Abstract
Background and Aims
Despite differences in physiology between dry and relative moist seeds, seed ageing tests most often use a temperature and seed moisture level that are higher than during dry storage used in commercial practice and gene banks. This study aimed to test whether seed ageing under dry conditions can be accelerated by storing under high-pressure oxygen.
Methods
Dry barley (Hordeum vulgare), cabbage (Brassica oleracea), lettuce (Lactuca sativa) and soybean (Glycine max) seeds were stored between 2 and 7 weeks in steel tanks under 18 MPa partial pressure of oxygen. Storage under high-pressure nitrogen gas or under ambient air pressure served as controls. The method was compared with storage at 45 °C after equilibration at 85 % relative humidity and long-term storage at the laboratory bench. Germination behaviour, seedling morphology and tocopherol levels were assessed.
Key Results
The ageing of the dry seeds was indeed accelerated by storing under high-pressure oxygen. The morphological ageing symptoms of the stored seeds resembled those observed after ageing under long-term dry storage conditions. Barley appeared more tolerant of this storage treatment compared with lettuce and soybean. Less-mature harvested cabbage seeds were more sensitive, as was the case for primed compared with non-primed lettuce seeds. Under high-pressure oxygen storage the tocopherol levels of dry seeds decreased, in a linear way with the decline in seed germination, but remained unchanged in seeds deteriorated during storage at 45 °C after equilibration at 85 % RH.
Conclusions
Seed storage under high-pressure oxygen offers a novel and relatively fast method to study the physiology and biochemistry of seed ageing at different seed moisture levels and temperatures, including those that are representative of the dry storage conditions as used in gene banks and commercial practice.
Keywords: Oxidative stress, seed ageing, seed longevity, seed quality, seed storage, seed test, tocopherol
INTRODUCTION
Many of our crops are reproduced through seeds, and throughout the world large quantities are produced, stored and transported. Seed ageing during storage may cause retardation of field establishment, and may eventually result in seedling abnormalities or even failure of emergence. During storage in gene banks, seed ageing may result in loss of genetic diversity. Extending and predicting the longevity of stored seeds is therefore highly relevant from a biodiversity as well as from an economic and social point of view. Seed ageing has been studied to a great extent. It is known that the rate of seed ageing is influenced by genetic and physiological factors and there is a need to understand the molecular mechanisms underlying these factors. Increased knowledge on seed ageing will aid breeders in improving shelf-life, the analysis of the effect of seed-production conditions and seed treatments and will provide gene banks with indicators for timely regeneration.
To test the effect of seed treatments or genetic variation on longevity, it is impractical to wait till the seeds have aged under their usual storage conditions. Storage experiments have been performed to estimate the longevity of seed lots in a large number of species. Based on these experimental results, a seed-viability equation has been developed, with species-specific constants, to determine the longevity of a seed lot, incorporating its initial viability, the storage temperature and the moisture content of the seeds during the storage period (Ellis and Roberts, 1980). To determine these species-specific constants, experimental treatments are used to accelerate the ageing process in such a manner that data can be collected within a relatively short period, preferably within a few months. Most often this involves storage of the seeds at an elevated moisture and/or temperature.
Variation in storage tolerance, e.g. between genotypes or due to seed treatments, is also frequently tested at a relative high moisture level and/or temperature. When the seeds are stored at an elevated temperature and the relative humidity (RH) of the atmosphere surrounding the seeds is 100 %, the test is generally called accelerated ageing (AA) (ISTA, 2012). When the seed moisture content is raised (generally to a level in equilibrium with 60 till 85 % RH) prior to the storage at elevated temperature, it is denoted as controlled deterioration (CD) (ISTA, 2012). AA and CD type tests (Powell and Matthews, 1981), are frequently used as indicators for seed longevity under conventional storage conditions, often with slight modifications in the procedure (Tesnier et al., 2002; Prieto-Dapena et al., 2006; Demir and Mavi, 2008).
In commercial seed storage of relatively expensive horticultural seeds, the warehouses are mostly conditioned at 30–35 % RH and 15–20 °C. For genetic conservation, seeds are also stored dry (can vary from 5 to 35 % RH) and cool (–20 to 5 °C). Since the physiology and biochemistry of seeds can vary with the water content and temperature, we questioned if artificial storage tests under high moisture or temperature reflect the ageing and tolerance mechanisms similarly to dry and cool or ambient storage conditions. Analysing longevity of 195 species, by rapid ageing at elevated temperature and relative humidity (either 45 °C and 60 % RH or 60 °C and 60 % RH) controlled ageing, showed that families with a generally short life span under gene-bank storage at the Millennium Seed Bank, also aged more rapidly under these artificially rapid ageing conditions (Probert et al., 2009). Others observe that predictions for longevity based on storage under more moist conditions and elevated temperatures may deviate from those observed in commercial practice and a general applicability of ageing tests based on CD procedures has been questioned (Niedzielski et al., 2009; Schwember and Bradford, 2010). The latter analysed genetic variation for storage under relatively ambient conditions (37 °C and 30 % RH) and under a CD type of procedure (4 d hermetically sealed at 50 °C after equilibration at 75 % RH) and observed a lack of correlation between tolerances to deterioration under the two conditions. Powell and Harman (1985) observed different changes in lipids in seed aged under different rapid ageing conditions and questioned whether the process of ageing is the same when seeds are aged rapidly compared with natural conditions. However, they did not examine naturally aged seeds.
Different seed lots from a same crop may vary in their shelf-life, due to previous deterioration during production and/or storage. The longevity pattern may deviate from the seed lot used for determination of the species-specific constants, either due to genetic variation, or to physiological changes. Seed priming for instance may result in a considerable shortening of shelf-life. To estimate an expected shelf-life of their seed lots or to modify seed priming treatments, seed companies perform artificial storage experiments at elevated moisture level and/or elevated temperature.
From food science it is known that some enzymes can still be active at a water activity of 0·5 (≅50 % RH) (Labuza, 1971). AA and CD procedures result in a medium-to-high water activity and enzymatic activity may occur that is at least strongly inhibited during storage at low seed moisture levels. Enzyme activity is influenced by temperature, which also influences fluidity of membranes and storage lipids. Both increases in temperature and seed moisture can induce phase transitions of the cytoplasm from the glass to the fluid state, thereby reducing shelf-life (Sun, 1997; Roos, 1998). It has been shown that above the glass transition temperature, the cytoplasm in seeds still exhibits a low molecular mobility and high stability, but the level and types of protection presumably differ from that during the glass phase (Buitink and Leprince, 2008).
The deterioration processes occurring in seeds, the protection mechanisms preventing it and the activity of enzymes involved in repair may vary with differences in either temperature or water activity (Walters and Engels, 1998; McDonald, 1999; Murthy et al., 2003; Kibinza et al., 2006). This may explain why tests following CD and AA procedures could have limitations in predicting seed shelf-life under rather dry conditions.
For a long time, low moisture levels and temperatures during storage have been considered the key factors in prolonging seed shelf-life [Justin and Bass, 1978; Cromarty et al., 1982 (revised 1990)]. Deterioration during seed ageing involves oxidation of lipids, cell and mitochondrial membranes, DNA, RNA and proteins and Maillard reactions (Osborne, 1994; Bailly, 2004; Rajjou and Debeaujon, 2008; Rajjou et al., 2008). Even though these seed-ageing processes are a direct or indirect consequence of oxidation, the role of oxygen during storage has hardly been considered in the practice of commercial or gene-bank seed storage.
