Abstract
Background and Aims
Reconstructions have identified the 20th century as being uniquely warm in the last 1000 years. Changes in the phenology of primary meristems converged toward increases in length of the growing season. Has the phenology of secondary meristem changed during the last century, and to what extent?
Methods
Timings of wood formation in black spruce, Picea mariana, were monitored for 9 years on a weekly timescale at four sites in the boreal forest of Quebec, Canada. Models for assessing xylem phenology were defined and applied to reconstruct onset, ending and duration of xylogenesis between 1950 and 2010 using thermal thresholds on chronologies of maximum and minimum temperatures.
Key Results
All sites exhibited increasing trends of both annual and May–September temperatures, with the greatest changes observed at the higher latitudes. Phenological events in spring were more affected than those occurring in autumn, with cambial resumptions occurring 0·5–0·8 d decade−1 earlier. The duration of xylogenesis has lengthened significantly since 1950, although the models supplied wide ranges of variations, between 0·07 and 1·5 d decade−1, respectively.
Conclusions
The estimated changes in past cambial phenology demonstrated the marked effects of the recent increase in temperature on the phenological traits of secondary meristems. In the long run, the advancement of cambial activity could modify the short time window for growth of boreal species and dramatically affect the dynamics and productivity of trees in these temperature-limited ecosystems.
Keywords: boreal forest, cell differentiation, Picea mariana, threshold temperature, wood formation, xylogenesis
INTRODUCTION
Hemispheric-scale reconstructions of surface temperature fluctuations over the last millennium have identified the 20th century as uniquely warm (cf. Hughes, 2002). Although an increase in global temperature of between 1·4 and 5·8 °C is expected during the period 1990–2100, the greater increases are predicted to occur at the higher latitudes of the northern hemisphere (IPCC, 2007). For the boreal forest of north-eastern Canada, climatic models predict increases in temperature of up to 3 °C over the next 50 years, with the largest increases occurring in winter and spring, when plant growth resumes (Plummer et al., 2006). In cold ecosystems, the growing season is strictly defined by the harsh winter temperatures (Rossi et al., 2008b, 2011b). Such climate modifications could thus severely affect the growth timings and dynamics of boreal species. As boreal forest represents 27 % of the world's forest cover and contains more than 30 % of all carbon present in the terrestrial biomes (Kasischke, 2000), any change in the productivity of this biotope has obvious ecological and economic relevance.
Interest in the cold forests of high altitude and latitude is linked to various causes such as the short growing season and high sensitivity of the species to climate change (Körner, 2003a). Plants of these ecosystems are assumed to be good indicators of changes in their environment (Pisaric et al., 2003). With the current changes in temperature, possible variations in phenology – the study of the timings of recurring seasonal biological events – have acquired particular importance worldwide. According to Forrest and Miller-Rushing (2010), interest has focused on documenting the recurrent appearance of the first flower blossoms or bud bursts in spring, the dates of animal migration, or timings of the first frost-damaged leaves in autumn. The longest monitoring periods in plants are concentrated in the botanical gardens of temperate Europe and concern the effects of such changes on the growth dynamics of the primary meristems (buds, leaves and flowers). There is no historical documentation on the phenology of the secondary meristem, the cambium, because it is not a macroscopically perceptible phenomenon like leaf development or flower maturation. Cambial activity occurs beneath the bark and, in high-latitude forests, produces a number of xylem cells that increase the stem diameter annually by one tree ring, which integrates the effects of climatic events occurring during the season when the cambium was active (Frankenstein et al., 2005). Tree rings have thus been used as a tool to explore the long-term growth reactions to historical climate variations (Huang et al., 2010). However, to our knowledge, retrospective studies concerning reconstructions of cambium phenology are still lacking due to the very recent identification and application of the standard procedures of analysis (Rossi et al., 2006a, b; Seo et al., 2008; Gričar et al., 2009).
Analyses of the variations in the concentrations of CO2 in the atmosphere showed that biospheric activity increased remarkably as a result of warming surface air (Myneni et al., 1997). This implies that even small changes in global temperature may be reflected by disproportionate responses at the regional level that can markedly influence all biological processes, in particular those concerning growth. Although several endogenous variables can influence the dynamics of xylem formation (Marion et al., 2007; Rossi et al., 2008a; Rathgeber et al., 2011; Anfodillo et al., 2012), temperature remains the main driving factor in cold environments. A local overheating in spring can reactivate cambium, inducing the conversion of starch reserves into sucrose for the activation of cell division and production of secondary xylem (Begum et al., 2007; Gričar et al., 2007). Deslauriers et al. (2008) observed that the higher temperatures occurring in spring 2003 led to earlier onsets of division and differentiation of xylem cells. The onset of xylogenesis influences the number of cells produced by the cambium, which, in turn, influences the ending of cell differentiation (Lupi et al., 2010; Rossi et al., 2012). Several studies in cold environments demonstrated that a certain temperature, in the form of heat sum or thermal threshold, is necessary to enable the cambium to divide (Rossi et al., 2008b; Seo et al., 2008; Swidrak et al., 2011). Rossi et al. (2011b) simulated several warming scenarios to predict changes in xylem phenology. The model predicted longer duration of xylem growth at higher temperatures, with increases of 8–11 d °C−1 because of an earlier onset and later ending of growth. Twenty-five per cent longer durations of xylogenesis were predicted with an increase of 3 °C in mean annual temperature (Rossi et al., 2011b).
Although substantial increases in temperature were observed during the last 100 years, with the mean surface temperature rising by 0·7 °C at global scale and by 0·5–1·5 °C across North America since the late 19th century (Zhang et al., 2000; IPCC, 2007), the rate of warming over the last 50 years (0·13 °C decade−1) is almost double that over the last 100 years (IPCC, 2007). For the northern regions of North America, McKenney et al. (2006) estimated increases attaining 0·26 °C decade−1 in the second half of the 20th century. The question is if and to what extent the modifications in air temperature during this period have affected cambial phenology. The model developed by Rossi et al. (2011b) simulated a potential xylem phenology under a possible and simplified climate warming, represented by a uniform increase in air temperature. However, no information was provided about changes in the timings of cambial growth occurring in the past. Moreover, there is evidence of divergent effects of climate change on seasonal temperatures, with spring having the greater warming (Zhang et al., 2000). Improvements in the precision of the models of cambial growth are thus expected to produce results consistent with the more realistic climatic scenarios.
