Abstract
Background and Aims
Gene determination of flowering is the result of complex interactions involving both promoters and inhibitors. In this study, the expression of flowering-related genes at the meristem level in alternate-bearing citrus trees is analysed, together with the interplay between buds and leaves in the determination of flowering.
Methods
First defruiting experiments were performed to manipulate blossoming intensity in ‘Moncada’ mandarin, Citrus clementina. Further defoliation was performed to elucidate the role leaves play in the flowering process. In both cases, the activity of flowering-related genes was investigated at the flower induction (November) and differentiation (February) stages.
Key Results
Study of the expression pattern of flowering-genes in buds from on (fully loaded) and off (without fruits) trees revealed that homologues of FLOWERING LOCUS T (CiFT), TWIN SISTER OF FT (TSF), APETALA1 (CsAP1) and LEAFY (CsLFY) were negatively affected by fruit load. CiFT and TSF activities showed a marked increase in buds from off trees through the study period (ten-fold in November). By contrast, expression of the homologues of the flowering inhibitors of TERMINAL FLOWER 1 (CsTFL), TERMINAL FLOWER 2 (TFL2) and FLOWERING LOCUS C (FLC) was generally lower in off trees. Regarding floral identity genes, the increase in CsAP1 expression in off trees was much greater in buds than in leaves, and significant variations in CsLFY expression (approx. 20 %) were found only in February. Defoliation experiments further revealed that the absence of leaves completely abolished blossoming and severely affected the expression of most of the flowering-related genes, particularly decreasing the activity of floral promoters and of CsAP1 at the induction stage.
Conclusions
These results suggest that the presence of fruit affects flowering by greatly altering gene-expression not only at the leaf but also at the meristem level. Although leaves are required for flowering to occur, their absence strongly affects the activity of floral promoters and identity genes.
Keywords: Alternate bearing, AP1, citrus, flowering, FT, FD, FLC, LFY, SOC1, TFL1, TFL2, TSF
INTRODUCTION
Under Mediterranean climatic conditions, Citrus floral evocation takes place in late autumn whereas differentiation and morphogenesis occur later, around early spring (Sherman and Beckman, 2003). Citrus species have a tropical–subtropical evergreen nature and, unlike deciduous fruit trees, their buds do not exhibit true dormancy, remaining in rest until initiation. The resting bud itself consists of an undifferentiated apical meristem, and initiation begins with cell division and differentiation of the primordium surrounding the meristematic dome (see revisions by Schneider, 1968; Lord and Eckard, 1985; Davenport, 1990). Previous research has demonstrated that fruit load acts as a strong inhibitor of flowering in many fruit-tree species, including citrus. This inhibition is determined by several factors such as environmental conditions, cultivar, number of fruits per tree and harvesting date (Martínez-Fuentes et al., 2010). Nutritional and hormonal factors also seem to be involved in the metabolic and biochemical pathways leading to fruit load-mediated inhibition of flowering (Koshita et al., 1999). Thus, seasonal changes in carbohydrate composition of buds or the mineral status of the tree are physiologically connected to flower differentiation (Vemmos, 1999; Yahata et al., 2004). At the molecular level, gene determination of flowering appears to be the result of complex interactions that involve both promoter and inhibitor genes in the context of an integrative system. Thus, specific homologues of FLOWERING LOCUS T (FT) (CiFT, Endo et al., 2005), TERMINAL FLOWER 1 (CsTFL, Pillitteri et al., 2004a), LEAFY (LFY) and APETALA (AP1) (CsLFY and CsAP1, Pillitteri et al., 2004b), and SUPRESSOR OF OVEREXPRESSION OF CONSTANS (SOC1) (CsSL1, Tan and Swain, 2007) have been previously isolated and characterized in Citrus. Moreover, a genetic framework for flowering-time pathways in Citrus has been proposed, and overviews of the relationships amongst the main genes involved have been given (Dornelas et al., 2007a, b). Additional effort has been recently made by our group to elucidate the molecular basis of floral regulation by fruit load (Muñoz-Fambuena et al., 2011), and we proposed that the promoter gene CiFT plays a pivotal role in the inhibition of flowering as a consequence of crop load through repressive mechanisms. Other promoter genes, such as SOC1, appear to exhibit minor responses to the presence of fruit. Hormonal compounds such as gibberellins have also been demonstrated to participate in the regulation processes (Muñoz-Fambuena et al., 2012). On the other hand, expression in leaves of those homologues of AP1 and LFY is strongly modulated by fruit load, although their activity appears to be more closely associated with floral identity. By contrast, inhibitors such as TFL1 and FLOWERING LOCUS C (FLC) do not appear to be correlated with fruit load.
However, although most of the studies have focused on the changes of gene activity at the leaf level, some recent studies indicate that the meristem might play a central role in the molecular determination of flowering. As large numbers of flowering-related gene transcripts have been described at this location, some authors have remarked on the importance of apices in controlling flowering time (Jang et al., 2009). Thus, some studies indicate that homologues of FT and SOC1 are expressed in the primordia of various species, and some others show that many organs of the plant are active producers of the associated proteins (Sreekantan and Thomas, 2006 in grapevine; Esumi et al., 2009 in apricot; Kotoda et al., 2010 in apple). Regarding identity genes, because the bud is the physical location for floral differentiation, more conventional research highlights the role and importance of gene expression at this level. Thus, it has been reported that floral identity genes are mainly expressed in the floral meristems of numerous plant species (Southerton et al., 1998 in Eucalyptus; Rottmann et al., 2000 in Populus; Wada et al., 2002 in apple; Yu et al., 2005 in papaya). In citrus, preliminary investigations have demonstrated that leaves are not exclusive in the production of transcripts of flowering-related genes (Nishikawa et al., 2007). However, there is a lack of information regarding the intensity, role and relative importance of the expression of flowering-related genes at the meristem level, and to what extent buds and leaves cooperate in flowering determination as a consequence of fruit bearing.