The detrimental effect of oxygen to stored seeds has been described by Abdalla and Roberts (1968), who showed that increased oxygen levels during storage resulted in more chromosome aberrations during cell division, likely induced by accumulation of DNA oxidation during the period of storage. In addition the vitality of lettuce seeds was better maintained by storage under carbon dioxide or nitrogen compared with packages containing air (Harrison and McLeish, 1954; Justin and Bass, 1978) and the long-term (36 years) survival of ultra-dry stored Brassicaceae seeds was at least partly due to a CO2 -enriched atmosphere in the flame-sealed glass vials (González-Benito et al., 2011). Stored wheat grains maintained germination capacity better if the silos were flushed with nitrogen gas compared with air (Shejbal, 1979) and, more recently, Schwember and Bradford (2011) showed that longevity of lettuce seeds stored at 32 % RH was extended by anaerobic environments.
If reduced oxygen levels can extend seed longevity in dry seed storage, then alternatively the seed ageing process might be expected to be accelerated by storing seeds under elevated oxygen concentration. Indeed, storage of barley seeds under increased pressure of oxygen was found to induce chromosome aberrations (Ehrenberg et al., 1957) and mutations (Kronstad et al., 1959). Storing Vicia faba seeds for 4 weeks at oxygen pressures of 3 or 6 MPa (1 MPa = 10 bar) resulted in increased chromosome defects the longer the duration of the treatment (Moutschen-Dahmen et al., 1959). Unfortunately, in these early publications no information was provided on the moisture level of the seeds or RH during the treatments. Berg et al. (1965) reported that 1 week of storage of barley seeds at various moisture levels under high-pressure (14 MPa) oxygen, resulted in retardation of seedling growth, especially after storage at very low moisture levels (2·1 or 3·1 % moisture content). Furthermore, the storage of soybean and safflower at elevated oxygen pressure (0·77 MPa) and 25 °C resulted in a loss of seed germination of relatively moist seeds (at 17 % moisture content, which is in equilibrium with about 94 % RH) within 3 weeks, whereas the decline in germination did not occur with the seeds stored under 0·77 MPa nitrogen (Ohlrogge and Kernan, 1982).
The study of physiological, molecular and genetic factors involved in seed ageing and tolerance and predictions for seed shelf-life might benefit from the development of a method to study seed ageing also in dry seeds and at temperate temperatures. If an increased pressure of oxygen accelerates the deterioration during storage, it might be used as an additional factor in artificial storage experiments and as an alternative method to understand seed ageing processes at different moisture levels, including those used in commercial and gene bank storage.
The objective of this study was to develop an artificial seed-ageing method using high-pressure oxygen storage. We tested the storage of seeds in steel tanks under elevated partial pressure of oxygen (EPPO) in combination with controlled humidity and temperature levels, and compared the test results and seed ageing symptoms with those obtained after ageing at elevated moisture and temperatures (comparable to CD procedures).
MATERIALS AND METHODS
Seed material
For our studies four crops species were selected. Lettuce (Lactuca sativa L.) and soybean [Glycine max (L.) Merr] have seeds with a relative short shelf-life in commercial seed storage. Lettuce seeds are also frequently primed, which negatively affects commercial shelf-life. Barley (Hordeum vulgare L.) seeds have a relatively long shelf-life. Cabbage (Brassica oleracea L.) seeds have an intermediate shelf-life and commercial harvests may contain seeds in different levels of maturity.
Lettuce seeds of different production years and cultivars were provided by the seed company Rijk Zwaan (The Netherlands). The seeds were from commercial seed productions and had been stored in the company's warehouse. Upon receipt, the seeds were stored at 20 °C and approx. 35 % RH. Five seed lots were used: Lot 1Y (1 year old), lot 2Y (2 years old), lot 3Y (3 years old), lot 4Y (4 years old) and lot 5Y (5 years old). Cabbage seeds (originally provided by Bejo Seeds, The Netherlands) had been stored in our laboratory at 5 °C and 35 % RH for approx. 10 years. An initial seed lot from the cultivar Bartolo had been divided in six maturity fractions, based on the level of chlorophyll fluorescence (Jalink et al., 1998). Seeds from the second least-mature fraction and from the most-mature fraction were used in the storage experiments. Other experimental cabbage seed samples, from different varieties, were also provided by Bejo. These samples had been stored on their laboratory bench for different periods, followed by storage at –20 °C. Soybean seeds were obtained from an experimental seed production at the Agricultural University of Teheran (Iran). Two barley seed lots (A and B) were provided by Holland Malt B.V. (The Netherlands).
To study the effect of EPPO storage on primed lettuce seeds, 1 g of seeds (lot 1Y; equilibrated at 35 % RH) were primed in 50-mL tubes with the addition of 0·2, 0·4 or 0·6 g water. A tube with seeds without water addition served as a control. The tubes were closed with an oxygen-permeable polyethylene membrane and placed on a roller bench at 20 °C. After 7 d, the seeds were re-dried and stored at 35 % RH and 20 °C for 3 months until EPPO storage was tested.
Storage treatments
To test the effect of the EPPO treatment on the seeds, they were stored in 1·5-L steel tanks for various periods under high-pressure air, oxygen or nitrogen gas (Fig. 1A). The seed samples were placed within the tanks in 13-mL polystyrene tubes (Sarstedt, Germany) perforated with a large number of approx. 1-mm-diameter holes and closed with low-density polyethylene push caps. The tanks were filled slowly (over approx. 1 h) with air, oxygen or nitrogen from large buffer tanks, until the tank pressure reached approx. 18 MPa (atmospheric pressure is about 0·1 MPa). Filling of the tanks with oxygen was performed by skilled staff at a local scuba diver shop (4Divers, Veenendaal, The Netherlands). Tanks filled with pure nitrogen, still contained the initial oxygen content (0·021 MPa partial pressure of oxygen) from the air already present in the tank, as tanks were not flushed before filling. Similarly, tanks filled with oxygen contained atmospheric amounts of nitrogen and other minor gasses present in air. As a control, seed samples were stored in air at atmospheric pressure in 1-L rubber sealed glass jars (Fig. 1B). The oxygen concentrations used in the various storage treatments are listed in Table 1. In each 1·5-L tank and 1-L glass jar, respectively, 200 g and 130 g of dry or moistened silica gel was added to buffer the relative humidity in the containers (Piechota, 1993). In the experiments either 5 % RH (created with dry silica gel) or 35 % RH were used. The first RH creates an ‘ultra-dry’ condition, whereas the latter is more in accordance with commercial seed-storage practice of vegetable seeds.
Fig. 1.

Storage containers used in the experiments. (A) Steel tank with an internal volume of 1·5 L which can be filled with gas to 20 MPa pressure. (B) Glass jar containing air at ambient pressure (0·1 MPa), showing the perforated tubes with seed samples, silica gel for RH buffering and a data logger for recording RH and temperature.
Table 1.