This study aimed to reconstruct timings of cambium phenology over the last 60 years in Quebec, Canada. This was done by (1) collecting and analysing a dataset of cambium phenology and wood formation in black spruce based on weekly monitoring for 9 years in four permanent sites at different latitudes and altitudes, (2) defining and validating a phenological model of xylem based on the air temperature measured at the sites, and (3) applying the phenological model on the chronologies of air temperature generated for the period 1950–2004 by the ANUSPLIN model (McKenney et al., 2006). The effects of climate change on plants have mainly been demonstrated by changes in the phenology of primary meristems, which have revealed marked increases in length of the growing season (Menzel, 2000; Zhou et al., 2001; Sparks and Menzel, 2002; Badeck et al., 2004). Thus, according to the results provided on primary meristems, the hypothesis that duration of xylogenesis has lengthened since 1950 was tested by the model.
MATERIALS AND METHODS
Study area and xylem sampling
The study was conducted on black spruce (Picea mariana) in the Saguenay-Lac-Saint-Jean area, in the boreal forest of Quebec, Canada. Four sites [Simoncouche (abbreviated as SIM), Bernatchez (BER), Mistassibi (MIS) and Camp Daniel (DAN)] were identified in mature even-aged stands at different altitudes and latitudes to obtain as wide as possible a range in the dynamics of tree growth (Table 1). Details on site characteristics are given by Rossi et al. (2011b).
Table 1.
Location of the four study sites, listed in order of decreasing latitude, and climatic characteristics measured during the period 2002–2010
| Site | Latitude | Longitude | Altitude (m a.s.l.) | Mean temperature |
Absolute annual temperature |
||
|---|---|---|---|---|---|---|---|
| Annual (°C) | May–September (°C) | Maximum (°C) | Minimum (°C) | ||||
| DAN | 50°41′N | 72°11′W | 487 | –0·9 | 11·0 | 34·2 | –47·1 |
| MIS | 49°43′N | 71°56′W | 342 | 1·0 | 12·7 | 35·1 | –42·4 |
| BER | 48°51′N | 70°20′W | 611 | 0·3 | 11·4 | 33·1 | –39·8 |
| SIM | 48°13′N | 71°15′W | 338 | 2·0 | 13·3 | 35·7 | –39·7 |
In each site, tree-ring formation was studied from April to October during 2002–2010 in five (2002–2005) and ten (2006–2010) trees. Wood microcores were collected weekly following a spiral trajectory on the stem from 30 cm below to 30 cm above breast height (1·3 m) using surgical bone sampling needles in 2002–2006 and Trephor in 2007–2010 (Rossi et al., 2006a). Samples usually contained the previous 4–5 tree rings and the developing annual layer with the cambial zone and adjacent phloem. Samplings were always taken at least 5 cm apart to avoid getting resin ducts on adjacent cores.
The microcores were stored in ethanol solution (10 % in water) at 5 °C to avoid tissue deterioration. Microcores were dehydrated with immersions in ethanol and d-limonene and embedded in paraffin (Rossi et al., 2006a). Transverse sections of 6–10 µm thickness were cut from the samples with a rotary microtome, stained with cresyl violet acetate (0·16 % in water) and examined within 10−25 min under visible and polarized light at magnifications of 400−500× to differentiate the developing and mature xylem cells. Occasionally, distorted rows of cells prevented adequate analysis of the sample. In these cases, the sections were gently stressed on the slide with thin needles to better observe all cells of the developing tree ring.
Microscopic observations
In each sample, the radial number of cells in the cambial zone, radial enlargement phase, cell-wall thickening phase and mature cells were counted along three radial rows. In cross-section, cambial cells were characterized by thin cell walls and small radial diameters (Rossi et al., 2006b). The dormant cambium was composed of 3–5 closely spaced cells. At the onset of cambial activity, the cambial zone began to widen rapidly as the number of cells increased, revealing that cell division had started. During cell enlargement, the tracheids were composed of a protoplast still enclosed in the thin primary wall but with radial diameter at least twice that of a cambial cell. Observations under polarized light discriminated between enlarging and cell-wall thickening tracheids (Thibeault-Martel et al., 2008). Because of the arrangement of cellulose microfibrils, the developing secondary walls were birefringent when observed under polarized light. Instead, no glistening was observed in enlargement zones, where the cells were still composed of just primary wall (Abe et al., 1997). Lignification was detected with cresyl violet acetate by a colour change from violet to blue. The colour change over the whole cell wall revealed the end of lignification and the tracheid reaching maturity (Gričar et al., 2005).
The cell number in the three rows was averaged for each tree and used to assess onset and ending of xylogenesis. In spring, when at least one tangential row of cells was observed in the enlargement, xylem formation was considered to have begun. In late summer, when no further cell was observed in wall thickening and lignification, xylem formation was considered complete. The duration of xylogenesis was assessed as the number of days occurring between onset and ending of xylogenesis and was calculated as the average among trees for each studied site and year.
Datasets of air temperature
Two datasets of air temperature were used in this study and consisted of time series (1) measured in the four sites and (2) estimated by a climatic model. Measured and estimated temperatures were used for the definition and application of the phenological model, respectively. For the first dataset, a standard weather station was installed in 2001 in a forest gap at each site. Air temperature data were collected at 3 m above ground level every 15 min and recorded as averages every hour by means of CR10X dataloggers (Campbell Scientific Corporation, Edmonton, Canada). Maximum and minimum values were later calculated from the 24 measurements per day. The second dataset consisted of air temperatures generated at a daily resolution for the period 1950–2004 by the ANUSPLIN model of the Canadian Forestry Service. This model used a multivariate non-parametric surface and point fitting approach to estimate the time series of maximum and minimum temperature corresponding to the location of each site according to the algorithm described by McKenney et al. (2006). To verify the consistency of the modelled time series, linear regressions were performed between the measured and estimated temperatures for the overlapping years (2002–2004).