In addition, because CiFT appears to be the central promoter regulating flowering in alternate-bearing citrus, and CsAP1 and CsLFY represent the main floral identity genes determining bud fate, we extend our study to other floral integrator genes, which modulate and control their function at the apex level in model species. Thus, paralogues of FT have been reported to contribute greatly to the production of the small globular FT protein, associated with the mythic florigen. This is the case of TWIN SISTER OF FT (TSF), the closest homologue of FT in Arabidopsis, which probably constitutes another independent and direct regulatory target of CONSTANS (Yamaguchi et al., 2005). Although TSF is reported to have similar functions to FT, it acts independently in flowering determination (Yu et al., 2006) and therefore it may play an important role in FT protein supply to meristems in both leaves and buds. Similarly, FLOWERING LOCUS D (FD) has been described to encode a bZIP protein required for FT function, and has recently been described to take part in the specific signalling pathways that occur at the shoot apex (Abe et al., 2005). Finally, TERMINAL FLOWER 2 (TFL2), which was defined over a decade ago as a main controller of the reproductive transition and meristem identity in Arabidopsis, is known to participate in flower induction and bud differentiation (Larsson et al., 1998). It encodes an HP1-like protein that negatively regulates FT expression and affects floral identity activity (Takada et al., 2002). Although the expression dynamics of those genes would appear to be very relevant to developing FT function and modulating floral morphogenetic processes, it is completely unknown in most tree species. Here we endeavour to shed light on the regulation of FT activity and floral identity at the meristem level by studying those complementary but necessary genes, and thus provide further insight into the underlying mechanisms that fine-tune FT function and floral identity in alternate-bearing citrus.
In summary, previous research has been conducted into the role of the main flowering promoters and inhibitors in citrus trees, but there is a complete lack of knowledge about the role and importance of buds in the regulation process. We investigate the role this tissue plays in flowering-related gene expression through the manipulation of flowering intensity and/or the presence of leaves. In addition, because a pivotal role has been proposed for some genes that strongly modulate the action of FT and cooperate with floral identity genes at the bud level in model species, we extend our study to the changes in expression of the putative orthologues in citrus.
MATERIAL AND METHODS
Plant material
This study was conducted with 12-year-old field-grown trees of ‘Moncada’ mandarin {Clementina Oroval (Citrus clementina Hort. ex Tan.) × ‘Kara’ mandarin [C. unshiu (Swingle) Marcow. × C. nobilis Lour.]} trees, grafted onto Carrizo citrange [C. sinensis Osbeck × Poncirus trifoliata (L.) Raf.] rootstock, planted 5 m × 5 m apart in a loamy clay soil, with drip irrigation. Experimental fields were located in the IVIA Research Station (Moncada, Spain). Trees of this cultivar exhibit a marked alternate-bearing behaviour.
Defruiting experiments were performed on a set of 12 trees, and two levels of fruit removal were employed (0 and 100 %, six trees per treatment). Defoliation treatments were performed on another 12 either fully loaded or unloaded trees (0 and 100 % leaf removal, six trees per treatment). Leaves were carefully removed to avoid bud damage and all of them started to develop the following spring. Both experiments were performed at the onset of the stage II of fruit development (July). Trees were selected for homogeneity in diameter, canopy height, size and shape and a randomized complete-block design was employed. In mid November and at the end of February, fully developed mature adult leaves (n = 30 per tree) and non-differentiated buds (≥100 mg f. wt per tree; see Fig. 1) from on (fully loaded) and off trees (without fruits) were collected for RNA extraction. Samples were immediately ground and stored at –80 °C until analysis.
Fig. 1.

Detail of the buds sampled in mid November and at the end of February for gene-expression analyses. Citrus buds, unlike those of deciduous fruit trees, do not exhibit a true dormancy, remaining in rest until initiation. The resting bud itself contains an undifferentiated primordium surrounding the meristematic dome.
Flowering evaluation
Flowering intensity was evaluated in spring considering four branches per tree of three ages (late spring, summer and autumn sprouts) with some 300 nodes per branch. Numbers of both sprouted nodes and sprouts were counted. The flowers per sprout were also counted, with the results given as the number of flowers per 100 nodes to compensate for the differences in size of the selected branches. Additionally, total yield per tree was determined by counting and weighing all fruits at harvest (April).
RNA extraction and RT-PCR
Total RNA was isolated from frozen tissue using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). RNA samples were treated with RNase-free DNase (Qiagen) through column purification following the manufacturer's instructions. RNA quality was tested based on OD260/OD280 and gel electrophoresis. RNA concentration was determined by fluorometric assays with the RiboGreen dye (Molecular Probes, Engene, OR, USA) according to the manufacturer's instructions. Three fluorometric assays per RNA sample were performed. Quantitative real-time RT-PCR was performed with a LightCycler 2·0 Instrument (Roche Diagnostic, Basel, Switzerland) equipped with LightCycler Software version 4·0. One-step RT-PCR was carried out. Reactions contained 2·5 U MultiScribe reverse transcriptase (Applied Biosystems, Carlsbad, CA, USA), 1 U RNase Inhibitor (Applied Biosystems), 2 µL LC FastStart DNA MasterPLUS SYBR Green I (Roche Diagnostic), 25 ng total RNA and 250 nm of the specific forward and reverse primers of each gene in a total volume of 10 µL. Incubations were carried out at 48 °C for 30 min and 95 °C for 10 min followed by 45 cycles at 95 °C for 2 s, 58 °C for 8 s and 72 °C for 8 s. Fluorescence intensity data were acquired during the 72 °C extension step and transformed to relative mRNA values using a ten-fold dilution series of RNA samples as the standard curve. Relative mRNA levels were then normalized to total mRNA amounts (Bustin, 2002; Hashimoto et al., 2004) and, in each case, an expression value of 1 was arbitrarily assigned to the sample collected in November corresponding to the buds from intact on trees. Beta actin was used as the reference gene, according to Yan et al. (2012). Specificity of the amplification reactions was assessed using post-amplification dissociation curves and by sequencing the reaction product.
Putative genes were identified through homology searches with related genes from an expressed sequence tag (EST) database of a random 5′ ‘Clemenules’ mandarin (C. clementina Hort ex. Tan.) full-length cDNA library (Terol et al., 2008). Synthetic oligonucleotides were designed to amplify the gene of the selected clones and, as stated before, sequenced for confirmation. Details of the forward and reverse primers are given in Table 1.
Table 1.
