binding activity of Escherichia coli: a determinant of attachment and ingestion of the bacteria by macrophages. Infect Immun. 1980 Aug;29(2):417–424. doi: 10.1128/iai.29.2.417-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigen-specific serotyping of Neisseria gonorrhoeae. I. Use of an enzyme-linked immunosorbent assay to quantitate pilus antigens on gonococci. J Infect Dis. 1978 Sep;138(3):319–315. doi: 10.1093/infdis/138.3.319. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Beachey E. H., Solomon S. S. Divergent effects of cyclic adenosine 3',5'-monophosphate on formation of type 1 fimbriae in different K-12 strains of Escherichia coli. J Bacteriol. 1981 Jan;145(1):620–623. doi: 10.1128/jb.145.1.620-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Dodd D. C. Pseudocatabolite repression of type 1 fimbriae of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1560–1567. doi: 10.1128/jb.151.3.1560-1567.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Ofek I., Beachey E. H. Loss of lectin-like activity in aberrant type 1 fimbriae of Escherichia coli. Infect Immun. 1981 Feb;31(2):792–797. doi: 10.1128/iai.31.2.792-797.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E. Factors affecting expression of the Escherichia coli pilus K99. Infect Immun. 1980 Apr;28(1):190–194. doi: 10.1128/iai.28.1.190-194.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Brown D. M., Glazer A. N. The formation of bacterial flagella. 3. Characterization of the subunits of the flagella of Bacillus subtilis and Spirillum serpens. J Mol Biol. 1967 Aug 28;28(1):45–51. doi: 10.1016/s0022-2836(67)80076-7. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Carnahan J., Brinton C. C., Jr Mechanical removal of F pili, type I pili, and flagella from Hfr and RTF donor cells and the kinetics of their reappearance. J Bacteriol. 1969 Jun;98(3):1294–1306. doi: 10.1128/jb.98.3.1294-1306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Ultraviolet irradiation disrupts somatic pili structure and function. Infect Immun. 1979 Sep;25(3):1060–1065. doi: 10.1128/iai.25.3.1060-1065.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Pisano J. J., Appella E. Analysis of amino acid phenylthiohydantoins by high speed liquid chromatography. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1220–1224. doi: 10.1016/s0006-291x(73)80024-5. [DOI] [PubMed] [Google Scholar]