Definition and validation of the phenological model
The model consisted in applying thermal thresholds for estimating xylem phenology using logistic regressions to calculate the probability of xylem growth being active at a given measured daily temperature. According to Rossi et al. (2011b), binary responses were coded as non-active (value 0) or active (value 1) growth, and temperature thresholds were calculated when the probability of xylem growth being active was 0·5. For each site and year, the model was fitted with minimum and maximum temperature series and results from each site were compared by analysis of variance (ANOVA) and Tukey's test. None of the 72 estimated functions was excluded because of lack of fit. Model validation was performed according to Legendre and Legendre (1998) by comparing the observations with the predicted values calculated using the estimated temperatures. A classification table was produced in the form of a contingency table, which for each day compared the observed active or non-active xylem growth with that predicted by the model.
Application of the phenological model
A quadratic logistic regression was applied on the time series of daily temperatures generated by the ANUSPLIN model, with binary responses coded as 0–1 if temperatures were lower or higher than the threshold, respectively. The two solutions of the quadratic regression corresponded to the days of the year when the probability of temperature being higher than the threshold was 0·5, and included the period of xylem growth (Rossi et al., 2011b). The phenological model was iteratively applied to the temperature series of each site to estimate changes in the timings of xylem growth. The resulting time series, which consisted of the dates of onset and ending of xylogenesis from 1950 to 2010, were tested for the presence of autocorrelation until the fourth order (McKenney et al., 2006). As no autocorrelation was observed for the onset of xylogenesis and the errors were only occasionally serially correlated at the second and third order for ending and duration of xylogenesis, the long-term trends were analysed by analysis of covariance (ANCOVA).
RESULTS
Observed and modelled temperatures
The region has a typical boreal climate with cold winters and cool summers (Table 1). The mean annual temperature in the four study sites varied between –0·9 and 2·0 °C while May–September temperature was 11·0–13·3 °C. The sites are characterized by long winters with temperatures close to or below zero, with the coldest generally being measured in January and reaching –47·1 °C in DAN in 2009. The summers are short with absolute temperatures exceeding 30 °C in all sites (Table 1). The warmest maximum temperatures were observed in 2002 in all sites. The temperature patterns were synchronous across the four study sites, with the coldest being DAN and BER, those located at the highest latitude and altitude, respectively. SIM was the warmest site both for annual and May–September temperatures (Supplementary data Fig. S1). The hottest year was 2010, which globally showed the highest annual temperatures, although high May–September maximum temperatures were also observed during 2005. The lowest maximum and minimum temperatures were detected in 2004.
The regressions indicated correlations between measured and modelled temperatures with R2 varying between 0·56 and 0·94 (Supplementary data Table S1). The stronger relationships were found for the annual maximum temperature, which on average showed an R2 of 0·93. SIM exhibited the lowest R2 while DAN and MIS were the sites with the higher R2 for both annual and May–September temperature. Overall, statistics confirmed that modelled data could represent the temperatures occurring in the four study sites during 1950–2001.
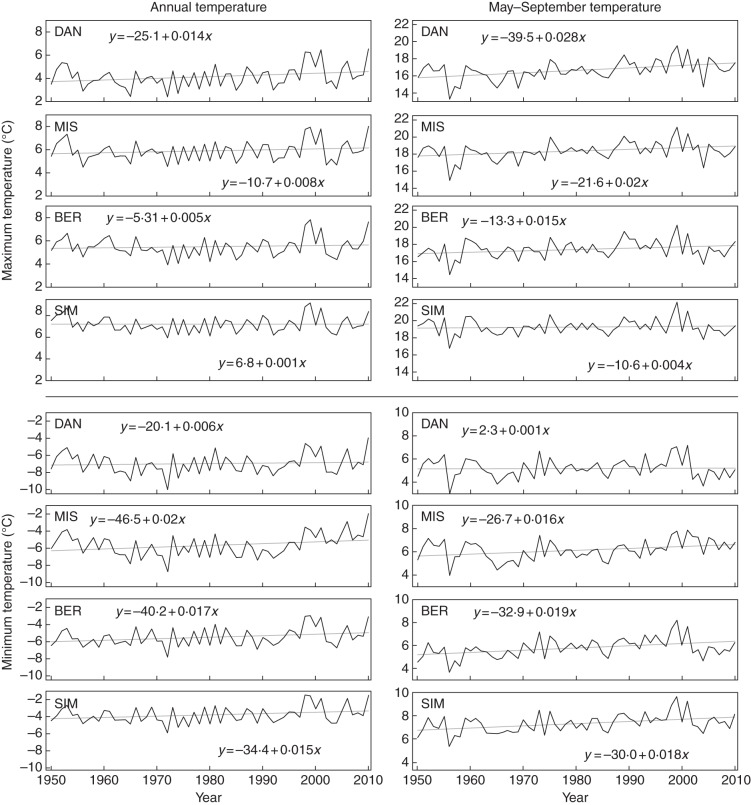
The positive slopes of the regressions performed on the temperature series for the period 1950–2010 indicated an increasing trend of both annual and May–September temperatures, although all models showed P > 0·05 (Fig. 1). The highest slopes were observed for maximum temperatures of May–September with increases of 0·04–0·28 °C decade−1. Overall, lower slopes were estimated for annual temperature than for May–September temperature except for the minimum temperature in DAN and MIS. A clear pattern of change in the slopes with latitude was noticeable, with the greater increases in maximum temperature being observed at the higher latitudes, although this pattern was less obvious for minimum temperature.
Fig. 1.
Temperatures during 1950–2010 at the four sampling sites (note different scales). The straight lines correspond to linear regression analyses.
The deviation from the 60-year average was calculated for the temperature series (Supplementary data Fig. S2). The 1960s were characterized by below-average values of both minimum and maximum May–September temperatures. However, a similar pattern was not detected for the annual temperature. From 1970 to 1998, values were located around the average. After those years, both annual and May–September temperatures were clearly above the historical average, with the greater deviations for the maximum temperature of May–September.
Model definition and validation
At the four sites, xylem growth lasted between 80 and 133 d, with SIM having the longest duration (Fig. 2). Overall, the onset of xylem growth occurred from mid-May to mid-June [days of the year (DOY) 139–166], covering a range of approx. 1 month. Later onsets of xylogenesis were detected in 2002 and 2009 and at the highest altitude and latitude, in BER and DAN, respectively. The ending of xylem growth differed by more than 1 month between the end of August in BER, MIS and DAN, and the beginning of October in SIM.
Fig. 2.
