Abstract
Neurons preconditioned with non-injurious hypoxia or the anesthetic isoflurane express different genes but are equally protected against severe hypoxia/ischemia. We hypothesized that neuroprotection would be augmented when preconditioning with isoflurane and hypoxic preconditioning are combined. We also tested if preconditioning requires intracellular Ca2+ and the inositol triphosphate receptor, and if gene expression is similar in single agent and combined preconditioning. Hippocampal slice cultures prepared from 9 day-old rats were preconditioned with hypoxia (95% N2, 5% CO2 for 15 min, HPC), 1% isoflurane for 15 min (APC) or their combination (CPC) for 15 min. A day later cultures were deprived of O2 and glucose (OGD) to produce neuronal injury. Cell death was assessed 48 hr after OGD. mRNA encoding 119 signal transduction genes was quantified with cDNA micro arrays. Intracellular Ca2+ in CA1 region was measured with fura-2 during preconditioning. The cell-permeable Ca2+ buffer BAPTA-AM, the IP3 receptor antagonist Xestospongin C and RNA silencing were used to investigate preconditioning mechanisms. CPC decreased CA1, CA3 and dentate region death by 64–86% following OGD, more than HPC or APC alone (P<0.01). Gene expression following CPC was an amalgam of gene expression in HPC and APC, with simultaneous increases in growth/development and survival/apoptosis regulation genes. Intracellular Ca2+ chelation and RNA silencing of IP3 receptors prevented preconditioning neuroprotection and gene responses. We conclude that combined isoflurane-hypoxia preconditioning augments neuroprotection compared to single agents in immature rat hippocampal slice cultures. The mechanism involves genes for growth, development, apoptosis regulation and cell survival as well as IP3 receptors and intracellular Ca2+.
1. Introduction
Preconditioning is a potent method of inducing ischemic tolerance in the brain and other tissues that involves a non-injurious stress (mild hypoxia, brief ischemia, etc.) that induces resistance to injurious ischemia. Ischemic preconditioning can be induced by a wide variety of stimuli and has been demonstrated in both in intact animals and in cells and tissues in culture. Cerebral preconditioning was first demonstrated with hypoxia (Dahl and Balfour, 1964; Gidday et al., 1994) and later with heat stress, excitotoxins such as glutamate, bacterial endotoxins, oxidative stress, volatile anesthetics and neuromodulators such as adenosine and nerve growth factors (Chopp et al., 1989; Gidday, 2006; Shpargel et al., 2008; Simon et al., 2007). Preconditioning can be achieved in heart, kidneys, lever and other organs. Isoflurane preconditioning of the myocardium is effective in humans (Landoni et al., 2008) but cerebral protection with isoflurane or hypoxia remains an experimental procedure that has not yet been tested in human clinical trials.
Preconditioning involves changes in gene expression. The phenotype of preconditioned brain includes up-regulated stress response genes, intracellular signaling genes, hypoxia-inducible genes, and others (Shpargel et al., 2008; Simon et al., 2007). The specific pattern of gene expression depends on the type of preconditioning stimulus. For example, the volatile anesthetic isoflurane increases genes regulating growth and development, while hypoxic preconditioning increases expression of genes involved in the regulation of cell survival and apoptosis (Bickler and Fahlman, 2009). Differences in gene expression with isoflurane and ischemic preconditioning have also been described in the myocardium (da Silva et al., 2004).
Combinations of preconditioning agents may produce greater neuroprotection than single agents, have fewer side effects and require briefer periods for induction of preconditioning protection. However, few studies have tested combinations of preconditioning agents. Studies of combined hypoxic and anesthetic preconditioning have involved the heart but rarely the brain (Inoue et al., 2004; Inoue et al., 2006). The purpose of this study was to determine, in a rat hippocampal slice model, if combined hypoxic and isoflurane preconditioning is more neuroprotective than either type of preconditioning alone. We also wished to define differences in the pattern of gene expression in single-agent and combined preconditioning. Because increased intracellular calcium is an upstream signal common to different types of preconditioning(Bickler and Fahlman, 2004), we measured intracellular calcium ([Ca2+]i) in cells in the CA1 region during preconditioning. Further, we tested whether preventing increases in [Ca2+]i with a cell permeable Ca2+ buffer (BAPTA-AM) prevents preconditioning neuroprotection in this cellular region. In addition, because endoplasmic reticulum IP3 receptors are responsible for the increase in cytosolic Ca2+ required for induction of neuroprotection in both isoflurane and hypoxic preconditioning (Gray et al., 2005), we tested if this is also true for combined preconditioning by using siRNA against the IP3 receptor.