List of primers used for quantitative real-time PCR
| Annotation | EST code* | 5′-Direct primer-3′ | Predicted product (bp) |
|---|---|---|---|
| 5′-Reverse primer-3′ | |||
| CiFT | aCL6275Contig1 | GGGAGGCAGACTGTTTATGC | 84 |
| CGGAGGTCCCAGATTGTAAA | |||
| CsTFL | aCL6873Contig1 | TCCGTCCACAGTTGTTTCAA | 105 |
| TCACTAGGGCCAGGAACATC | |||
| CsAP1 | aCL2870Contig1 | CAAAACCAGGTTCCCAACAC | 139 |
| ACGAACATACGGGTTCAAGG | |||
| CsLFY | aC34107C06EF_c | TCTTGATCCAGGTCCAGAACATC | 63 |
| TAGTCACCTTGGTTGGGCATT | |||
| CsSL1 | aC31703B03EF_c | TCAGCTTCTTCCCATTTTGG | 135 |
| AAGGTGACTTGCCTGCTTGT | |||
| FLC | aC01009F11SK_c | TCTTTGGTGCAGACACAACTG | 74 |
| TCTTCACTCAGCAGCTTTTCC | |||
| TSF | aIC0AAA15CC06RM1_c | TGCATGCAGGCCAAGAGATTGTGA | 97 |
| TGCCTTCCAAGTTGCCGGAACAA | |||
| FD | aCL3553Contig1 | TGGAAGAAGTTTGGCAGGA | 69 |
| TGTTTGGGATGGCAGTGTT | |||
| TFL2 | aKN0AAP8YL12FM1_c | GCGGCCGAAGCTTGATGAAGGATT | 62 |
| TGCGAACCCTTTTCCGACGAATGG |
* EST code refers to the database entry available in the Citrus Functional Genomics Project (CFGP; http://bioinfo.ibmcp.upv.es/genomics/cfgpDB/).
Statistical analyses
The experiment was laid out in randomized blocks, with single-tree plots and six replicates per treatment. Parameters were statistically tested by analyses of variance (ANOVA), using the least significant differences (LSD) test for separation of means. When required, percentages were arcsin-transformed to homogenize the variance. StatGraphics Plus software for Windows, version 5·1 (Statistical Graphics, Englewood Cliffs, NJ, USA), was used.
RESULTS
Effect of fruit load on flowering
The effect of fruit load on flowering is shown in Fig. 2. The removal of all fruits severely affected fruit load. Thus, the presence of fruit drastically inhibited the number of flowers produced in spring (1·3 vs. 153·6 flowers/100 nodes in on and off trees, respectively). The effects were also evident when crop load was examined the following year: those off trees produced a mean of 104·1 kg fruit per tree, in contrast to the nil yield produced by on trees.
Fig. 2.

Effect of fruit removal on flowering intensity of ‘Moncada’ mandarin trees (on: fully loaded; off: without fruit). Defruiting treatment consisted of removing the whole yield in the selected trees at the onset of the previous fruit development stage II (July). Data are the means ± s.e. of six trees per treatment and different letters indicate significant differences (P ≤ 0·05).
Expression of flowering-related genes
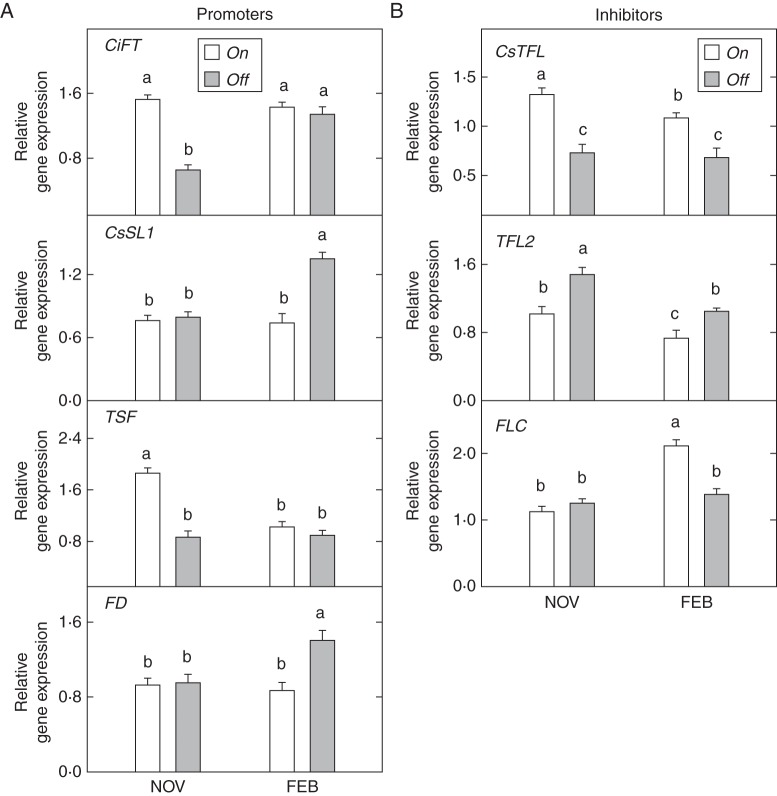
CiFT and TSF expression was strongly affected by fruit load throughout the study period (Fig. 3). Significant differences in the number of mRNA transcripts were detected between on and off trees in both buds and leaves. Thus, the activity registered in off trees in November was more than ten-fold higher than that in on trees in both tissues. In February, although still statistically significant, differences in buds decreased notably (about five-fold), whereas in leaves increased up to 17-fold. TSF expression dynamics strongly paralleled that of CiFT, and off trees presented a significantly higher number of transcripts in all cases. Differences between on and off trees were greater in buds than in leaves. Thus, differences in buds ranged from 4·9- to 2·5-fold in November and February respectively, whereas leaves ranged from 1·9- to 1·5-fold on the same dates.
Fig. 3.

Expression of flowering promoters (CiFT, TSF, CsSL1, FD) in buds from fully loaded (on) and without-fruit (off) ‘Moncada’ mandarin trees in November and February. Insets: activity in leaves. Data are means ± s.e. of three independent replicates (n = 3). Different letters indicate statistically significant differences (P ≤ 0·05).