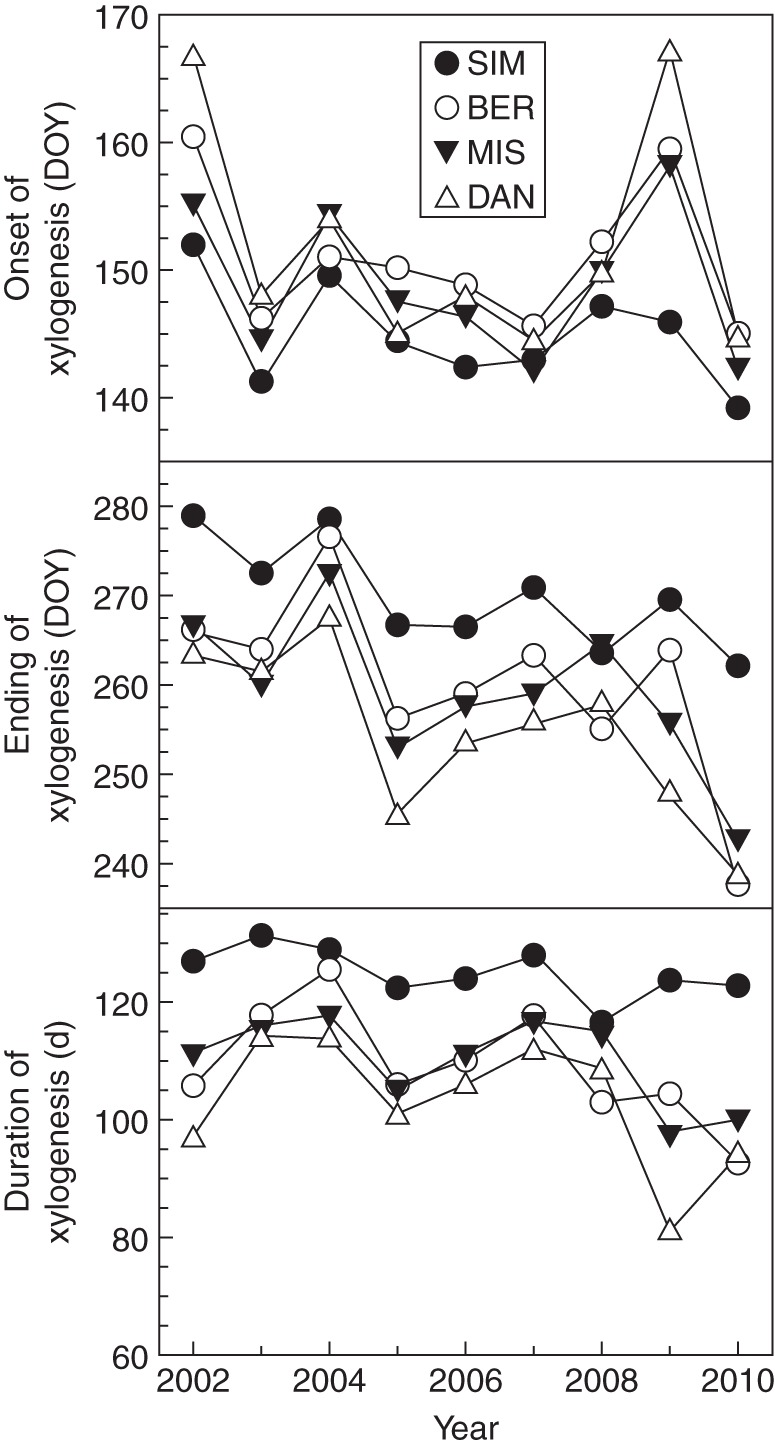
Cambial phenology in black spruce during 2002–2010 in the boreal forest of Quebec, Canada. DOY = day of year (Julian day).
On average, the logistic regressions assessed the thresholds at 4·4 and 15·5 °C for minimum and maximum temperature, respectively (Table 2). For a temperature above the calculated thresholds, xylem growth was more likely to be active than non-active. Although MIS had slightly higher values compared with the other sites, no significant difference was detected by ANOVA for either minimum or maximum temperature (P > 0·05, Table 2).
Table 2.
ANOVA comparisons performed among the threshold temperatures occurring when the probability of xylem growth in black spruce being active was 0·5; thresholds were estimated on a dataset of cambial phenology collected during 2002–2010 in the boreal forest of Quebec, Canada. Values are means ± s.d.
| Threshold temperature (°C) | SIM | BER | MIS | DAN | F-value | P |
|---|---|---|---|---|---|---|
| Minimum | 4·2 ± 1·0 | 4·0 ± 1·9 | 5·4 ± 1·3 | 4·0 ± 1·3 | 2·68 | 0·06 |
| Maximum | 15·1 ± 0·7 | 15·2 ± 1·4 | 16·3 ± 1·4 | 15·4 ± 1·4 | 1·64 | 0·19 |
The results generated by the logistic regressions were verified by forecasting the presence or absence of xylem growth in the study sites and comparing results by means of a contingency table (Table 3). Overall, observations showed that xylem growth was active during about one-third of the year and not active during 72·3 % of the year. For minimum and maximum temperatures, 94·9 and 95·0 % of the predictions were confirmed by observations, respectively, confirming that the model with both temperatures produced reliable estimations of the thresholds and suitably predicted timings of the phenological phases of xylem. On average, not active and active xylem growth was correctly predicted for 69·0 and 25·9 % of days, respectively, while the predictions were not confirmed for only 1·5–3·1 % of days.
Table 3.
Proportions of observed and predicted days with non-active (first and second row) or active (third and fourth row) xylem growth in black spruce
| Observed xylogenesis | Predicted xylogenesis | Model using minimum temperatures (%) | Model using maximum temperatures (%) |
|---|---|---|---|
| No | No | 69·2 | 68·8 |
| No | Yes | 3·1 | 3·5 |
| Yes | No | 2·0 | 1·5 |
| Yes | Yes | 25·7 | 26·2 |
Predictions were obtained using the minimum and maximum temperatures estimated during 2002–2010 in the boreal forest of Quebec, Canada. The first and fourth rows correspond to the days correctly predicted by the model.