2. Results
Survival and intracellular Ca2+ after preconditioning
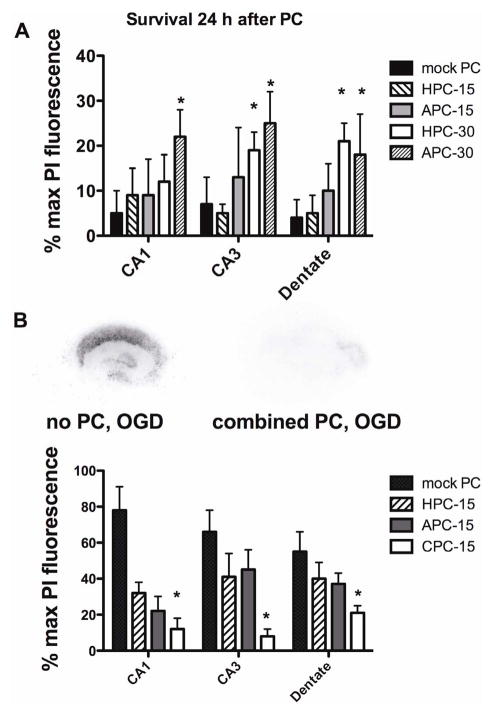
The effects of preconditioning duration on cell death in CA1, CA3 and dentate regions was evaluated following hypoxic (HPC) and isoflurane preconditioning (APC). Fifteen min of either preconditioning agent did not increase cell death 24 hr later, but 30 min of APC increased death in all cell regions and 30 min of HPC increased death in CA3 and dentate (Fig. 1, panel A). Accordingly, a preconditioning duration of 15 min was used for the remaining studies.
Figure 1.
Panel A Effects of hypoxic and isoflurane preconditioning duration on cell death 24 hrs later. HPC is hypoxic preconditioning 15 min, APC-10 is 1% isoflurane for 15 min and HPC-30 and APC-30 are 30 min duration preconditioning treatments. * indicates significant increase in PI fluorescence compared to mock PC group. Panel B. The combination of hypoxic preconditioning (HPC) and anesthetic preconditioning with 1% isoflurane (APC) decreases cell death in hippocampal slice cultures exposed to oxygen/glucose deprivation (OGD) in CA1, CA3 and dentate more than HPC or APC alone. * indicates significant difference compared to HPC and APC groups, with 12–18 observations in each group. Images of PI fluorescence in mock preconditioned and combination preconditioned slice cultures are also presented.
Preconditioning with a combination of isoflurane and hypoxia (CPC) for 15 min yielded greater neuroprotection than did 15 min of HPC or APC alone (Fig. 1, Panel B). This protection was observed in all cell regions (CA1, CA3 and dentate) of the cultures.
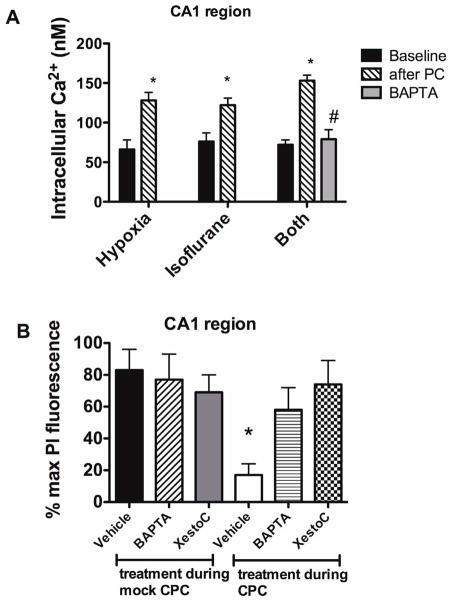
Increases in intracellular Ca2+ in CA1 neurons of approximately 50 nM above pre-exposure baseline were observed with APC and HPC. A moderately larger increase (79±13 nM, p<0.05 compared to APC and HPC) was found with combined preconditioning (Fig. 2 panel A). When added to the cultures 30 min before preconditioning, the cell-permeable Ca2+ buffer BAPTA-AM prevented the increase in [Ca2+]i.
Figure 2.
Panel A Intracellular Ca2+ concentration in CA1 region cells at baseline and at the completion of preconditioning. * indicates significant increase compared to baseline, # indicates significant difference compared to HPC and APC groups. N=6–8 observations in each group. Panel B. Chelation of intracellular Ca2+ changes and antagonism of IP3 receptors with Xestospongin C (XestoC) during combined hypoxia and isoflurane preconditioning (CPC) prevents neuroprotection. Slice cultures were treated with BAPTA-AM (25 μM) or Xestospongin C (10 μM) for 30 min before and during the CPC. Cell death was assessed 24 hours after oxygen glucose deprivation, which was 24 hr after the preconditioning. * indicates significant reduction in cell death compared to mock preconditioning groups.