In buds, relative expression of CsSL1 and FD was not affected by fruit load. Interestingly, CsSL1 expression level in buds and leaves was similar, whereas FD activity was much higher at the meristem (about five- and three-fold higher in November and February, respectively).
Expression of the inhibitors, although not always significant, exhibited a systematic decline in buds from off trees (Fig. 4). Thus, the expression of CsTFL in this tissue was significantly lower in off trees in both November and February (22 and 32 %, respectively). A similar pattern was observed for TFL2 expression in buds, although differences were only significant in February. CsTFL and TFL2 expression were not significantly different in leaves from on and off trees, irrespective of the sample date. Finally, FLC expression in buds from off trees exhibited a maintained, significant decline throughout the study period (about 16 % lower).
Fig. 4.
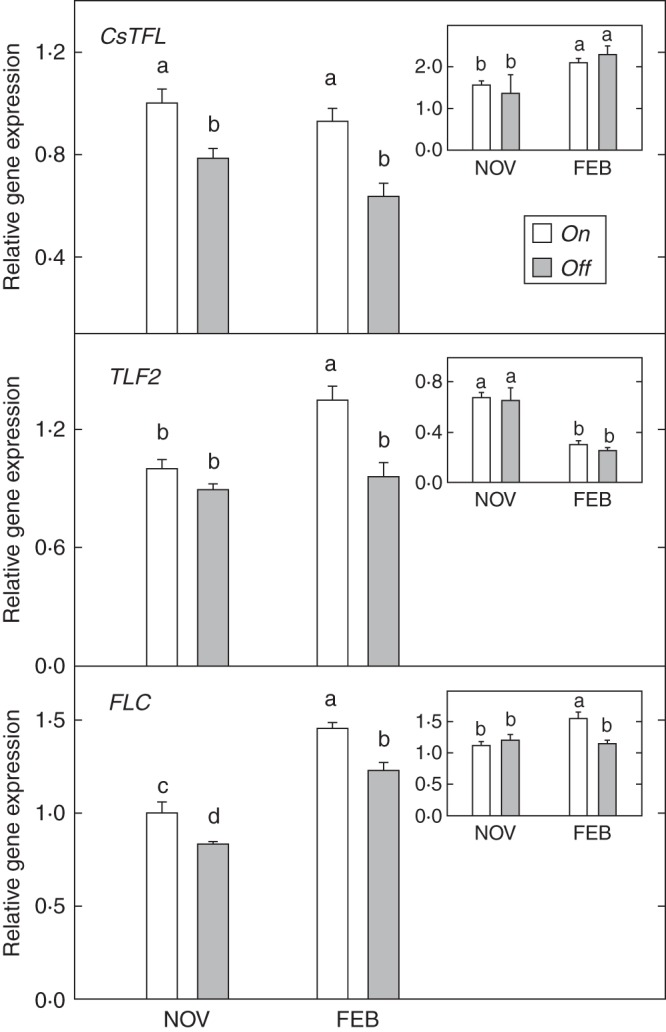
Expression of flowering inhibitors (CsTFL1, TFL2, FLC) in buds from fully loaded (on) and without-fruit (off) ‘Moncada’ mandarin trees in November and February. Insets: activity in leaves. Data are means ± s.e. of three independent replicates (n = 3). Different letters indicate statistically significant differences (P ≤ 0·05).
The activity of floral identity genes in buds and leaves revealed remarkable differences between on and off trees (Fig. 5). On the one hand, CsAP1 expression in both tissues greatly increased from November to February, and the accumulation of transcripts was markedly higher in buds than in leaves. Through the study period, those buds from off trees showed significantly higher expression values than those from on trees, ranging from 2·7- to 4·3-fold higher. Differences between on and off trees in leaves were minor, although the trend was similar. CsLFY, like CsAP1, exhibited higher expression levels in buds than in leaves. Significant changes due to fruit load were detected only in February in both tissues; by this time, activity had increased more than 50 % in buds from off trees.
Fig. 5.
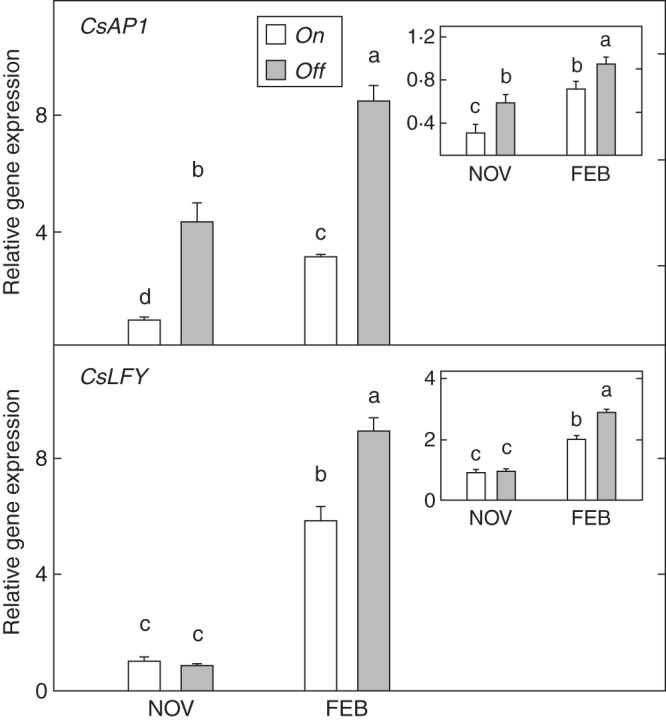
Expression of floral identity genes (CsAP1, CsLFY) in buds from fully loaded (on) and without-fruit (off) ‘Moncada’ mandarin trees in November and February. Insets: activity in leaves. Data are means ± s.e. of three independent replicates (n = 3). Different letters indicate statistically significant differences (P ≤ 0·05).