Model application
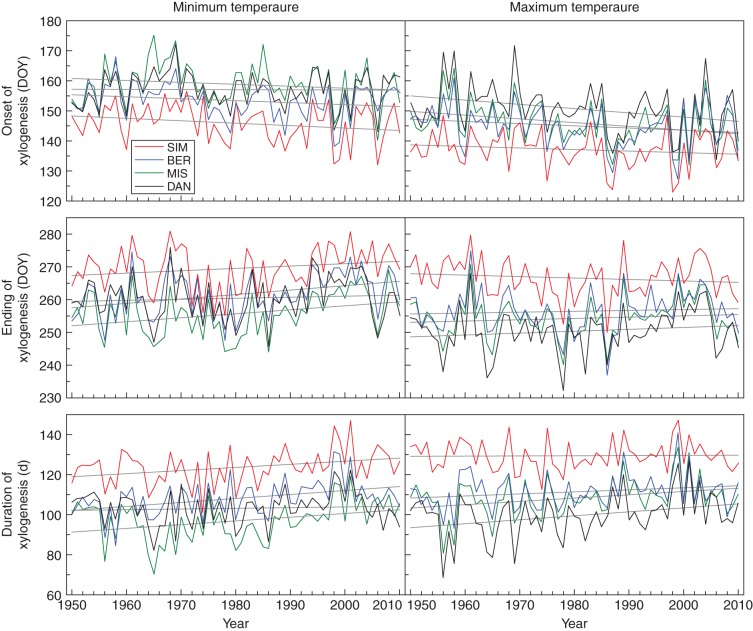
The model generated using the threshold temperatures was used for estimating chronologies of cambium phenology (onset, ending and duration of xylogenesis) for the period 1950–2010 (Fig. 3). Long-term trends of all series were tested using ANCOVA, which calculated models with R2 ranging between 0·36 and 0·60 and significance P < 0·0001 (Table 4). Predictions of the models using both minimum and maximum temperatures showed significant negative trends in all sites (P < 0·05), revealing an earlier onset of xylogenesis that was estimated by the slope of the regression at 0·5–0·8 d decade−1. Significant slopes for the ending of xylogenesis were detected only with the model using minimum temperatures, which indicated a delay of 0·7 d decade−1 (Table 4). Longer durations of xylogenesis were predicted for the period 1950–2010, with the higher (1·5 d decade−1) and lower (0·07 d decade−1) increases estimated by the model using minimum and maximum temperatures, respectively. Significant differences among sites were found for all phenological models (P < 0·0001, Table 4), with SIM having earlier onsets, later endings and longer durations of xylogenesis over all chronologies (Fig. 3). Differences in cambial phenology among BER, MIS and DAN were less marked. No significant year × site interaction was observed by ANCOVA (P > 0·05, Table 4).
Fig. 3.
Estimation of cambial phenology for the period 1950–2010 (onset, ending and duration of xylogenesis) using daily minimum and maximum temperatures. Lines correspond to regression analyses performed by ANCOVA. DOY = day of year (Julian day).
Table 4.
ANCOVA models comparing the chronologies of cambial phenology (onset, ending and duration of xylogenesis) of black spruce predicted for 1950–2010 using thresholds of minimum and maximum temperature
| Xylogenesis | Temperature | Source | Regressors |
Statistics |
Coefficients |
|||
|---|---|---|---|---|---|---|---|---|
| Type I SS | F-value | F-value | R2 | Intercept | Slope (101) | |||
| Onset | Minimum | Year | 219·9 | 5·6* | 22·9*** | 0·40 | 307·9 | –0·8 |
| Site | 6000·0 | 51·1*** | ||||||
| Year × site | 64·2 | 0·5 | ||||||
| Maximum | Year | 713·5 | 14·3** | 19·1*** | 0·36 | 237·5 | –0·5 | |
| Site | 5852·0 | 39·1*** | ||||||
| Year × site | 100·8 | 0·6 | ||||||
| Ending | Minimum | Year | 627·0 | 15·3** | 23·6*** | 0·41 | 125·3 | 0·7 |
| Site | 60303·2 | 49·0*** | ||||||
| Year × site | 125·8 | 1·0 | ||||||
| Maximum | Year | 36·8 | 0·9 | 32·3*** | 0·48 | 351·9 | –0·4 | |
| Site | 8754·5 | 74·1*** | ||||||
| Year × site | 116·4 | 0·9 | ||||||
| Duration | Minimum | Year | 1589·6 | 21·4*** | 49·3*** | 0·59 | –182·6 | 1·5 |
| Site | 23747·7 | 106·7*** | ||||||
| Year × site | 308·2 | 1·3 | ||||||
| Maximum | Year | 1074·9 | 13·0** | 52·5*** | 0·60 | 114·4 | 0·07 | |
| Site | 28846·8 | 116·6*** | ||||||
| Year × site | 409·3 | 1·6 | ||||||
Asterisks indicate statistically significant differences at *P < 0·05, **P < 0·001 and ***P < 0·0001.
DISCUSSION
Compared with the primary meristems such as leaves and buds, analyses on the phenology of the secondary meristem have only been emerging in the last decade. At the time of writing, the chronologies of cambial phenology deriving from direct observations are spatially and temporally fragmented (Rossi et al., 2008b; Moser et al., 2010; Rathgeber et al., 2011; Swidrak et al., 2011), hampering understanding of long-term responses of the cambium to environmental changes and any interpretation of the trends over time (Sparks and Menzel, 2002). This study thus aimed to reconstruct timings of cambium phenology of black spruce over the last 60 years to test the hypothesis that duration of xylogenesis has lengthened since 1950. In the boreal forest, wood formation is restricted to 3–4 months by thermal limits that characterize the change between favourable and unfavourable periods for growth. In spring, cambial reactivation occurs from mid-May to mid-June, when the temperatures allow snow to melt and soil to warm up (Rossi et al., 2011a). Mature xylem is observed in September, when all cells have concluded differentiation, which corresponds to night-time temperatures of 4–5 °C, and maximum temperatures reaching 15–16 °C. For the first time, long-term chronologies of cambial phenology are provided, which allows investigation of the impact of past increases in temperature on wood formation. The dynamics and periods of xylem growth and their eventual changes over time are of particular interest for the global carbon budget as they define the period of main biomass accumulation in wood, during which trees act as an important sink of the carbon sequestrated from the atmosphere. An extended period of tree growth associated with higher temperature could allow cambial cells to divide more vigorously and longer, thus producing wider tree rings and greater amounts of wood.