Ca2+ and IP3 receptors are involved in the mechanisms of combined preconditioning
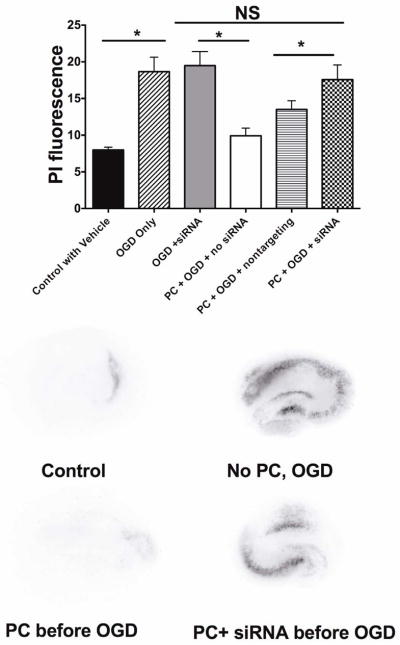
Fig. 2, panel B shows that chelation of intracellular Ca2+ with BAPTA-AM and antagonism of IP3 receptors with Xestospongin C during CPC prevents neuroprotection. The reagents used, BAPTA-AM and Xestospongin C, had no effect on survival when present during a mock preconditioning protocol. Further, slice cultures treated with RNA silencing reagents directed against IP3 receptors were not protected against OGD by preconditioning with a combination of isoflurane and hypoxia (Fig. 3). The RNA silencing protocol resulted in a ~90% decrease in ITPR1 mRNA levels at the time of preconditioning, as determined by quantitative PCR.
Figure 3.
RNA interference against IP3 receptor gene expression prevents a combination of hypoxia and isoflurane from preconditioning hippocampal slice cultures. Each group represents mean PI fluorescence intensity (a measure of cell death) and SDs for 12–16 cultures. Examples of PI fluorescence from the different treatment groups are also shown.
Patterns of gene expression following preconditioning
Table 1 presents the fold-changes in the expression of signal transduction genes 24 hr following HPC, APC, CPC and CPC with Ca2+ chelation pre-treatment. In this table, only genes exhibiting significant changes in expression (1.5 fold increase or decrease) following one of the types of preconditioning are included. A total of 40 of 119 genes on the array were significantly increased or decreased by at least one type of preconditioning. Of these, 30 genes were significantly changed following CPC, 23 by HPC and 25 by APC.
Table 1.
Changes in expression of signal transduction genes following hypoxic (HPC), isoflurane (APC) and combined preconditioning in hippocampal slice cultures. + or − indicates at least 1.5 fold increase or decrease in gene expression compared to mock preconditioned cultures. The fold increase was calculated as the mean fold change in 3–5 separate array experiments. The CPC + BAPTA column refers to cultures pre-treated with the cell-permeant Ca2+ chelator BAPTA-AM before combined preconditioning with hypoxia and isoflurane.
| HPC | APC | Combined (CPC) | CPC+BAPTA | |
|---|---|---|---|---|
| Survival and Apoptosis | ||||
| Bax | + | + | + | |
| Bcl2 | + | + | ||
| Bcl2a1 | + | |||
| Bcl2L1 | − | |||
| Cebpb | + | + | ||
| Fos | + | |||
| Fn1 | + | + | ||
| Mdm2 | + | + | + | |
| P53 | + | + | ||
| Birc3 | + | − | + | |
| Ppia | + | + | ||
| Tank | + | + | ||
| Growth and Development | ||||
| Atf2 | − | |||
| Egr | − | + | + | |
| Pten | − | + | + | |
| Rpl32 | + | + | ||
| Cdkn1a | + | + | ||
| Ccnd1 | + | + | ||
| Igfbp3 | + | + | ||
| Myc | + | + | + | + |
| Bmp4 | + | |||
| Gadd45a | + | + | ||
| Rbp1 | + | |||
| Rbp2 | + | |||
| Irf1 | + | + | ||
| Egfr | + | + | ||
| Signal Transduction | ||||
| Ccl2 | + | + | + | |
| Prkcb1 | + | + | ||
| Pkce | + | + | + | |
| Il4r | + | + | + | |
| Jun | + | |||
| Vcam | + | + | ||
| Odc1 | + | + | ||
| Stress | ||||
| Response | ||||
| Nfkb1 | + | + | + | |
| Nfkb1a | + | |||
| Ptgs2 | + | + | + | |
| Hsf1 | + | + | + | |
| Hspb1 | + | + | ||
| Hspca | + | + | ||
| Hspcal3 | + | + | + | |
HPC induced genes regulating apoptosis and survival, whereas APC increased the expression of genes regulating growth and development. Of 12 apoptosis or cell survival-associated genes showing a significant change in expression following at least one type of preconditioning, HPC increased 10 genes compared to control. In contrast, APC increased the expression of only 2 genes in the apoptosis/survival regulation group (Bax and Mdm2), and decreases in one (Birc3).