Gas concentrations in the storage tests performed, under the assumption that the air contained 21 % oxygen, 78 % nitrogen and 1 % other gasses
| Storage container | Total pressure | Oxygen | Nitrogen | Other gasses |
|---|---|---|---|---|
| Glass jar | 0·1 MPa | 0·021 MPa | 0·078 MPa | 0·001 MPa |
| Steel tank filled with air | 18·0 MPa | 3·780 MPa | 14·040 MPa | 0·180 MPa |
| Steel tank filled with oxygen | 18·0 MPa | 17·921 MPa | 0·078 MPa | 0·001 MPa |
Tanks and jars containing seed samples were stored at 20 °C for a predefined time period. Unless noted otherwise, the tank pressure was released with a rate of 1 L per minute per tank using an adjustable oxygen valve (Mediline, France). As this rate of pressure release appeared to cause seed coat rupture with the cabbage seeds, in a later experiment the pressure was released at a maximum rate of 5 % per minute using a computer-controlled relative flow rate.
Between experiments and prior to germination testing, seeds were stored in a cabinet with air circulating above a saturated CaCl2 salt solution (RH 35 %) and a temperature of 20 °C. A CD procedure with a mature fraction of cabbage seeds was applied according to Soeda et al. (2005). Briefly, seeds were equilibrated at 85 % RH and 20 °C for 3 d, packaged in sealed laminated foil pouches and subsequently stored for 0, 3, 6, 9 or 12 d at 40 °C. After the CD treatment the seeds were re-dried at 35 % RH.
Germination tests
Unless indicated otherwise, germination was tested within 2 months after EPPO storage. Lettuce, cabbage and barley seeds were incubated on filter paper (T300; Schut, The Netherlands) at 20 °C on a Jacobsen table under 8 h of light. Soybean seeds were rolled between water-saturated blotting paper (Schut) and placed in beakers with the addition of approx. 20 mL water in a climate-controlled cabinet with 8 h light. Unless otherwise indicated, three replicates of 50 cabbage seeds, two replicates of 100 lettuce seeds and two replicates of 50 seeds of barley and soybean were tested. The rate of seed germination was determined by examining radicle protrusion, twice a day at the beginning of the experiment and once a day in the later stages. Seedling quality was evaluated according to standards of the International Seed Testing Association (ISTA, 2012). The proportion of germinated seeds, normal seedlings, the time to reach 50 % of the maximum germination (T50) and the statistical significance were calculated using the software program Germinator (Joosen et al., 2010).
Tocopherol analysis
Tocopherol levels were quantified by HPLC analysis using three replicates (Bino et al., 2005).
RESULTS
Symptoms of warehouse-aged lettuce seeds
Lettuce seeds are known to have a relatively short shelf-life in seed company warehouses. The symptoms of warehouse-aged seeds were analysed with five lettuce seed lots, aged for different periods in a seed-company warehouse (15–20 °C and 30–35 % RH). Germination, as measured by radicle protrusion, occurred more slowly with increasing age of the seed lot. The seedlings from the 1- and 2-year-old seed lots (1Y and 2Y) appeared normal and healthy (Fig. 2A), whereas the 3-year-old lot (lot 3Y) produced only 25 % normal seedlings and the 4-year-old seed lot (lot 4Y) even fewer. Normal seedlings were not observed for the 5-year-old seed lot (5Y). Besides a delay in germination and a reduced total germination, the main symptoms observed for the abnormal lettuce seedlings included reduced elongation of the hypocotyl and cotyledons and necrosis occurring at the mid-vein of the cotyledons (Fig. 2B). In more severe cases, only radicle protrusion and very little embryo growth occurred.
Fig. 2.

Lettuce seedling quality after (A) 2 years (Lot 2Y) or (B) 4 years (Lot 4Y) of warehouse ageing.
Lettuce seed storage under high-pressure oxygen or nitrogen
To analyse the effect of EPPO treatment, dry lettuce seeds of different ages (seed lots 2Y, 3Y, 4Y and 5Y) were stored in tanks filled with high-pressure air, oxygen or nitrogen at 18 MPa for 4 weeks at 20 °C and 35 % RH. Control seeds were stored in glass jars with air at atmospheric pressure at 20 °C and 35 % RH.
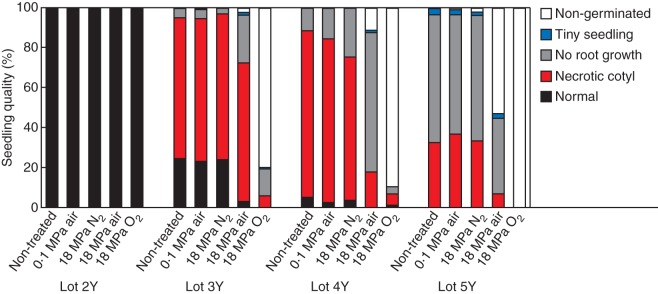
For all four seed lots, seed germination and seedling quality (i.e. normal or abnormal seedlings, non-germinated seeds) was not visibly influenced by storage for 4 weeks at atmospheric pressure in the glass jars or under high-pressure nitrogen, compared with that of the non-treated seeds (Fig. 3 and Supplementary Data Fig. S1). Storage under 18 MPa air (3·8 MPa pO2, partial pressure of O2) showed a slight retardation of germination for seed lot 2Y (time to reach 50 % of maximum germination T50 increased from 28·4 ± 0·4 h for the non-treated seeds to 30·0 ± 0·4 h for the 18 MPa air-stored seeds), but no obvious decline in seedling quality was observed. For lots 3Y, 4Y and 5Y storage under 18 MPa air resulted in delayed germination (from 53·6 ± 4·6 h to 84·6 ± 2·0 h, 31·0 ± 0·6 h to 83·0 ± 2·0 h and 46·6 ± 1·4 h to 118·0 ± 0·7 h, respectively), comparing non-treated seeds and seeds stored under 18 MPa air and a decline in seedling quality. Storage under high-pressure oxygen (18 MPa pO2) delayed the germination of lot 2Y even further (T50 = 35·9 ± 0·7 h) compared with storage under 18 MPa air. It also had a greater deteriorative effect on lots 3Y, 4Y and 5Y, resulting in a further decrease in seedling quality. The main symptoms of deterioration were cotyledons with mid-vein necrosis or lack of root growth (Supplementary Data Fig. S1). Most seeds from lot 4Y and all seeds from lot 5Y failed to show radicle protrusion after storage under 18 MPa pO2, in some cases the pericarp was split revealing a swollen endosperm envelope filled with liquid and a floating embryo. The size of the embryo had not increased, indicating severe damage to its cell membranes.
Fig. 3.
Lettuce seedling quality after storage under various conditions. Initially the seeds had been stored for 2, 3, 4 or 5 years under warehouse conditions (lots 2Y, 3Y, 4Y and 5Y). Subsequently the seeds were stored as indicated, in glass jars containing air at ambient pressure (0·1 MPa Air), in tanks filled with high-pressure nitrogen (18 MPa N2), air (18 MPa Air) or oxygen (18 MPa O2). Seedling quality was determined 10 d after the start of imbibition. Abnormal seedlings were divided over three categories: seedlings with necrotic spots on the cotyledons and growing roots, seedlings without root growth (with or without necrotic spots on the cotyledons) and seedlings where the radicle had protruded, but without further growth. Seeds showing no radicle protrusion were scored as non-germinated.