Trends of temperature and phenology
The chronologies generated by the ANUSPLIN model (McKenney et al., 2006) for the four study sites exhibited increasing trends of both annual and May–September temperatures between 1950 and 2010. In some cases, the modelled climatic data were only partially correlated with measurements, and this may be explained by the remote location of the sites and by the scarcity of nearby weather stations used for the climatic modelling. The greatest changes were observed for maximum temperature at the higher latitudes, attaining increases of up to 0·28 °C decade−1 in DAN, the most northern site. For a similar period (1950–1998), Zhang et al. (2000) estimated that the higher increases in temperature for this region occurred in summer, with values ranging between 1·0 and 1·5 °C. Plummer et al. (2006) showed different trends for Quebec, with temperature rising by up to 6 °C in all seasons except in early spring, before the observed onset of xylogenesis. Our temperature chronologies exhibited a higher warming than the estimations of Zhang et al. (2000) and were more conservative than those of Plummer et al. (2006).
Changes in phenology of the primary meristems represents one of the best-documented effects of climate change on plants, with results converging toward increases in length of the growing season (Zhou et al., 2001; Sparks and Menzel, 2002; Badeck et al., 2004). In Canada, Beaubien and Freeland (2000) reported that the first flowering date of aspen poplar showed a marked trend of earlier flowering with an advance of 26 d over the period 1900–1997. For the northern hemisphere, Schwartz et al. (2006) estimated that during 1955–2002 the dates of first leaf and first bloom have been 1·2 and 1·0 d decade−1 earlier. Greater advances of 2·1 d decade−1 were calculated between 1951 and 1996 for leaf unfolding in Europe (Menzel, 2000). Despite similar lengths of the study periods, the results vary widely, which could be explained by the complex origin (observations or estimations) and nature (leaf or flower buds) of the datasets and species over the broad spatial scales of analysis. Moreover, a high heterogeneity in change of temperature has been observed across North America, with lower effects of warming occurring in the eastern part of the continent, where this work was carried out (Schwartz et al., 2006). Overall, the hypothesis that duration of xylogenesis has lengthened over the last 60 years has been confirmed, although the estimated increasing trends of cambial phenology exhibited lower slopes than those observed in the primary meristems.
Does phenological cascade prevent estimating the end of xylogenesis?
In Europe, the beginning of the growing season has advanced by 2·7 d decade−1 in the last 30 years, while its ending showed smaller annual variations and has occurred just 1 d decade−1 later (Chmielewski and Rötzer, 2001). Sparks and Menzel (2002) definitely confirmed that earlier events were more variable and changed faster than later events. This was consistent with our results, which showed more marked changes in the spring onset than in late-summer ending of xylogenesis, and contrasting slopes and significances were observed over the study period from the trends of ending of xylogenesis. During development, the cambial derivatives (i.e. the cells produced by cambial division) alter both morphologically and physiologically, progressively assuming definite features. In other words, cells differentiate into the specific elements of the stem tissues, represented by the phases of enlargement, wall thickening and lignification. Investigations into xylem phenology and climate–growth relationships have focused mainly on the onset of the growth process, i.e. onset of xylem production or differentiation, while the end of growth still remains partly or completely unexplored (Gričar et al., 2007; Rossi et al., 2007; Seo et al., 2008; Turcotte et al., 2009). According to our findings, this could essentially be due to a greater number of significant responses being obtained between onset of growth and climate rather than a mere lack of interest in the final phases of the growth process (Hänninen and Tanino, 2011).
In cold environments, cell production is closely related to xylem phenology (Lupi et al., 2010; Rossi et al., 2012). The date of onset of xylogenesis affects the number of cells produced by the cambium, which, in turn, influences the ending of cell differentiation. As a result, earlier cambial resumptions lengthen the period available for cell division in the secondary meristem, increasing the growth potential during the year (Gričar et al., 2005; Deslauriers et al., 2008). In conifers, wider tree rings (i.e. with higher number of cells) require a longer period for differentiating and maturing the tracheids, which delays the ending of wood formation. Thus, any environmental factor affecting the resumption of growth in spring could indirectly influence the production and temporal dynamics of cell differentiation by affecting all successive phenological phases of xylem (Rossi et al., 2006b). The hypothesis of an indirect effect of environment on the chain of phenological events in the xylem provides valuable cues for identifying the relative importance of the factors affecting timings and dynamics of xylem growth, and makes the relationships between the temperatures occurring in late summer and the date of ending of xylem growth more complex.
Model and thermal predictors of xylogenesis
Several methods have been applied to investigate plant growth and its changes over time. Definitions of the growing season differ according to plant species, and are calculated in different ways, either directly (bud or cambial phenology) or indirectly (thermal sums, days with air and soil temperatures above certain thresholds, freezing days) (Nizinski and Saugier, 1988; Körner and Paulsen, 2004; Schwartz et al., 2006; Seo et al., 2008). In our reconstruction of past cambial phenology, the applied temperature thresholds were not defined a priori. They were instead statistically assessed on a wide dataset of observations collected weekly from four permanent plots over 9 years, by defining a binary response of presence or absence of growth, and modelling the logistic response probability according to a vector of the explanatory variable, either minimum or maximum temperature. The resulting temperature thresholds have the advantage of being objectively assessed and statistically validated, and are as close as possible to the biological limits of the growth process in the stem. However, the definition of the model assumed a linear response of cambial phenology to temperature, which is expected to occur only for narrow thermal ranges.
In this study, the minimum and maximum temperature thresholds allowing xylogenesis ranged between 4 and 5 °C and 15 and 16 °C, respectively, which confirm previous findings from other conifer species of cold climates (Rossi et al., 2008b; Swidrak et al., 2011) but contrast with the hypothesis of a cumulative effect of temperatures for cambial resumption (i.e. heat sums, Seo et al., 2008; Swidrak et al., 2011). Cambium is a sink for carbohydrates, and its activity requires a continuous supply of energy in the form of sucrose, which, for the first cells to be formed, is extracted from the storage tissues or produced by photosynthesis (Oribe et al., 2003; Deslauriers et al., 2009). During cell maturation, trees assign a large amount of carbon obtained from photosynthesis to the deposition of cellulose microfibrils in order to provide the developing cells with secondary walls. The thresholds estimated in this study could represent the critical temperatures limiting the demand for photo-assimilates by the metabolic processes involved in cell growth. Moreover, as xylogenesis is the most important net accumulation of biomass in forest ecosystems, knowledge about the climatic factors on the verges of the growing season is crucial to determine the time window during which the carbon sequestrated by the atmosphere is permanently stocked in trees.