Growth and development genes were preferentially increased by APC. Fourteen genes in this category were changed by preconditioning, with 12 of the 14 genes increasing following APC, and only 3 following HPC. Combined preconditioning increased both survival/apoptosis regulation and growth/development groups of genes.
Genes in the intracellular signaling and stress response groups were activated by both HPC and APC. Many of both gene groups were activated with CPC; that is, with CPC the pattern of gene response was generally the additive of HPC and APC expression patterns.
Treatment of slice cultures with BAPTA-AM before preconditioning prevented the increased expression of mRNA of almost all genes normally increased with preconditioning. Only Ptgs2 and Myc were still increased in these cultures.
Quantitative PCR was used to validate the gene array methodology for a genes in several different groups. Table 2 demonstrates good general agreement between the qPCR and array methods.
Table 2.
Comparison of microarray and quantitative polymerase chain reaction (PCR) data. Data are fold-changes (means±SE) in mRNA levels in gene arrays (n=6 data sets) and in quantitative PCR (n=4) of genes from apoptosis regulation (Birc-3), cell division and differentiation pathways (Myc) and cell activation signaling (Jun). Quantitative PCR fold-change is expressed relative to actin gene mRNA, with fold change calculated as 2ΔΔCt.
| Fold Change in Microarray
|
Fold Change in qPCR
|
|||||
|---|---|---|---|---|---|---|
| Gene | Hypoxia | Isoflurane | Combined | Hypoxia | Isoflurane | Combined |
| Birc-3 | 3.7±1.9 | −5.2±6.1 | 4.3±0.9 | 6.1±2.5 | −1.2±0.5 | 3.3±3.0 |
| Myc | 3.9±2.8 | 2.5±1.4 | 4.3±2.0 | 2.1±1.0 | 2.95±2.1 | 1.9±0.8 |
| Jun | 1.0±0.4 | 4.2±3.5 | 1.2±0.9 | 0.52±0.45 | 3.4±2.1 | 1.4±1.7 |
3. Discussion
Combined preconditioning of rat hippocampal slice cultures with hypoxia and isoflurane increased survival compared to single agent preconditioning and involved a unique pattern of gene expression. The pattern of gene expression following combined preconditioning is a combination or amalgam of HPC and APC, in that both survival and apoptosis regulating genes are increased. Whereas hypoxia alone increased 10 survival/apoptosis genes, and isoflurane increased 12 development/growth genes, the combination increased 19 genes from both groups. This pattern is seen in Table 1. We also found that combined preconditioning required the involvement of IP3 receptors and moderate increases in intracellular Ca2+, the same as with hypoxic preconditioning (Bickler et al., 2009) or isoflurane preconditioning (Gray et al., 2005).
Differences in signal gene expression with similarly neuroprotective paradigms of isoflurane and hypoxic preconditioning were reported previously (Bickler and Fahlman, 2009). The present study involved a combination of hypoxia and isoflurane that increased the neuroprotective outcome and also involved a larger increase in intracellular Ca2+ during the preconditioning period. Because of this outcome, it is unknown whether the unique pattern of gene expression observed in this study was a result of the greater neuroprotective signaling or if it is related to the separate effects of each type of preconditioning. For example, it could be that a larger preconditioning “dose” (concentration or duration of isoflurane or hypoxia) would have produced gene changes, increases in [Ca2+]I or survival benefits similar to the combined regimen studied here. This seems unlikely however, because longer preconditioning with either isoflurane or hypoxia increased cell death, probably from the inherent toxicity of hypoxia and anesthetics (Wei et al., 2008).