Effect of defoliation on flowering and gene expression
Defoliation had a dramatic effect on flowering in spring. Those trees whose leaves were fully removed dramatically abolished flower production: neither on nor off trees flowered in spring. In addition, defoliation experiments revealed that the absence of leaves severely affected the expression of several pivotal flowering-related genes (Figs 6 and 7). Thus, bud CiFT expression was markedly reduced in defoliated (Fig. 6A) compared with non-defoliated (Fig. 3) off trees throughout the study period (15- and three-fold in November and February, respectively). Interestingly, differences in gene activity between on and off trees only reached significance in November. By this time, expression in buds from defoliated off plants was severely reduced with respect to on planbts (close to 60 %). An analogous tendency was observed when TSF expression was examined. Activity in buds from defoliated trees was significantly reduced (Fig. 6A) with respect to control intact trees (Fig. 3) and, like FT, only in November did defoliated off trees show a significant decrease in TSF expression compared with defoliated on trees (about 50 %). Regarding CsSL1 and FD, activity in buds did not show important changes as a consequence of defoliation in November (Figs 3 and 6A). By contrast, CsSL1 and FD expression in buds of defoliated trees changed as a consequence of fruit load in February in both cases, and at this time on trees exhibited low activities compared with off trees (about 50 %).
Fig. 6.
Effect of defoliation on the expression of floral promoters (A: CiFT, TSF, CsSL1, FD) and inhibitors (B: CsTFL1, TFL2, FLC) in buds from fully loaded (on) and without-fruit (off) ‘Moncada’ mandarin trees in November and February. Data are means ± s.e. of three independent replicates (n = 3). Different letters indicate statistically significant differences (P ≤ 0·05).
Fig. 7.

Effect of defoliation on the expression of floral identity genes (CsAP1, CsLFY) in buds from fully loaded (on) and without-fruit (off) ‘Moncada’ mandarin trees in November and February. Data are means ± s.e. of three independent replicates (n = 3). Different letters indicate statistically significant differences (P ≤ 0·05).
In general, expression intensity of flowering-inhibitors was not noticeably affected by leaf removal (Figs 4 and 6B); however, on and off trees responded differently in terms of gene expression. Thus, whereas CsTFL expression in defoliated off trees exhibited a marked decrease with respect to on trees in both November and February (45 and 37 %, respectively), TFL2 followed a significant, opposite trend. FLC variations were only observed in February (like control non-defoliated trees, Fig. 4), and by this time expression in off plants was significantly reduced (close to 35 %).
The activity of floral identity genes was also noticeably altered as a consequence of leaf removal (Fig. 7). On the one hand, CsAP1 expression patently decreased in defoliated off trees in both November and February compared with control plants (four-fold, see Fig. 5). Differences between on and off trees were only found in February, buds from defoliated off trees showing higher expression values (1·5-fold). Surprisingly, CsLFY expression in buds from defoliated trees showed a marked increase also in November with respect to control foliated trees (more than five-fold, see also Fig. 5). Interestingly, off trees exhibited significant increases in CsLFY expression values with respect to on trees in both November and February, with differences ranging from 28 to 39 %, respectively.
DISCUSSION
Effect of fruit load on flowering
The presence of fruit is commonly described to affect flowering in citrus species (Moss, 1971). In the present study, the number of flowers produced in spring was modulated by manipulating the number of fruits. Thus, non-defruited fully loaded trees did not flower at all, whereas completely defruited trees displayed the highest blossoming intensity, associated with higher fruit load (Fig. 2). The results obtained herein support previous observations, confirming the effect of alternate bearing, and therefore fruit load, on flowering dynamics.
Previous research has shown that differences in gene expression in leaves arise as a consequence of alternate bearing in citrus trees (Muñoz-Fambuena et al., 2011). The present study shows that active expression of the main genes involved in floral induction and differentiation also occurs in citrus tree buds. Our findings provide further evidence for the control of flowering not only in time but also in space, and shed light on the role of the meristem in the regulation process. Additionally, we describe changes in the activity of several genes that influence the principal flowering pathways at the apical level.
Regulation of the expression of flowering-related genes at the meristem
Regarding the primary response to floral induction, which is highly conserved in plant species, the activation of FT has been shown to play a central role in the flowering process (Matsuda et al., 2009; Trankner et al., 2010). In citrus, the corresponding orthologue CiFT is a key gene determining flowering (Nishikawa et al., 2010), and previous studies demonstrate that, at least at the leaf level, it exerts rigorous control under alternate-bearing conditions (Muñoz-Fambuena et al., 2011). The results presented herein further indicate that, like leaves, buds from unloaded trees exhibit high CiFT activity. This was observed throughout the study period, suggesting that active, constitutive FT protein production also occurs at the apical level, which finally leads to high flowering intensity in spring. Particularly at the inductive stage (November), the differences between on and off trees were maximum in both leaves and buds (about ten-fold higher in off plants). Later, in February, activity declined in buds compared with leaves and, although still significant, differences between on and off trees decreased. As expected, the expression pattern registered for TSF, the closest homologue of FT in Arabidopsis, strongly paralleled that of CiFT in all cases, although the intensity and the magnitude of the differences between treatments was minor. It is assumed that TSF in Arabidopsis is a main promoter of the flowering processes together with FT, but makes a distinct contribution (Mathieu et al., 2007; Yu et al., 2005). As the greatest differences in FT and TSF activity between on and off trees were found in November in both buds and leaves, our data also suggest a critical and complementary role at the floral inductive stage, supporting previous observations in model species (Notaguchi et al., 2008; Jang et al., 2009). Acting together on a different spatial scale, their heightened activity would increase the pool of FT protein necessary to induce flowering at the apex, either directly or through import processes, at least during the stages closest to the inductive period. In fact, recent research suggests a long-distance action for FT protein, involving complex and selective trafficking pathways that physically connect leaves to meristems (Corbesier and Coupland, 2006; Goto, 2006; Jang et al., 2009). Once the bud is induced, the increased CiFT expression found in both buds and leaves (Fig. 3) would be responsible for a continuous flux of FT protein to the developing meristems up until the floral morphogenetic phase is initiated (Jaeger et al., 2006; Giakountis and Coupland, 2008).