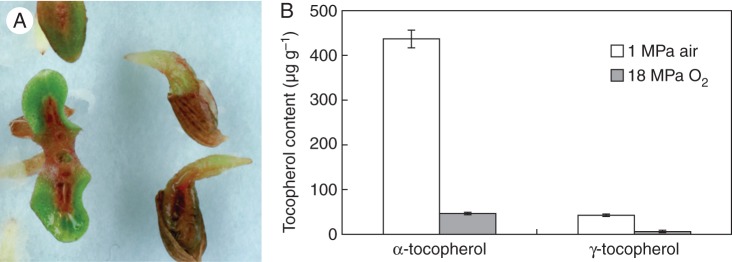
Seed lot 1Y was stored for 7 weeks at 35 % RH under ambient pressure air or 18 MPa pO2 and analysed for tocopherol levels. The 7 weeks EPPO treatment resulted in the induction of severe cotyledon mid-vein necrosis (Fig. 4A), not visible in the control seeds. For the lettuce seeds stored under ambient air pressure α-tocopherol was the main form of these lipophilic anti-oxidants, while γ-tocopherol was present in lower amounts (Fig. 4B). Both tocopherol levels had dropped considerably for seeds stored for 7 weeks under 18 MPa pO2.
Fig. 4.
(A) Abnormal seedlings from 1-year-old lettuce seeds (lot 1Y) treated for 7 weeks at 18-MPa EPPO storage. (B) Tocopherol levels in 1-year-old lettuce seeds subsequently stored for 7 weeks at ambient pressure air or 18-MPa EPPO conditions.
Thus storage of lettuce seeds under EPPO conditions resulted in symptoms comparable to those observed with the lettuce seeds after long-term dry storage in a commercial warehouse: delay in radicle protrusion, reduction of total germination, smaller seedlings, abnormal seedlings with mid-vein necrosis and cell membrane leakage. The effects were more pronounced after 18 MPa pO2 storage compared with 3·8 MPa pO2 storage. Moreover, older seeds were more sensitive to the induction of this damage.
Different durations of EPPO storage for cabbage and soybean seeds
The EPPO treatment was tested with soybean and cabbage seeds held at 5 % RH for 1–4 weeks. For cabbage, samples of different maturity were available. After EPPO storage, some of the cabbage seeds showed rupture of the testa. This was also observed with high-pressure nitrogen-stored cabbage seeds and a similar rate of pressure release (data not presented). The dry soybean seeds showed no morphological differences between EPPO and control treatments.
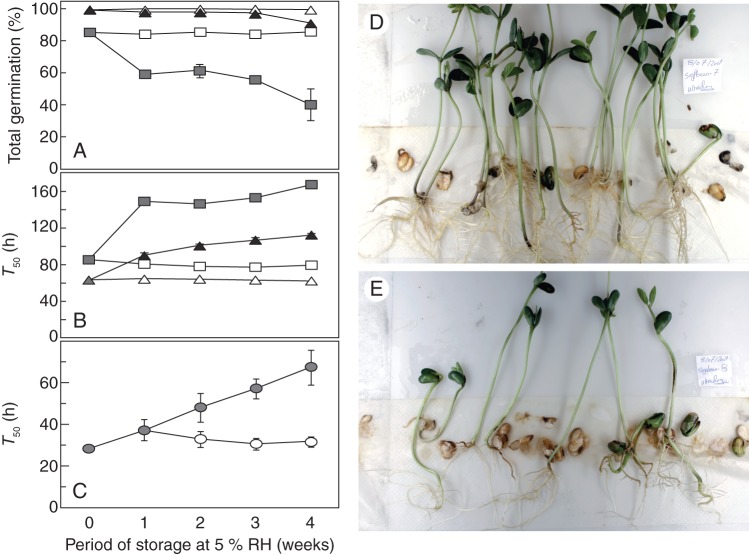
Less-mature cabbage seeds showed a lower total germination and slower germination (higher T50) as compared with mature seeds (Fig. 5A, B) throughout 1–4 weeks rather dry storage at ambient air pressure. Total germination of the less-mature seeds was reduced after 1 week of dry storage at 18 MPa pO2 and declined further with prolonged EPPO storage (Fig. 5A). Total germination of the mature seeds was only reduced after 4 weeks of EPPO storage. The EPPO storage delayed radicle protrusion for both maturity fractions after only 1 week at 18 MPa pO2, and the less-mature seeds were more sensitive compared with the most-mature seeds (Fig. 5B).
Fig. 5.
Effect of cabbage and soybean seed storage at elevated partial pressure of oxygen on germination behaviour and seedling quality. Seeds were stored for 4 weeks at 5 % RH, either at ambient air conditions (open symbols) or in steel tanks with 18 MPa oxygen (closed symbols). (A) Effect on total germination for the most- (triangles) and the less-mature (squares) cabbage seed fractions. (B) Effect of storage conditions and period on the rate of germination (T50) for the two cabbage seed fractions. (C) Effect of storage conditions and period on the speed of germination (T50) for the soybean seeds (circles). (D) Seedling quality of control soybean seeds after 4 weeks of storage under ambient conditions (32 % RH, 20 °C and 0·1 MPa air). (E) Soybean seedling quality after 4 weeks storage at 18 MPa oxygen, 5 % RH and 20 °C. Error bars represent the standard error in the germination assay with three replicates of 50 (cabbage) or 25 (soybean) seeds.
Four weeks of dry storage of soybeans in jars at ambient pressure had little or no effect on the rate of radicle protrusion in the germination assay (Fig. 5C). After dry storage at 18 MPa pO2, the germination rate was clearly reduced (higher T50) and this delay increased with storage duration. Furthermore, seedling quality was considerably reduced by dry storage at 18 MPa pO2 compared with soybean seeds stored at ambient pressure (Fig. 5D, E).
Thus in cabbage and soybean seeds, EPPO ageing also resulted in a delay of the germination, which increased with the duration of the treatment and, in cabbage, less-mature seeds were more sensitive to the EPPO treatment.
Barley seeds under EPPO storage
In general, barley seeds have a longer shelf-life compared with lettuce seeds. It was tested if this is also the case under EPPO storage conditions. Lettuce seed lot 1Y and two barley seed samples were stored for 7 weeks at 18 MPa oxygen and 35 % RH. Seeds stored under ambient-pressure air served as a control. Compared with ambient storage, the EPPO treatment reduced the seed quality of both lettuce and barley seeds, as revealed by reduced total germination, an increased T50 value and a reduced proportion of normal seedlings (Table 2). The quality loss was stronger with the lettuce seeds compared with the barley seeds and there was a difference in response between the two barley seed lots. With the barley seeds the EPPO-induced deterioration was visible as retarded seedling growth (Supplementary Data Fig. S2) and small chlorotic spots on the coleoptiles.
Table 2.
Barley and lettuce seed quality after 7 weeks of storage at either ambient air pressure (0·1 MPa air, 35 % RH, 20 °C) or EPPO (18 MPa oxygen, 35 % RH 20 °C)
| Maximum germination (%) |
T50 (h) |
Normal seedlings (%) |
||||
|---|---|---|---|---|---|---|
| 0·1 MPa air | 18 MPa O2 | 0·1 MPa air | 18 MPa O2 | 0·1 MPa air | 18 MPa O2 | |
| Barley lot A | 99 ± 1 | 64 ± 0 | 60 ± 1 | 131 ± 8 | 99 ± 1 | 8 ± 4 |
| Barley lot B | 100 ± 0 | 88 ± 2 | 58 ± 2 | 121 ± 8 | 100 ± 0 | 49 ± 3 |
| Lettuce lot 1Y | 100 ± 0 | 47 ± 0 | 22 ± 0 | 130 ± 1 | 100 ± 0 | 0 ± 0 |
High-pressure oxygen storage of primed lettuce seeds
Lettuce seeds are frequently primed to break high-temperature dormancy, but the priming treatment reduces the shelf-life under commercial storage conditions. To study the effect of EPPO storage on primed seeds, three levels of hydro-priming treatments were performed with seed lot 1Y and the seeds were stored at 20 °C and 35 % RH under ambient conditions for 2 or 4 weeks at 18 MPa PO2.