Changes in gene expression in hippocampal slice cultures during hypoxia or isoflurane preconditioning are similar to patterns in studies that have analyzed whole brain responses to preconditioning in intact animals. For example, increased expression of heat shock protein genes, cell signaling genes, and other stress response genes have been noted (Hamaya et al., 2000; Pan et al., 2006; Rampil et al., 2006; Sakamoto et al., 2005) and are similar to our general findings. The overall response of the brain transcriptome remains controversial, with recent reports (Stapels et al., 2010; Stenzel-Poore et al., 2003) suggesting that widespread transcriptional suppression is key to the conditioned state in post-ischemic brain. Our data would argue otherwise, although without genome-wide expression analysis, and analysis of gene expression after simulated ischemia, this cannot be verified. The genomic responses of the brain to anesthesia exposure also has been reported to vary substantially between studies, with some reports (e.g. (Rampil et al., 2006)) reporting widespread changes in multiple gene families (including some of the signaling genes found to be altered in this study), whereas other studies reported few changes(Sakamoto et al., 2005). The data in Table 1 would suggest relatively broad effects. This impression may be biased because although a small percentage of the total genome may be altered by anesthesia, many of the altered genes are in the signaling pathways examined in the present study.
Study limitations
It is important to note that this study is limited because only signal transduction genes were examined; i.e. we did not quantify expression of all genes in the rat hippocampus transcriptome. The main value of our study design is the generation of specific hypotheses concerning the mechanisms involved in preconditioning. While we have described correlations between gene expression and selected changes in gene expression during preconditioning, it was beyond the scope of the study to prove that changes in any gene or group of genes is related mechanistically to neuroprotection. Our analysis did show that the pattern of gene expression normally seen after preconditioning was not present when cultures were treated with a cell permeable Ca2+ chelator, but because a broad collection of genes was affected by chelation, it remains uncertain which of the genes are key to the preconditioned phenotype. Another limitation of the study is that we measured gene response and not changes in the levels of proteins encoded by those genes. Because many signaling pathway proteins (e.g. protein kinases) are regulated by phosphorylation, a comprehensive understanding of the effects of altered gene expression on function would in addition require analysis of phosphorylation state and the functional effects of the activated phospho-proteins. An analysis of the consequences of increased expression of specific genes is required to understand the neuroprotective basis of preconditioning. Blocking the expression of specific genes, for example with RNA interference, is one approach that could be used to obtain that information. Finally, our results may only apply to the immature brain. The effect of development and aging on the capacity of the brain to be preconditioned against ischemic damage is important, but little studied.
Although we did not analyze the entire genome’s response to preconditioning, the 119 signal transduction genes is a sufficient sample to indicate broad patterns of responses, i.e. that both growth and development genes and apoptosis/survival regulation genes are part of the pattern of gene expression following combined isoflurane and hypoxic preconditioning. We suggest that measuring whole genome responses is unnecessary to define such important patterns in gene expression, especially when the focus is on a narrow question such differences in signaling gene activation between different types of preconditioning (Hess et al., 2001).
Role of Ca2+ in inducing gene expression changes
Differences in upstream initiators of gene expression during HPC, APC and CPC, except for the broadly important role of increases in intracellular Ca2+, remain undefined. One may ask how a common upstream signal (increases in [Ca2+]i) can elaborate different gene responses depending on the stimulus. We speculate that Ca2+ signals arising during preconditioning with isoflurane and hypoxia are actually separate, as they arise from different mechanisms causing the increases in intracellular Ca2+, and cause different patterns of gene expression. Hypoxia releases Ca2+ from the endoplasmic reticulum as does isoflurane(Bickler and Fahlman, 2004) but hypoxia involves changes in mitochondrial and cytosolic redox balance (Mayevsky and Rogatsky, 2006), resulting in a broad range of responses including cellular stasis such a spindle checkpoint arrest in development (Fischer et al., 2004) and a diverse group of cell defense mechanisms(Bickler and Donohoe, 2002). In contrast, signaling involving increases in intracellular Ca2+ produced by isoflurane preconditioning may be more similar to developmental signaling such as that following growth factor receptor activation, cell fate/differentiation decisions, and synaptic strengthening in the developing nervous system (Berglund and Augustine, 2008). It is also important to note that IP3 receptor-mediated processes can have deleterious effects as well as beneficial ones. Several studies have shown that IP3 receptor-mediated Ca2+ release can contribute to neuronal death following prolonged exposure to volatile anesthetics (Wei et al., 2008; Yang et al., 2008); this may be a function of exposure duration or anesthetic concentration.
Gene expression patterns in combined HPC and APC
Combined preconditioning increased the expression of numerous signaling genes. Combined preconditioning was associated larger numbers of genes expressed (40 of 119), rather than increases in the magnitude of gene expression compared to single agent preconditioning (data not presented). This increase in the total number of genes upregulated may be the basis for enhanced neuroprotection following combined preconditioning.