The models using maximum and minimum temperature produced similar results in terms of onset of xylogenesis, but calculated different endings, and hence different durations of xylogenesis for the last 60 years (Table 4). Both models provided equally reliable estimations of xylem phenology, which prevented a definitive choice of the most suitable model. Unlike photosynthesis, which is able to maintain high assimilation rates even at temperatures below 5 °C, xylem formation necessitates large amounts of available sucrose to be allocated in the growing tissues to complete growth, which is a temperature-limited process (Körner, 2003a; Deslauriers et al., 2009). Cell doubling time remains quite constant at temperatures of 10−25 °C, but triples when temperatures fall from 10 to 5 °C, and cell division stops at 1−2 °C (Körner, 2003b). Moreover, comparing the daily growth responses of conifers with maximum and minimum temperatures, better results were observed with the latter (Deslauriers and Morin, 2005; Wei et al., 2007; Rossi et al., 2008b). However, the effects of the two variables have not yet been experimentally and definitively disentangled, and whether maximum or minimum temperatures mostly control the length of the growing period remains unresolved.
Conclusions
This study used the phenological model developed by Rossi et al. (2011b) and weekly observations performed for 9 years in four permanent sites in Quebec, Canada, to reconstruct the timings of cambium phenology over the last 60 years. All sites exhibited increasing trends of both annual and May–September temperatures, with the greatest changes occurring for maximum temperature at the higher latitudes. Accordingly, earlier cambial resumptions by 0·5–0·8 d decade−1 were estimated, while significant delays for the ending of xylogenesis were calculated only with the model using minimum temperatures. Phenological events in spring were confirmed to be more variable and changing faster than those occurring in autumn. Results confirmed the initial hypothesis that duration of xylogenesis has lengthened since 1950. However, contrasting extents were observed, ranging between 0·07 and 1·5 d decade−1, calculated with the model based on maximum and minimum temperatures, respectively. To our knowledge, this is the first time that past cambial phenology has been modelled and reconstructed.
Consistent with the findings reported for leaf and flower buds, changes in cambial phenology showed increasing trends in length of the growing season, demonstrating the effects of the recent global warming on secondary meristems of trees. If the observed trend is maintained unaltered in the long term, the demonstrated advancement of cambial activity could dramatically modify the short time window for growth of boreal species and markedly affect cell production of the secondary meristem. Our findings showed that long-term increases in temperature can substantially extend wood formation and, consequently, the dynamics and productivity of cold ecosystems, by removing the thermal constraints to the activity of carbon sinks in trees. However, evidence of these trends in forest ecosystem productivity needs to be confirmed by further specific investigations.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was funded by the Consortium de Recherche sur la Forêt Boréale Commerciale and Fonds de Recherche sur la Nature et les Technologies du Québec. We thank B. Dufour, G. Dumont-Frenette, F. Gionest, C. Lupi, S. Pedneault, P.-Y. Plourde, G. Savard, C. Soucy and M. Thibeault-Martel for technical support. Special thanks are extended to D. McKenney, K. Lawrence and P. Papadopol for sharing their dataset with the temperature chronologies, to J. Pedlar for his recommendations on data analysis, and to A. Garside for checking the English text.
LITERATURE CITED
- Abe H, Funada R, Ohtani J, Fukazawa K. Changes in the arrangement of cellulose microfibrils associated with the cessation of cell expansion in tracheids. Trees. 1997;11:328–332. [Google Scholar]
- Anfodillo T, Deslauriers A, Menardi R, Tedoldi L, Petit G, Rossi S. Widening of xylem conduits in a conifer tree depends on the longer time of cell expansion downwards along the stem. Journal of Experimental Botany. 2012;63:837–845. doi: 10.1093/jxb/err309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badeck FW, Bondeau A, Böttcher K, et al. Responses of spring phenology to climate change. New Phytologist. 2004;162:295–309. [Google Scholar]
- Beaubien EG, Freeland HJ. Spring phenology trends in Alberta, Canada: links to ocean temperature. International Journal of Biometeorology. 2000;44:53–59. doi: 10.1007/s004840000050. [DOI] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii×P. grandidentata) Annals of Botany. 2007;100:439–447. doi: 10.1093/aob/mcm130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski F-M, Rötzer T. Response of tree phenology to climate change across Europe. Agricultural and Forest Meteorology. 2001;108:101–112. [Google Scholar]
- Deslauriers A, Morin H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees. 2005;19:402–408. [Google Scholar]
- Deslauriers A, Rossi S, Anfodillo T, Saracino A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiology. 2008;28:863–871. doi: 10.1093/treephys/28.6.863. [DOI] [PubMed] [Google Scholar]
- Deslauriers A, Giovannelli A, Rossi S, Castro G, Fragnelli G, Traversi L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiology. 2009;29:1223–1235. doi: 10.1093/treephys/tpp061. [DOI] [PubMed] [Google Scholar]
- Forrest J, Miller-Rushing AJ. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philosophical Transactions of The Royal Society. 2010;365:3101–3112. doi: 10.1098/rstb.2010.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenstein C, Eckstein D, Schmitt U. The onset of cambium activity – a matter of agreement? Dendrochronologia. 2005;23:57–62. [Google Scholar]
- Gričar J, Čufar K, Oven P, Schmitt U. Differentiation of terminal latewood tracheids in silver fir trees during autumn. Annals of Botany. 2005;95:959–965. doi: 10.1093/aob/mci112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Oven P. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Science and Technology. 2007;41:463–475. [Google Scholar]
- Gričar J, Krže L, Čufar K. Number of cells in xylem, phloem and dormant cambium in silver fir (Abies alba), in trees of different vitality. IAWA Journal. 2009;30:121–133. [Google Scholar]