Storage under ambient conditions had no influence on seedling quality (Fig. 6). Apart from a retardation of germination, the seedling quality of non-primed seeds or from those primed with the addition of only 0·2 g water per 1 g seeds was also not influenced by the EPPO treatment. However, 18 MPa EPPO-treated seeds from the priming treatments in which 0·4 or 0·6 g water were added per 1 g seeds all produced abnormal seedlings (Fig. 6). Abnormalities included necrosis of both the shoot apical meristem and root meristem, although root hairs still developed on the stunted roots. Only a few seedlings were observed with necrosis on the cotyledons. Thus primed lettuce seeds are more sensitive to EPPO storage and the abnormalities resembled those observed for commercially primed lettuce seeds after prolonged storage at ambient conditions (H. Bruggink, Incotec, NL, pers. comm.).
Fig. 6.

Sensitivity of primed lettuce seeds (lot 1Y) to high-pressure oxygen storage. (A) Seedling morphology of non-primed seeds after ambient storage. (B) Seedling morphology of non-primed seeds after 2 weeks storage at 18 MPa pO2. (C) Seedling morphology of primed seeds (1 g seeds with 0·6 g water) after ambient storage. (D) Seedling morphology of primed seeds after 2 weeks of storage at 18 MPa pO2. The arrows point to lack of root growth.
Comparison of EPPO with CD storage
The EPPO treatment, with rapid ageing under dry conditions, might be an alternative to a CD procedure with storage at higher moisture levels. An initial test was performed with cabbage seed (‘Bartolo’) to compare the effect of both treatments. Two maturity fractions from the cabbage seed lot were subjected both to CD-type (air, 85 % RH and 40 °C) or EPPO (18 MPa pO2, 35 % RH and 20 °C) storage. To prevent testa rupture in this experiment, the high-pressure nitrogen or oxygen was released with a maximum of 5 % relative pressure decline per minute. This modification indeed prevented an increase in the frequency of seeds with testa rupture by the high-pressure storage treatments.
Five weeks of storage under high-pressure nitrogen delayed radicle protrusion, but total germination remained high (Fig. 7A and Supplementary Data Table S1). Storing the seeds under 18 MPa pO2 reduced the frequency of germinating seeds nearly linearly with storage time, resulting in a decline of about 20 % after 5 weeks for the mature seeds and about 43 % for the less-mature seeds. The rate of radicle protrusion was slower for seed treated with EPPO as compared with high-pressure nitrogen or no treatment (Supplementary Data Table S1). Again less-mature seeds were more sensitive to the EPPO treatment compared with mature seeds.
Fig. 7.
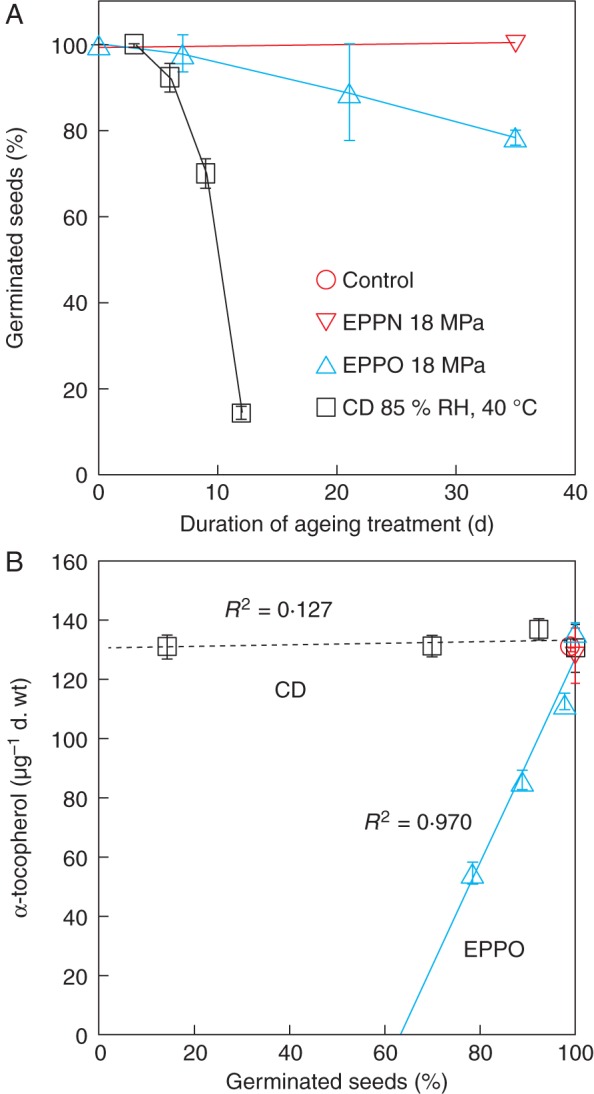
Comparison of cabbage seed ageing under CD-type, 18-MPa O2 (EPPO) or 18-MPa N2 (EPPN) storage conditions. (A) Frequency of germinating seeds in relation to the duration of storage. (B) α-Tocopherol levels in seeds after CD or EPPO storage in relation to germination. Details and data for less-mature seeds are presented in Supplementary Data Table S1.
Under the applied CD conditions the seeds deteriorated rather fast, and after 12 d CD <20 % of the seeds showed radicle protrusion (Fig. 7A and Supplementary Data Table S1). Less-mature seeds were also more sensitive to the CD treatment compared with seeds from the mature fraction.
Cabbage seeds ‘Bartolo’ contained mainly α- and γ-tocopherol, and lower amounts of δ-tocopherol (Supplementary Data Table S1). The α- and γ-tocopherol levels in seeds from the less-mature fraction were about three-quarters of those from the mature seeds. Neither 5 weeks of storage at ambient pressure nor storage under high-pressure nitrogen had any effect on the tocopherol levels (Fig. 7B and Supplementary Data Table S1). CD treatment also did not influence the tocopherol levels, despite a very significant drop in the frequency of germinating seeds after 12 d of CD treatment. However, both α- and γ-tocopherol levels declined markedly after EPPO-storage treatment, showing for both maturity fractions a linear relationship with the decline in the frequency of germinating seeds (Fig. 7B and Supplementary Data Table S1).
Laboratory bench-aged seeds
After detecting differences in tocopherol degradation between CD-stored and EPPO-stored cabbage seeds, tocopherol levels were investigated for seeds from three other seed lots that had undergone a slow ageing treatment at the laboratory bench of the seed company. During 3 years laboratory-bench storage, total germination had slightly declined only for the seeds from seed lot 1 (Supplementary Data Table S2). For the seeds from lot 1 and lot 2, the germination rate had dropped after 3 years of laboratory-bench storage, but no loss in germination rate was observed for lot 3. The three seed lots, originating from different varieties, differed in their tocopherol content (Supplementary Data table S2). Lot 1 mainly contained α-tocopherol, whereas for lot 2 and lot 3 it was mainly γ-tocopherol. Minor amounts of δ-tocopherol were observed in seeds from all three lots. None of the seed lots showed a significant decline in tocopherol contents after 3 years of laboratory-bench storage as compared with 5 months of laboratory-bench storage followed by 31 months of storage at –20 °C.