A greater number of cell survival and apoptosis regulating genes were increased following combined preconditioning than by single-agent preconditioning. These genes included Bcl2, Bcl2a1, Fn1 and p53. These responses are, on balance, consistent with pro-survival and anti-apoptosis signaling following preconditioning, even though p53 can be pro-apoptotic by up-regulating Bax. The relative levels of these proteins complexly influence survival or apoptosis (Basu and Haldar, 1998). Bcl2, p53 and Mdm2 were all increased 24 hr after CPC. Bcl2 is an important survival signal following hypoxic preconditioning (Meller et al., 2005). In intact rodents, isoflurane preconditioning increases Bcl2 levels (Li et al., 2008). The p53 gene product regulates apoptosis by interacting with a number of different proteins (Banasiak and Haddad, 1998). p53 mRNA increased following HPC and CPC, but not after APC. One of the genes induced by p53 is Bax, which is pro-apoptotic; translocation of Bax to mitochondria is a crucial step in p53-mediated apoptosis. Bax mRNA levels increased following APC, HPC and CPC. The pro-apoptotic actions of p53 and Bax must therefore be countered by the anti-apoptotic actions of other genes (e.g. Bcl2) or signals because, on balance, preconditioning enhances cell survival. Our preconditioning model could be used to determine the relative importance of these proteins in neuroprotection or injury.
Tank and Ppia were both increased by combined preconditioning. Tank is a scaffolding protein that binds TRAF proteins, and is a key activator of NfκB (Bonif et al., 2006; Pomerantz and Baltimore, 1999), which promotes survival. Similarly, Ppia, which encodes a widely expressed scaffolding/protein folding gene (Colgan et al., 2000), was upregulated by HPC and CPC. This could suppress apoptosis following preconditioning, since deletion of unfolded of proteins is an adaptive response believed to increase cell survival following hypoxic endoplasmic reticulum stress (Feldman et al., 2005).
Isoflurane increased more genes associated with regulation of cell proliferation and development than did hypoxia, and CPC also increased the mRNA levels of these genes. Genes in this group included Egr1 (an early growth response gene), Pten (a tumor suppressor gene associated with developmental regulation) Rbp1 (retinol binding protein, an important developmental regulator), the Irf1 (interferon regulatory factor), Ccnd1 (cell cycle protein cyclin d1), Egfr (epidermal growth factor receptor), Igfbp3 insulin like growth factor receptor and Cdkn1 (cyclin dependent kinase inhibitor, significant because it decreased after isoflurane). However, bone morphogenic protein (Bmp4) was not increased following CPC.
All three types of preconditioning increased the expression of Myc, a gene regulating both growth and survival. The Myc-Max heterodimer binds to the promoter of ornithine decarboxylase (ODC1) a growth/cell metabolism gene (Dang, 1999; Grandori et al., 2000). ODC1 was increased by CPC. An unexplained finding was that Myc continued to be up-regulated (as was Ptgs2) by preconditioning in Ca2+-chelated cultures. This illustrates that important Ca2+-independent processes probably are normally involved in preconditioning processes.
Expression of genes in the NfκB pathway also showed greater quantitative expression following combined preconditioning. This may be significant for neuronal apoptotic/anti-apoptotic responses: NfκB has both pro and anti apoptotic functions, activating genes with death-inducing properties like p53, c-myc, Fas and the survival genes Bcl-2, Bcl-x, and MnSOD. NfkB induction of these survival genes may play a role in excitatory, chemical and ischemic preconditioning (Marini et al., 2007). In contrast, acutely inhibiting NfκB delays p53 induced death. NfκB therefore has a dual role, maintaining neuron survival under baseline conditions, but signaling death after DNA damage. The Jnk/JunD pathway works with NfκB to increase expression of anti-apoptotic genes (Lamb et al., 2003). Gadd45 is involved in cellular response to DNA damage or oxidative stress. CPC increased Gadd45a mRNA.
Conclusions
Combining hypoxia with isoflurane in a preconditioning model with hippocampal slice cultures produces greater neuroprotection than either agent alone and a pattern of gene expression that is a unique amalgam of the two. Intracellular Ca2+ may be involved in the neuroprotective actions of this type of preconditioning. While the complete mechanistic basis for these observations was not revealed in this study, the findings have significant implications for the long terms effects of anesthesia and for the use of hypoxia or isoflurane as preconditioning agents against cerebral ischemia.
4. Experimental Procedures
The studies were approved by the University of California San Francisco Committee on Animal Research and conform to relevant National Institutes of Health guidelines for the use of animals in research.