- Hänninen H, Tanino K. Tree seasonality in a warming climate. Trends in Plant Science. 2011;16:412–416. doi: 10.1016/j.tplants.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Huang J, Tardif JC, Bergeron Y, Denneler B, Berninger F, Girardins MP. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Global Change Biology. 2010;16:711–731. [Google Scholar]
- Hughes MK. Dendrochronology in climatology – the state of the art. Dendrochronologia. 2002;20:95–116. [Google Scholar]
- IPCC. Climate change 2007: synthesis report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. Geneva: IPCC; 2007. [Google Scholar]
- Kasischke ES. Boreal ecosystems in the global carbon cycle. In: Kasischke ES, Stocks BJ, editors. Fire, climate change, and carbon cycling in the boreal forest. New York: Springer; 2000. pp. 19–30. [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. Berlin: Springer; 2003a. [Google Scholar]
- Körner C. Carbon limitation in trees. Journal of Ecology. 2003b;91:4–17. [Google Scholar]
- Körner C, Paulsen J. A world-wide study of high altitude treeline temperatures. Journal of Biogeography. 2004;31:713–732. [Google Scholar]
- Legendre P, Legendre L. Numerical ecology. Amsterdam: Elsevier; 1998. [Google Scholar]
- Lupi C, Morin H, Deslauriers A, Rossi S. Xylem phenology and wood production: resolving the chicken-or-egg dilemma. Plant, Cell and Environment. 2010;33:1721–1730. doi: 10.1111/j.1365-3040.2010.02176.x. [DOI] [PubMed] [Google Scholar]
- Marion L, Gričar J, Oven P. Wood formation in urban Norway maple trees studied by the micro-coring method. Dendrochronologia. 2007;25:97–102. [Google Scholar]
- McKenney DW, Pedlar JH, Papadopol P, Hutchinson MF. The development of 1901–2000 historical monthly climate models for Canada and the United States. Agricultural and Forest Meteorology. 2006;138:69–81. [Google Scholar]
- Menzel A. Trends in phenological phases in Europe between 1951 and 1996. International Journal of Biometeorology. 2000;44:76–81. doi: 10.1007/s004840000054. [DOI] [PubMed] [Google Scholar]
- Moser L, Fonti P, Buentgen U, et al. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiology. 2010;30:225–233. doi: 10.1093/treephys/tpp108. [DOI] [PubMed] [Google Scholar]
- Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature. 1997;386:698–702. [Google Scholar]
- Nizinski JJ, Saugier B. A model of leaf budding and development for a mature Quercus forest. Journal of Applied Ecology. 1988;25:643–652. [Google Scholar]
- Oribe Y, Funada R, Kubo T. Relationships between cambial activity, cell differentiation and the localisation of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees. 2003;17:185–192. [Google Scholar]
- Pisaric MFJ, Holt C, Szeicz J, Karst T, Smol JP. Holocene treeline dynamics in the mountains of northeastern British Columbia, Canada, inferred from fossil pollen and stomata. The Holocene. 2003;13:161–173. [Google Scholar]
- Plummer DA, Caya D, Frigon A, et al. Climate and climate change over North America as simulated by the Canadian RCM. Journal of Climate. 2006;19:3112–3132. [Google Scholar]
- Rathgeber CBK, Rossi S, Bontemps J-D. Tree size influences cambial activity in a mature silver fir plantation. Annals of Botany. 2011;108:429–438. doi: 10.1093/aob/mcr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. Trephor: a new tool for sampling microcores from tree stems. IAWA Journal. 2006a;27:89–97. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA Journal. 2006b;27:383–394. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carraro V. Evidence of threshold temperatures for xylogenesis in conifers at high altitude. Oecologia. 2007;152:1–12. doi: 10.1007/s00442-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carrer M. Age-dependent xylogenesis in timberline conifers. New Phytologist. 2008a;177:199–208. doi: 10.1111/j.1469-8137.2007.02235.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Gričar J, et al. Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography. 2008b;17:696–707. [Google Scholar]
- Rossi S, Morin H, Deslauriers A. Multi-scale influence of snowmelt on xylogenesis of black spruce. Arctic, Antarctic, and Alpine Research. 2011a;43:457–464. [Google Scholar]
- Rossi S, Morin H, Deslauriers A, Plourde P-Y. Predicting xylem phenology in black spruce under climate warming. Global Change Biology. 2011b;17:614–625. [Google Scholar]
- Rossi S, Morin H, Deslauriers A. Causes and correlations in cambium phenology: towards an integrated framework of xylogenesis. Journal of Experimental Botany. 2012;63:2117–2126. doi: 10.1093/jxb/err423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Ahas R, Aasa A. Onset of spring starting earlier across the Northern Hemisphere. Global Change Biology. 2006;12:343–351. [Google Scholar]
- Seo J-W, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U. Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiology. 2008;28:105–112. doi: 10.1093/treephys/28.1.105. [DOI] [PubMed] [Google Scholar]
- Sparks TH, Menzel A. Observed changes in seasons: an overview. International Journal of Climatology. 2002;22:1715–1725. [Google Scholar]
- Swidrak I, Gruber A, Kofler W, Oberhuber W. Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiology. 2011;31:483–493. doi: 10.1093/treephys/tpr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeault-Martel M, Krause C, Morin H, Rossi S. Cambial activity and intra-annual xylem formation in roots and stems of Abies balsamea and Picea mariana. Annals of Botany. 2008;102:667–674. doi: 10.1093/aob/mcn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte A, Morin H, Krause C, Deslauriers A, Thibeault-Martel M. The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agricultural and Forest Meteorology. 2009;149:1403–1409. [Google Scholar]
- Wei X, Yanhui W, Pengtao Y, Hailong L, Zhongjie S, Wei G. Growth in stem diameter of Larix principis-rupprechtii and its response to meteorological factors in the south of Liupan Mountain, China. Acta Ecologica Sinica. 2007;27:432–441. [Google Scholar]
- Zhang X, Vincent L, Hogg WD, Niitsoo A. Temperature and precipitation trends in Canada during the 20th century. Atmosphere Ocean. 2000;38:395–429. [Google Scholar]
- Zhou L, Tucker CJ, Kaufmann RK, Slayback D, Shabanov NV, Myneni RB. Variations in northern vegetation activity inferred from satellite data of vegetation index during 1981–1999. Journal of Geophysical Research. 2001;106:20069–20083. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.