DISCUSSION
Seed longevity is important to seed companies and gene banks and there is a need to identify seed lots with poor shelf-life. Presently, most often tests to predict shelf-life are based on storing seeds at an elevated moisture level and temperature. We tested if seed ageing can also be analysed by storing seeds dry and at normal temperatures, but under an elevated pressure of oxygen. Our experiments confirm that an increase in oxygen concentration can also accelerate the rate of seed deterioration and we show that the method can be applied with dry seeds of several non-related species. A few weeks of EPPO storage of dry lettuce seeds mimicked the morphological ageing symptoms observed after storage in seed company warehouses (Figs 2, 4 and 6 and Supplementary Data Fig. S1). For barley, lettuce and soybean seeds, the observed relative sensitivity to deterioration under EPPO conditions is in agreement with their observed relative shelf-life during storage in warehouses or gene banks (Walters et al., 2005; Nagel and Börner, 2010). Cabbage seeds were not considered in this comparison, since these seeds had already been stored in our laboratory for about 10 years at 5 °C before performing the EPPO- and CD-storage experiments.
Similarly the faster ageing of primed lettuce seeds under ambient conditions was mimicked by a relative rapid deterioration under EPPO conditions, resulting in symptoms of root and shoot meristem abnormalities that are typical for primed lettuce seeds that have undergone ageing (Fig. 6). The relationship between seed maturity and seed longevity (Hay and Probert, 1995; Jalink et al., 1998) was also successfully reproduced by the EPPO experiments (Fig. 5 and Supplementary Data Table S1).
Gas pressure
The EPPO treatment offers a novel tool to study dry seed ageing, especially those processes that are influenced by oxygen. Storage under high-pressure nitrogen can be performed as a control for potential artefacts induced by high pressure or its release. Dry seeds may contain air-filled spaces, as has been demonstrated by X-ray for primed tomato seeds (van der Burg et al., 1994). Quantitative phase tomography with dry arabidopsis seeds has shown the presence of intercellular air channels in the embryonic tissues (Cloetens et al., 2006). As arabidopsis and brassica crops are taxonomically closely related within the family of Cruciferae, cabbage seeds may be expected also to contain such air channels. If these cavities are filled with gasses during the high-pressure storage, expansion will occur upon a reduction in pressure (Boyle's law). This may explain the observed occurrence of seed-coat cracking of cabbage seeds after a fast relative decrease in pressure, which was not observed for the barley and soybean seeds. To prevent this physical damage a computer-controlled pressure-release system was developed that regulates a relative pressure release. When the relative pressure release was at a maximum of 5 % per minute, no additional seed-coat cracking occurred for cabbage seeds that had been stored under 18 MPa oxygen or nitrogen gas. This, however, does not exclude minor internal fractures, which may be responsible for the delay in radicle protrusion still observed for the high-pressure nitrogen-stored cabbage seeds (Supplementary Data Table S1). To avoid artificial effects unrelated to seed ageing, for seeds with air cavities the relative pressure decrease can be reduced further with the device developed.
One other issue concerning the EPPO system is whether the increased pressure influences the glass-phase transition temperature. If the thermodynamic behaviour of synthetic ionic liquids can be compared with natural deep eutectic solvents in seeds, as suggested by Choi et al. (2011), the pressure effect on the melting point (or glass-phase transition) will probably not be very large. For the synthetic ionic liquids 1-ethyl-3-methylimidazolium tosylate and 1,3-dimethylimidazolium methylsulfate, a pressure increase of 20 MPa increases the melting point with, respectively, 5 °K or 4 °K [calculated from data presented in Domanska and Morawski 2007)].
Partial gas pressure
According to the gas law formulated in 1801 by John Dalton, the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture. The pO2 under atmospheric conditions is about 0·021 MPa. This pO2 can be increased by either increasing the relative amount of oxygen or by increasing the total pressure of a gas mixture (e.g. air) that contains oxygen. According to the physics gas law formulated by William Henry in 1803, the amount of a gas in a liquid at a constant temperature is directly proportional to the partial pressure of the gas above the liquid. Therefore the internal oxygen concentration in dry seeds will reach equilibrium with the storage atmosphere. When storing seeds under pure oxygen at atmospheric pressure (0·1 MPa pO2), the oxygen concentration in the seeds is almost 5-fold compared with storing in air. If the rate of seed deterioration is at least for a part proportional to the partial pressure of oxygen, then the rate of ageing should be accelerated by increasing the oxygen concentration, or decreased by reducing it. The latter has been observed by Roberts (1961) and Shrestha et al. (1985). Studies on food lipid oxidation, performed at relative ambient or lower partial pressures, indicate that the effect of pO2 on lipid oxidation differs with the pressure. Milk lipid and soybean oil oxidation declines upon reduction of the headspace partial pressure of oxygen (Kacyn et al., 1983; Koelsch et al., 1991).
Dry seeds are a much more complex system compared with oil or aqueous solutions. Apart from lipids, phospholipids, nucleic acids, proteins and other molecules are also oxidized during seed ageing. Oxygen is much more soluble in non-polar lipids than in aqueous systems (Min and Boff, 2002). Moreover, seeds contain different types of anti-oxidant molecules which are not homogeneously distributed and which will decline in activity during prolonged storage in the presence of oxygen. Therefore, one should be cautious about making direct extrapolations based on observations with aqueous test systems or vegetable oils.
Comparison with CD ageing procedures
Both dry EPPO and relatively moist CD-type storage resulted in seed deterioration, progressing with the duration of the treatment. However, differences were observed in the effects on tocopherol levels. Tocopherols are lipid-soluble anti-oxidants that are important to protect the phospholipids in cell and organelle membranes and are essential for longevity of dry arabidopsis seeds (Sattler et al., 2004). Basically no decline in tocopherol levels were observed for cabbage seeds stored under CD type of conditions (Fig. 7 and Supplementary Data Table S1), whereas tocopherol levels declined both with dry lettuce and cabbage seeds stored under high-pressure oxygen conditions (Figs 4 and 7). The observation that tocopherol levels were not reduced after CD type of storage does not exclude the possibility that oxidation of tocopherols had occurred, since oxidized tocopherols may have been regenerated. In animal systems, tocopherol is regenerated by the interaction with vitamin C at the interface of the cell membrane and the water phase, maintaining vitamin E levels in tissues (Packer et al., 1979; Nagaoka et al., 2007). Another explanation for the absence of a decline in tocopherol levels during CD-type ageing can be that, at high moisture contents, water can act as a buffer between oxidation-generated free radicles and target molecules. This possibility was suggested by Murthy et al. (2003) who showed for mung bean seeds that the rate of lipid peroxidation also varies with the moisture level.
Following storage at the laboratory bench (temperatures between 15–22 °C and unknown RH) cabbage seeds showed no or, at most, a limited decline in tocopherol levels. Again the RH conditions might have been (temporally) high enough during the storage or processing to allow recuperation of oxidized tocopherol.