Preparation of hippocampal slice cultures
Organotypic cultures of the hippocampus were prepared by standard methods (Laake et al., 1999; Stoppini et al., 1991) and slightly modified by our laboratory (Sullivan et al., 2002). Briefly, 7 to 9 day old Sprague Dawley rats (Charles River Laboratories, Hollister, CA) were anesthetized with 2–5% isoflurane until they did not move in response to a vigorous tail pinch. Following decapitation, the hippocampi were quickly removed and placed in 4 °C Gey’s Balanced S alt Solution (GBSS) with 20 mM glucose. The isolated hippocampi were transversely sliced (400 μm thick) with a tissue slicer (Siskiyou Design Instruments, Grants Pass, OR), and returned to GBSS at room temperature. The slices were placed on 30-mm diameter membrane inserts (Millicell-CM, Millipore, Billerica, MA) in 6-well culture trays with 1.2 ml of slice culture medium per well. The slice culture medium consisted of 50% Eagle’s Minimal Essential Medium, 25% Earle’s balanced salt solution), 25% heat inactivated horse serum (all from the UCSF cell culture facility) with 6.5 mg/ml glucose and 5mM KCl. After 2 days in culture the serum concentration was decreased to 10%. 7–10 days later the slices were preconditioned.
Study design: preconditioning organotypic cultures of hippocampus
Slice cultures were preconditioned by placing them in a Billups-Rothenberg modular incubator chamber (Del Mar, CA) filled with humidified 95% N2/5% CO2 (HPC), or 1% isoflurane in humidified air and 5% CO2 (APC) for 15 min. Combined preconditioning (CPC) was done similarly with 94% N2, 5% CO2 and 1% isoflurane, also for 15 min. RNA was extracted for gene array analysis 24 hr after preconditioning.
Simulation of ischemia with in vitro oxygen-glucose deprivation
Ischemia was simulated in vitro by one of 2 techniques: 1) immersion of cultures for 10 min into glucose-free media bubbled with 95% N2/5% CO2 (oxygen/glucose deprivation, OGD); 2) transfer of cultures to glucose free media and exposure to humidified 95% N2/5% CO2 for 30 min. The later was used with cultures treated with RNA interference or BAPTA, as these reagents sometimes caused slices to float off the culture membranes. The temperature of the media was 37°C, measured with a thermocouple thermometer. The partial pressure of oxygen, measured with a polarographic oxygen electrode, was 0–0.2 mmHg. After this insult, the cultures were returned to standard slice culture media. The percentages of dead and living neurons in CA1, CA3 and dentate was measured 48 h after OGD.
Assessment of cell death in cultured hippocampal slice
Cell death was measured with propidium iodide (PI) fluorescence. PI, a highly polar dye, penetrates damaged plasma membranes and binds to DNA. 2.3 μM PI was added to the wells of the culture trays 30 min before imaging. After 30 minutes digital images of PI fluorescence 9excitation light 520 nm, emission 600 nm) were taken with and inverted microscope and a SPOT Jr. Digital Camera (Diagnostic Instruments, Sterling Heights, MI). The camera sensitivity and the excitation light intensity were identical from day to day. Slices were discarded if they showed more than slight PI fluorescence in after 7–10 days in culture. PI fluorescence images were recorded in the dentate gyrus, CA1, and CA3 regions of the hippocampal slices prior to OGD (signal assumed to represent 0 % cell death), and 48h following OGD. In previous studies, we found that maximum post-OGD death consistently occurs at about day 2 or 3, and declines over the next 11 days (Sullivan et al., 2002). Serial measurements of PI fluorescence intensity were made in pre-defined areas (selecting CA1, CA3 and dentate separately) for each slice using NIH Image-J software (U.S. National Institutes of Health). After the measurement of PI fluorescence on the 2nd post-OGD day, all the neurons in the slice were killed to produce a fluorescence signal equal to 100% neuron death in the regions of interest. This was done by adding 100-μM potassium cyanide and 2 mM sodium iodoacetate to the cultures for 20 minutes. One hr later, final images of PI fluorescence (equated to 100% cell death) were acquired. Percent of dead cells 48 hr after OGD were then calculated based on these values. PI fluorescence intensity is a linear function of cell death (Laake et al., 1999; Newell et al., 1995).
Statistical Analysis of Cell Death
The percentages of living and dead neurons in the different regions of the slices sometimes is not be normally distributed. Therefore, the Kruskal-Wallis test followed by the Mann-Whitney U-test (JMP, SAS Institute, Cary, N.C.) was used to compare the medians of different treatment groups. T-tests or ANOVA were used to compare the means of normally distributed data, and allowance was made for multiple comparisons (Tukey-Kramer multiple comparison or Dunnett’s test). Differences were considered significant for P<0.05.