Other promoter genes, such as SOC1, have also been reported to contribute to the promotion of flowering (Tan and Swain, 2007), and previous studies have analysed their participation in the regulation of flowering under alternate-bearing conditions (Muñoz-Fambuena et al., 2011). Our data indicate that the citrus homologue CsSL1 shows a constant and similar activity in both buds and leaves, suggesting a minor role in relation to fruit load at the apical level. Probably after flowering induction takes place, expression of CsSL1 not only supports the continuous promoting action of CiFT at the shoot apex (Fig. 3), but also participates in the regulation of floral morphogenesis through the activation of identity genes (e.g. CsLFY, see Fig. 5). By contrast, unlike CsSL1, FD expression in buds was markedly higher than in leaves in both November and February. As in other species (Abe et al., 2003), this observation reinforces the hypothesis that its role is decisive at the apical level, possibly because the transition from vegetative to floral meristem mainly occurs there. In fact, this gene has been described as a strong modulator of FT action specifically in the meristem (Abe et al., 2005; Jaeger et al., 2006), integrating temporal and spatial signals and mediating promoter and floral identity molecular pathways (Wigge et al., 2005; Li et al., 2008). The continuous expression observed over time coincides with studies in other species that report a constitutive activity in the shoot apex even before floral induction (see Wigge et al., 2005).
In general, differences were revealed in the expression of inhibitors in buds between on and off trees. Thus, whereas no important effects were observed as a consequence of alternate bearing in leaves, there was a continuous decline in expression levels in all genes considered in buds from off trees during the study period. This observation supports the hypothesis that floral repressors ensure the correct reproductive timing by controlling promoters, and the reduced expression found in buds from off trees facilitates the action of promoters, thereby contributing to flowering induction. Indeed, the lower expression levels in buds than in leaves may suggest that the observed changes in CsTFL expression are not a direct consequence of fruit load (Muñoz-Fambuena et al., 2011) but may also be associated with juvenility processes and/or with the maintenance and development of vegetative growth (Pillitteri et al., 2004a; Esumi et al., 2010). In contrast to CsTFL, TFL2 expression levels were always higher in buds than in leaves, suggesting a pivotal role for this gene at this location. In fact, TFL2 was first described as a main controller of both reproductive transition and meristem identity in Arabidopsis (Larsson et al., 1998), and appears to actively and constitutively repress FT expression throughout development (Takada et al., 2002; Takada and Goto, 2003). We also extend this observation to the fact that activity was much higher in buds than in leaves in February and that, at that moment, differences between on and off trees were also highly accentuated there. Effects upon morphogenetic processes have also been reported for this gene, strongly influencing the role of identity genes such as AP1 and contributing to the maintenance of inflorescence meristem identity and floral architecture (Larsson et al., 1998). Finally, it is interesting to note that FLC did not display differences in activity between leaves and buds, concomitant with a function associated with flowering regulation through the vernalization pathway (Michaels et al., 2005), possibly at any plant level. Regardless, lower expression levels at the buds may also continuously activate and facilitate the action of promoters (Michaels et al., 2005).
Study of the expression of floral identity genes, CsAP1 and CsLFY, has corroborated their pivotal role at the bud level (Fig. 5), as a higher production of transcripts in this tissue was found compared with leaves. Irrespective of fruit load, CsAP1 and CsLFY activities were significantly higher in buds than in leaves in February, whereas in November this trend was only encountered for CsAP1. The increased number of CsAP1 transcripts found in off trees, more notable in buds, suggests a main correlation with flowering, as reported for other species (Jaya et al., 2010). Together with the reduction in the suppressive action of inhibitor genes, promoter genes such as FT and/or FD probably contribute to the development of floral morphogenesis (Yamamoto et al., 2004; Notaguchi et al., 2008). By contrast, the absence of differences in CsLFY activity between on and off trees in November suggests a more delayed response. In fact, although high expression levels of both genes coincided in the shoot apex with the stage of flower differentiation and morphogenesis, previous studies in citrus report CsAP1 to be more effective than CsLFY in terms of flowering induction (Peña et al., 2001).
Defoliation-induced changes in flowering intensity and gene expression
Because data revealed that buds actively express the main genes involved in flowering-related processes, defoliation experiments were conducted to further investigate the role of leaves in flowering determination. The results presented herein indicate that leaf removal severely affected flowering-gene expression in buds, and resulted in the complete inhibition of blossoming. Therefore, it can be concluded that leaves are necessary for floral induction and differentiation, independently of the alternate-bearing condition and variations in gene expression in buds might play a complementary role at least during the induction and differentiation stages. As expected, given their role in the flowering process, CiFT and TSF activities were strongly affected by leaf removal (Fig. 6A). Thus, defoliation treatments dramatically inhibited CiFT expression in buds from off trees, which did not flower after leaf removal, but not in those from on trees, which did not flower in either foliated or defoliated trees. The decrease in the activity was much more accentuated in November than in February, confirming the key role of leaves at the floral inductive stage. Treatment also reduced the expression of TSF. Although a certain amount of protein is directly produced at the shoot apex it is not enough to induce flowering and the FT protein pool produced by the leaf appears to be essential to flower development (Notaguchi et al., 2008). Other promoter genes, such as CsSL1 and FD, did not show marked responses to defoliation (Figs 3 and 6A). Significant differences were only observed in February, probably associated with a residual and delayed effect of the early inhibition of FT and TSF. The defoliation experiment also confirmed that the roles of CsTFL and FLC seem not to be directly associated with fruit load, as those genes did not substantially change their expression pattern in either on or off trees due to treatment (Figs 4 and 6B). Interestingly, TFL2 expression pattern was inverted in on and off trees as a consequence of leaf removal (see Figs 4 and 6B), concomitantly with the decrease in FT protein levels (Fig. 6A), reinforcing its inhibitory role in the absence of leaves.
Defoliation also confirmed the main role of identity genes at the bud level (Fig. 7). The data presented herein show that their expression in buds was drastically affected as a consequence of leaf removal. Thus, CsAP1 expression decreased greatly in off trees in both November and February (about four-fold) with respect to control foliated trees. Interestingly, the effect of CsLFY on floral identity has been associated with dates around floral differentiation (February, see Fig. 5), whereas leaf removal induced a reduction of CsLFY in buds from on and off leaves in both November and February, suggesting that latent expression of this gene at the inductive stage, after leaf removal, could contribute to the observed inhibition of flowering.