In the scientific literature there are contrasting reports about seed-storage effects on tocopherol levels. In soybean seeds, for example, no change in the tocopherol levels was observed in comparing long-term (‘cool and dry’) stored seeds and seeds that had been exposed to high temperature and humidity (accelerated ageing at 100 % RH and 40 °C) (Priestley et al., 1980). However, beech seeds stored dry in sealed laminated aluminium foil bags at −10 °C showed a decline in tocopherol levels after several years of storage (Pukacka and Ratajczak, 2007). Another hypothesis is that the activity of tocopherol synthesis is influenced by water activity of the seeds during storage. Under dry storage conditions, including the EPPO treatments in this paper, no enzyme activity would be expected, whereas it cannot be excluded that some synthetic enzyme activity may occur under the more moist and warm CD-type storage conditions.
In addition to water activity in the seeds, temperature may also have an effect on tocopherol degradation or regeneration. More research is needed in this area and our data show that it is important to take the temperature, water activity and oxygen availability into account. All three parameters should constantly be monitored during the experiment and sample preparation. With Digitalis purpurea seeds, a temporary increase in seed moisture level by polyethylene glycol priming or short storage in a humid atmosphere was found to increase subsequent shelf-life (Butler et al., 2009).
All together these data support the idea that tests to predict the shelf-life of seeds should ideally be performed at the seed moisture level, at which they are aimed to be stored and moisture levels should remain as such during the entire experiment.
Comparison with ‘natural’ ageing
It has been questioned to what extent EPPO storage mimics long-term ‘natural’ ageing of dry seeds. To answer this question ‘natural’ and ‘dry’ need to be defined. As indicated in the introduction, ‘natural ‘storage conditions differ considerably for seeds in gene banks, high-value horticultural seeds, for seeds from tropical field crops or for seeds in the soil bank. Most often the exact storage history of ‘naturally’ aged seed samples is unknown.
Given the long seed storage potential of many plant species, experimental time frames need to be shortened artificially in order to study ageing processes and components providing tolerance to ageing. Compared with other artificial seed ageing procedures, EPPO storage offers the advantage that these processes can also be studied at low seed moisture levels and ambient temperatures and which can be changed accordingly between different experiments. EPPO storage experiments performed with morphologically distinct types of seeds of barley, cabbage, lettuce and soybean, showed that the experimental time frame is well within the limits of most research projects and commercial applications. Comparable results have also been obtained for arabidopsis, pepper, pumpkin and tomato (results not shown).
Reduced ageing under anoxia
An increase in oxygen concentration during storage hastens the deterioration of seeds. As cited before, several studies have shown that storing seeds under reduced or zero oxygen levels can prolong their shelf-life. Although this will not provide a full alternative for storing at low temperatures, zero oxygen levels may be an additional tool to prolong longevity of stored seeds. With seed lots exhibiting a relatively poor shelf-life, either due to genetic variation or seed treatments, it may be worthwhile storing the seeds under low oxygen levels, once they are dried, in order to improve their shelf-life.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The seed companies Rijk Zwaan and Bejo Zaden are acknowledged for kindly providing lettuce and cabbage seed samples and Lex Schotema from 4Divers Veenendaal for technical advice. Fiona Hay (International Rice Research Institute, Philippines) and Rob van Treuren (Centre for Genetic Resources, the Netherlands) are acknowledged for their stimulating discussions and critical comments on the draft manuscript. The work described in this study was carried out in the framework of the Fundamental Research Program on Sustainable Agriculture (KB-12-005·03-004) funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation. R.C.H.d.V. acknowledges the Centre for Biosystems Genomics, which is part of the Netherlands Genomics Initiative/Netherlands Organization of Scientific Research, for additional funding.
LITERATURE CITED
- Abdalla FH, Roberts EH. Effects of temperature, moisture, and oxygen on the induction of chromosome damage in seeds of barley, broad beans, and peas during storage. Annals of Botany. 1968;32:119–136. [Google Scholar]
- Bailly C. Active oxygen species and antioxidants in seed biology. Seed Science Research. 2004;14:93–107. [Google Scholar]
- Berg CC, Nilan RA, Konzak CF. The effect of pressure and seed water content on the mutagenic action of oxygen in barley seeds. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1965;2:263–273. doi: 10.1016/0027-5107(65)90037-0. [DOI] [PubMed] [Google Scholar]
- Bino R, De Vos C, Lieberman M, et al. The light-hyperresponsive high pigment-2dg mutation of tomato: alterations in the fruit metabolome. New Phytologist. 2005;166:427–438. doi: 10.1111/j.1469-8137.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- Buitink J, Leprince O. Intracellular glasses and seed survival in the dry state. Comptes Rendus Biologies. 2008;331:788–795. doi: 10.1016/j.crvi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- van der Burg WJ, Aartse JW, van Zwol RA, Jalink H, Bino RJ. Predicting tomato seedling morphology by X-ray analysis of seeds. Journal of the American Society for Horticultural Science. 1994;119:258–263. [Google Scholar]
- Butler LH, Hay FR, Ellis RH, Smith RD, Murray TB. Priming and re-drying improve the survival of mature seeds of Digitalis purpurea during storage. Annals of Botany. 2009;103:1261–1270. doi: 10.1093/aob/mcp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, van Spronsen J, Dai Y, et al. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiology. 2011;156:1701–1705. doi: 10.1104/pp.111.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloetens P, Mache R, Schlenker M, Lerbs-Mache S. Quantitative phase tomography of Arabidopsis seeds reveals intercellular void network. Proceedings of the National Academy of Sciences of the USA. 2006;103:14626–14630. doi: 10.1073/pnas.0603490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromarty AS, Ellis RH, Roberts EH. The design of seed storage facilities for genetic conservation. Rome, Italy: International Plant Genetic Resources Institute; 1982 (revised 1990) [Google Scholar]
- Demir I, Mavi K. Controlled deterioration and accelerated aging tests to estimate the relative storage potential of cucurbit seed lots. Hortscience. 2008;43:1544–1548. [Google Scholar]
- Domanska U, Morawski P. Influence of high pressure on solubility of ionic liquids: experimental data and correlation. Green Chemistry. 2007;9:361–368. [Google Scholar]
- Ehrenberg L, Moutschen-Dahmen J, Moutschen- Dahmen M. Aberrations chromosomiques produites dans des graines par de hautes pressions d'oxygène. Acta Chemica Scandinavica. 1957;11:1428–1429. [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Annals of Botany. 1980;45:13–30. [Google Scholar]
- González-Benito ME, Pérez-García F, Tejeda G, Gómez-Campo C. Effect of the gaseous environment and water content on seed viability of four Brassicaceae species after 36 years storage. Seed Science and Technology. 2011;39:443–451. [Google Scholar]
- Harrison BJ, McLeish J. Abnormalities of stored seeds. Nature. 1954;173:593–594. [Google Scholar]