Together, these are the first results to suggest that the presence of fruit not only affects flowering by severely alters gene expression at the leaf and meristem level in alternate-bearing citrus trees. Thus, CiFT and TSF expression were strongly inhibited in buds as a consequence of fruit load, and expression of floral identity genes (CsAP1 and CsLFY) was considerably reduced. The flowering inhibitors CsTFL, TLF2 and FLC generally exhibited lower expression in off trees, probably supporting the action of promoter genes. Finally, although leaves are essential for flowering, fruit defoliation strongly reduced the activity of floral promoters CiFT, TSF and CsAP1 in buds, especially at the inductive stage.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Instituto Nacional Investigaciones Agrarias, Spain (RTA2009-00147). M. C. González was the recipient of a contract by the Fundación Agroalimed (Conselleria d’Agricultura, Pesca i Alimentació, Generalitat Valenciana).
LITERATURE CITED
- Abe M, Yamamoto S, Kobayashi Y, Nakabayashi H, Ichinoki H, Araki T. Flowering-time gene FD encodes a bZIP protein which is required for the function of a floral pathway integrator FT. Plant and Cell Physiology. 2003;44 [Google Scholar]
- Abe M, Kobayashi Y, Yamamoto S, et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. Journal of Molecular Endocrinology. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Coupland G. The quest for florigen: a review of recent progress. Journal of Experimental Botany. 2006;57:3395–3403. doi: 10.1093/jxb/erl095. [DOI] [PubMed] [Google Scholar]
- Davenport TL. Citrus flowering. Horticultural Reviews. 1990;12:349–408. [Google Scholar]
- Dornelas MC, Camargo RLB, Figueiredo LHM, Takita MA. A genetic framework for flowering-time pathways in Citrus spp. Genetics and Molecular Biology. 2007a;30:769–779. [Google Scholar]
- Dornelas MC, Luciana R, Camargo B, Berger IJ, Takita MA. Towards the identification of flower-specific genes in Citrus spp. Plant Physiology. 2007b;3:761–768. [Google Scholar]
- Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M. Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.) Transgenic Research. 2005;14:703–712. doi: 10.1007/s11248-005-6632-3. [DOI] [PubMed] [Google Scholar]
- Esumi T, Hagihara C, Kitamura Y, et al. Identification of an FT ortholog in Japanese apricot (Prunus mume Sieb. et Zucc.) Journal of Horticultural Science & Biotechnology. 2009;84:149–154. [Google Scholar]
- Esumi T, Kitamura Y, Hagihara C, Yamane H, Tao R. Identification of a TFL1 ortholog in Japanese apricot (Prunus mume Sieb. et Zucc.) Scientia Horticulturae. 2010;125:608–616. [Google Scholar]
- Giakountis A, Coupland G. Phloem transport of flowering signals. Current Opinion in Plant Biology. 2008;11:687–694. doi: 10.1016/j.pbi.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Goto K. Protein trafficking of FT/TFL1 and flowering signal transmission. Comparative Biochemistry and Physiology: A. Molecular & Integrative Physiology. 2006;143:S167–S167. [Google Scholar]
- Hashimoto JG, Beadles-Bohling AS, Wiren KM. Comparison of RiboGreen and 18S rRNA quantitation for normalizing real-time RT-PCR expression analysis. BioTechniques. 2004;36:54–60. doi: 10.2144/04361BM06. [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Graf A, Wigge PA. The control of flowering in time and space. Journal of Experimental Botany. 2006;57:3415–3418. doi: 10.1093/jxb/erl159. [DOI] [PubMed] [Google Scholar]
- Jang S, Torti S, Coupland G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant Journal. 2009;60:614–625. doi: 10.1111/j.1365-313X.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- Jaya E, Clemens J, Song JC, Zhang HB, Jameson PE. Quantitative expression analysis of meristem identity genes in Eucalyptus occidentalis: AP1 is an expression marker for flowering. Tree Physiology. 2010;30:304–312. doi: 10.1093/treephys/tpp117. [DOI] [PubMed] [Google Scholar]
- Koshita Y, Takahara T, Ogata T, Goto A. Involvement of endogenous plant hormones (IAA, ABA, GAs) in leaves and flower bud formation of satsuma mandarin (Citrus unshiu Marc.) Scientia Horticulturae. 1999;79:185–194. [Google Scholar]
- Kotoda N, Hayashi H, Suzuki M, et al. Molecular characterization of FLOWERING LOCUS T-like genes of apple (Malus domestica Borkh.) Plant and Cell Physiology. 2010;51:561–575. doi: 10.1093/pcp/pcq021. [DOI] [PubMed] [Google Scholar]
- Larsson AS, Landberg K, Meeks-Wagner DR. The TERMINAL FLOWER2 (TFL2) gene controls the reproductive transition and meristem identity in Arabidopsis thaliana. Genetics. 1998;149:597–605. doi: 10.1093/genetics/149.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu C, Shen L, et al. A repressor complex governs the integration of flowering signals in Arabidopsis. Developmental Cell. 2008;15:110–120. doi: 10.1016/j.devcel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Lord EM, Eckard KJ. Shoot development in Citrus sinensis L. (Washington navel orange). I. Floral and inflorescence ontogeny. Botanical Gazette. 1985;146:320–326. [Google Scholar]
- Martínez-Fuentes A, Mesejo C, Reig C, Agusti M. Timing of the inhibitory effect of fruit on return bloom of ‘Valencia’ sweet orange (Citrus sinensis L. Osbeck) Journal of the Science of Food and Agriculture. 2010;90:1936–1943. doi: 10.1002/jsfa.4038. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Kuttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Current Biology. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Ikeda K, Kurosaka M, et al. Early flowering phenotype in transgenic pears (Pyrus communis L.) expressing the CiFT gene. Journal of the Japanese Society for Horticultural Science. 2009;78:410–416. [Google Scholar]
- Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM. Integration of flowering signals in winter-annual Arabidopsis. Plant Physiology. 2005;137:149–156. doi: 10.1104/pp.104.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss GI. Effect of fruit on flowering in relation to biennial bearing in sweet orange (Citrus sinensis) Journal of Horticultural Science & Biotechnology. 1971;46:177. [Google Scholar]
- Muñoz-Fambuena N, Mesejo C, González-Mas MC, Primo-Millo E, Agustí M, Iglesias DJ. Fruit regulates seasonal expression of flowering genes in alternate-bearing ‘Moncada’ mandarin. Annals of Botany. 2011;108:511–519. doi: 10.1093/aob/mcr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Fambuena N, Mesejo C, González-Mas MC, Iglesias J, Primo-Millo E, Agustí M. Gibberellic acid reduces flowering intensity in sweet orange (Citrus sinensis L. Osbeck) by repressing CiFT gene expression. Plant Growth Regulation. 2012 doi:10.1007/s00344-012-9263-y. http://dx.doi.org/10.1007/s00344-012-9263-y . [Google Scholar]
- Nishikawa F, Endo T, Shimada T, et al. Increased CiFT abundance in the stem correlates with floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.) Journal of Experimental Botany. 2007;58:3915–3927. doi: 10.1093/jxb/erm246. [DOI] [PubMed] [Google Scholar]
- Nishikawa F, Endo T, Shimada T, et al. Transcriptional changes in CiFT-introduced transgenic trifoliate orange (Poncirus trifoliata L. Raf.) Tree Physiology. 2010;30:431–439. doi: 10.1093/treephys/tpp122. [DOI] [PubMed] [Google Scholar]
- Notaguchi M, Abe M, Kimura T, et al. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant and Cell Physiology. 2008;49:1645–1658. doi: 10.1093/pcp/pcn154. [DOI] [PubMed] [Google Scholar]
- Peña L, Martin-Trillo M, Juárez J, Pina JA, Navarro L, Martínez-Zapater JM. Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nature Biotechnology. 2001;19:263–267. doi: 10.1038/85719. [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Lovatt CJ, Walling LL. Isolation and characterization of a TERMINAL FLOWER homolog and its correlation with juvenility in citrus. Plant Physiology. 2004a;135:1540–1551. doi: 10.1104/pp.103.036178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Lovatt CJ, Walling LL. Isolation and characterization of LEAFY and APETALA1 homologues from Citrus sinensis L. Osbeck ‘Washington. Journal of the American Society for Horticultural Science. 2004b;129:846–856. [Google Scholar]
- Rottmann WH, Meilan R, Sheppard LA, et al. Diverse effects of overexpression of LEAFY and PTLF, a poplar (Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. Plant Journal. 2000;22:235–245. doi: 10.1046/j.1365-313x.2000.00734.x. [DOI] [PubMed] [Google Scholar]
- Schneider H. The anatomy of Citrus. In: Reuther W, Batchelor LD, Webber HJ, editors. The citrus industry. II. Berkeley, CA: University of California; 1968. pp. 1–86. [Google Scholar]
- Sherman WG, Beckman TG. The climatic adaptation in fruit crops. Acta Horticulturae. 2003;622:411–428. [Google Scholar]
- Southerton SG, Strauss SH, Olive MR, et al. Eucalyptus has a functional equivalent of the Arabidopsis floral meristem identity gene LEAFY. Plant Molecular Biology. 1998;37:897–910. doi: 10.1023/a:1006056014079. [DOI] [PubMed] [Google Scholar]
- Sreekantan L, Thomas MR. VvFT and VvMADS8, the grapevine homologues of the floral integrators FT and SOC1, have unique expression patterns in grapevine and hasten flowering in Arabidopsis. Functional Plant Biology. 2006;33:1129–1139. doi: 10.1071/FP06144. [DOI] [PubMed] [Google Scholar]
- Takada S, Goto K. TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Kotaka T, Ohto M, et al. Arabidopsis flowering gene TERMINAL FLOWER2 (TFL2) encodes an HP1-like protein and negatively regulates FT expression. Plant and Cell Physiology. 2002;43 [Google Scholar]
- Tan FC, Swain SM. Functional characterization of AP3, SOC1 and WUS homologues from citrus (Citrus sinensis) Physiologia Plantarum. 2007;131:481–495. doi: 10.1111/j.1399-3054.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- Terol J, Naranjo MA, Ollitrault P, Talón M. Development of genomic resources for Citrus clementina: characterization of three deep-coverage BAC libraries and analysis of 46000 BAC end sequences. BMC Genomics. 2008;9(423) doi: 10.1186/1471-2164-9-423. http://dx.doi.org/10.1186/1471-2164-9-423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trankner C, Lehmann S, Hoenicka H, et al. Over-expression of an FT-homologous gene of apple induces early flowering in annual and perennial plants. Planta. 2010;232:1309–1324. doi: 10.1007/s00425-010-1254-2. [DOI] [PubMed] [Google Scholar]
- Vemmos SN. Carbohydrate content of inflorescent buds of defruited and fruiting pistachio (Pistacia vera L.) branches in relation to biennial bearing. Journal of Horticultural Science & Biotechnology. 1999;74:94–100. [Google Scholar]
- Wada M, Cao QF, Kotoda N, Soejima J, Masuda T. Apple has two orthologues of FLORICAULA/LEAFY involved in flowering. Plant Molecular Biology. 2002;49:567–577. doi: 10.1023/a:1015544207121. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, et al. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- Yahata D, Matsumoto K, Ushijima K. Relationship between flower-bud differentiation and carbohydrate contents in spring shoots of very-early, early and late maturing cultivars of satsuma mandarin. Journal of the Japanese Society for Horticultural Science. 2004;73:405–410. [Google Scholar]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant and Cell Physiology. 2005;46:1175–1189. doi: 10.1093/pcp/pci151. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Abe M, Ichinoki H, Kobayashi Y, Araki T. Effect of the Arabidopsis flowering-time gene FD on the expression of meristem identity gene AP1. Plant and Cell Physiology. 2004;45 [Google Scholar]
- Yan J, Yuan F, Long G, Qin L, Deng Z. Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Molecular Biology Reports. 2012;39:1831–1838. doi: 10.1007/s11033-011-0925-9. [DOI] [PubMed] [Google Scholar]
- Yu QY, Moore PH, Albert HH, Roader AHK, Ming R. Cloning and characterization of a FLORICAULA/LEAFY ortholog, PFL, in polygamous papaya. Cell Research. 2005;15:576–584. doi: 10.1038/sj.cr.7290327. [DOI] [PubMed] [Google Scholar]
- Yu XH, Klejnot J, Lin CT. Florigen: one found, more to follow? Journal of Integrative Plant Biology. 2006;48:617–621. [Google Scholar]