- Hay FR, Probert RJ. Seed maturity and the effects of different drying conditions on desiccation tolerance and seed longevity in foxglove (Digitalis purpurea L.) Annals of Botany. 1995;76:639–647. [Google Scholar]
- ISTA. International rules for seed testing. 2012 edn. Bassersdorf, Switzerland: The International Seed testing Association (ISTA); 2012. [Google Scholar]
- Jalink H, van der Schoor R, Frandas A, van Pijlen JG, Bino RJ. Chlorophyll fluorescence of Brassica oleracea seeds as a non-destructive marker for seed maturity and seed performance. Seed Science Research. 1998;8:437–443. [Google Scholar]
- Joosen RVL, Kodde J, Willems LAJ, Ligterink W, Van Der Plas LHW, Hilhorst HWM. Germinator: a software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. The Plant Journal. 2010;62:148–159. doi: 10.1111/j.1365-313X.2009.04116.x. [DOI] [PubMed] [Google Scholar]
- Justin OL, Bass LN. US Dept of Agriculture, Science and Education Administration. Principles and practices of seed storage. 1978 [Google Scholar]
- Kacyn LJ, Saguy I, Karel M. Kinetics of oxidation of dehydrated food at low oxygen pressures. Journal of Food Processing and Preservation. 1983;7:161–178. [Google Scholar]
- Kibinza S, Vinel D, Côme D, Bailly C, Corbineau F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiologia Plantarum. 2006;128:496–506. [Google Scholar]
- Koelsch CM, Downes TW, Labuza TP. Hexanal formation via lipid oxidation as a function of oxygen concentration: measurement and kinetics. Journal of Food Science. 1991;56:816–820. [Google Scholar]
- Kronstad WE, Nilan RA, Konzak CF. Mutagenic effect of oxygen on barley seeds. Science. 1959;129:1618. doi: 10.1126/science.129.3363.1618. [DOI] [PubMed] [Google Scholar]
- Labuza TP. Kinetics of lipid oxidation in foods. CRC Critical Reviews in Food Science and Technology. 1971;2:355–405. [Google Scholar]
- McDonald MB. Seed deterioration: physiology, repair and assessment. Seed Science and Technology. 1999;27:177–237. [Google Scholar]
- Min DB, Boff JM. Lipid oxidation of edible oil. In: Akoh CC, Min DB, editors. Food lipids: chemistry, nutrition, and biotechnology. 2nd. New York. NY: Marcel Dekker; 2002. pp. 335–363. [Google Scholar]
- Moutschen-Dahmen M, Moutschen J, Ehrenberg L. Chromosome disturbances and mutation produced in plant seeds by oxygen at high pressures. Hereditas. 1959;45:230–244. [Google Scholar]
- Murthy UMN, Kumar PP, Sun WQ. Mechanisms of seed ageing under different storage conditions for Vigna radiata (L.) Wilczek: lipid peroxidation, sugar hydrolysis, Maillard reactions and their relationship to glass state transition. Journal of Experimental Botany. 2003;54:1057–1067. doi: 10.1093/jxb/erg092. [DOI] [PubMed] [Google Scholar]
- Nagaoka S-i, Kakiuchi T, Ohara K, Mukai K. Kinetics of the reaction by which natural vitamin E is regenerated by vitamin C. Chemistry and Physics of Lipids. 2007;146:26–32. doi: 10.1016/j.chemphyslip.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Nagel M, Börner A. The longevity of crop seeds stored under ambient conditions. Seed Science Research. 2010;20:1–12. [Google Scholar]
- Niedzielski M, Walters C, Luczak W, Hill LM, Wheeler LJ, Puchalski J. Assessment of variation in seed longevity within rye, wheat and the intergeneric hybrid triticale. Seed Science Research. 2009;19:213–224. [Google Scholar]
- Ohlrogge JB, Kernan TP. Oxygen-dependent ageing of seeds. Plant Physiology. 1982;70:791–794. doi: 10.1104/pp.70.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne DJ. DNA and desiccation tolerance. Seed Science Research. 1994;4:175–185. [Google Scholar]
- Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278:737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- Piechota D. Relative humidity control in cases: buffered silica gel versus saturated salt solutions1. WAAC Newsletter. 1993;15:19–21. [Google Scholar]
- Powell AA, Harman GE. Absence of a consistent association of changes in membranal lipids with the aging of pea-seeds. Seed Science and Technology. 1985;13:659–667. [Google Scholar]
- Powell AA, Matthews S. Evaluation of controlled deterioration, a new vigour test for small seeded vegetables. Seed Science and Technology. 1981;9:633–640. [Google Scholar]
- Priestley DA, McBride MB, Leopold AC. Tocopherol and organic free radical levels in soybean seeds during natural and accelerated ageing. Plant Physiology. 1980;66:715–719. doi: 10.1104/pp.66.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Dapena P, Castaño R, Almoguera C, Jordano J. Improved resistance to controlled deterioration in transgenic seeds. Plant Physiology. 2006;142:1102–1112. doi: 10.1104/pp.106.087817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert RJ, Daws MI, Hay FR. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany. 2009;104:57–69. doi: 10.1093/aob/mcp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukacka S, Ratajczak E. Age-related biochemical changes during storage of beech (Fagus sylvatica L.) seeds. Seed Science Research. 2007;17:45–53. [Google Scholar]
- Rajjou L, Debeaujon I. Seed longevity: survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biologies. 2008;331:796–805. doi: 10.1016/j.crvi.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Rajjou L, Lovigny Y, Groot SPC, Belghazi M, Job C, Job D. Proteome-wide characterization of seed aging in Arabidopsis: a comparison between artificial and natural aging protocols. Plant Physiology. 2008;148:620–641. doi: 10.1104/pp.108.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EH. The viability of rice seed in relation to temperature, moisture content, and gaseous environment. Annals of Botany. 1961;25:381–390. [Google Scholar]
- Roos YH. Phase transitions and structure of solid food matrices. Current Opinion in Colloid & Interface Science. 1998;3:651–656. [Google Scholar]
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. The Plant Cell. 2004;16:1419–1432. doi: 10.1105/tpc.021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwember AR, Bradford KJ. Quantitative trait loci associated with longevity of lettuce seeds under conventional and controlled deterioration storage conditions. Journal of Experimental Botany. 2010;61:4423–4436. doi: 10.1093/jxb/erq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwember AR, Bradford KJ. Oxygen interacts with priming, moisture content and temperature to affect the longevity of lettuce and onion seeds. Seed Science Research. 2011;21:175–185. [Google Scholar]
- Shejbal J. Preservation of cereal grains in nitrogen atmospheres. Resource Recovery and Conservation. 1979;4:13–29. [Google Scholar]
- Shrestha KB, Shepherd KR, Turnbill JW. Controlled atmosphere storage for Pinus radiata seed. Commonwealth Forestry Review. 1985;64:141–150. [Google Scholar]
- Soeda Y, Konings MC, Vorst O, et al. Gene expression programs during Brassica oleracea seed maturation, osmopriming, and germination are indicators of progression of the germination process and the stress tolerance level. Plant Physiology. 2005;137:354–368. doi: 10.1104/pp.104.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WQ. Glassy state and seed storage stability: the WLF kinetics of seed viability loss at T>Tg and the plasticization effect of water on storage stability. Annals of Botany. 1997;79:291–297. [Google Scholar]
- Tesnier K, Strookman-Donkers HM, et al. A controlled deterioration test for Arabidopsis thaliana reveals genetic variation in seed quality. Seed Science and Technology. 2002;30:149–165. [Google Scholar]
- Walters C, Engels J. The effects of storing seeds under extremely dry conditions. Seed Science Research. 1998;8(Suppl. 1):3–8. [Google Scholar]
- Walters C, Wheeler LM, Grotenhuis JM. Longevity of seeds stored in a genebank: species characteristics. Seed Science Research. 2005;15:1–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.