Measurement of intracellular Ca2+ in CA1 neurons
[Ca2+]i in CA1 was measured before, during and after preconditioning. Estimates of [Ca2+]i in CA1 neurons in slice cultures were made using the indicator fura-2-AM and a dual excitation fluorescence spectrometer (Photon Technology International) coupled to a Nikon Diaphot inverted microscope. Slice cultures were incubated with 5–10 μM fura-2 AM plus 1% pleuronic acid for 30 min before measurements. Cultures for these measurements were grown on Nunc Anopore (Nalge Nunc) membranes because of their low ultraviolet fluorescence. Slit apertures in the emission light path were adjusted to restrict measurement of light signals to those coming from the CA1 cell body region. Calibration of [Ca2+]i was performed using the KD of fura-2 determined in vitro with a Ca2+ buffer calibration kit (Invitrogen). The calibration process involved using the same light source, optical path and filters as used with the slice culture measurements. The KD for fura-2 was 311 nM, similar to published values (Hyrc et al., 1997). Background fluorescence (i.e. fluorescence in the absence of fura) was subtracted from total fluorescence signals prior to calculation of [Ca2+]i as described previously (Bickler and Hansen, 1998). Estimates of [Ca2+]i with this technique are accurate to about ±10 nM(Grynkiewicz et al., 1985). Measurements of [Ca2+]I were made briefly at discrete periods during the preconditioning, to avoid photobleaching of fura-2. These were at baseline, at mid-point and termination of preconditioning, and after 10 min washout of preconditioning medium. Peak [Ca2+]i always occurred at the end of the preconditioning period.
In some studies, the Ca2+ chelator BAPTA in the cell permeant form BAPTA-AM (10–25 μM) was added to cultures 30 min before preconditioning and remained during preconditioning. In others, the IP3 receptor antagonist Xestospongin C was included in the media during preconditioning. The cultures were transferred to fresh media after the preconditioning period.
RNA interference
Pools of interfering RNA (Custom smart pool RNAi, Dharmacon, Lafayette, CO) directed at the IP3 receptor subtype 1 (ITPR1) were used to transfect slice cultures with the Trans IT-neural transfection reagent (Mirus Bio Corp, Madison, WI). Three treatment groups were studied: target RNAi, nonsense (nontargeting) RNAi, and transfection reagents only. Preliminary studies defined time of maximal knockdown of IP3 receptors; RT-PCR was used to quantify transcript levels at 12, 24 48, 72, and 96 hours after addition of transfection reagents to slice culture medium. Maximal reduction in mRNA and IP3 receptor protein was at 72 hr after transfection in both cases. Slices were transfected on days 7, 8 and 9 in culture. To assess the effectiveness of target mRNA depletion, slices were transferred into −80°C RNAlater-ICE (Ambion, Austin, TX) and stored until RNA was extracted with Trizol (Invitrogen, Carlsbad, CA). DNA was synthesized using an OmniScript Reverse Transcriptase kit (Qiagen, Valencia, CA). Quantitative PCR was run using a QuantiTect SYBR Green PCR kit (Qiagen) and the following primers, created using Primer3 online software (Roizen and Skaletsky, 2000): IP3r1 Forward: tcgtggatgttctacacaga, IP3r1 Reverse: agctgcttggtgtgttttat
(Product size: 112 bp, Accession number 1054962) and for β-actin forward: acagctgagagggaaatcgt β-actin reverse: ttctccagggaggaagagg
(Product size: 107 bp, Accession number: 031144).
Microarray analysis
RNA for microarray analysis was extracted from slice cultures 24 hrs after mock preconditioning (control), hypoxic preconditioning, isoflurane preconditioning and combined preconditioning as follows. Pooled tissue slices (12–18) were homogenized in 1 ml of TriZol Reagent. The RNA was precipitated from the aqueous phase with isopropyl alcohol, rinsed with 75% ethanol, and then re-suspended in DEPC-treated water. RNA was further purified by means of the ArrayGrade™ Total RNA isolation kit (SuperArray, SA Biosciences, Frederick, MD), and concentrated down to a final volume of 50 μl in RNASE-free water.
cDNA was synthesized using 0.1 to 2 μg of total RNA, by means of the TrueLabelingTM LinearRNA Amplification Kit (SuperArray). From this cDNA an amplified Biotin-Labeled cRNA was synthesized. Biotinylated UTP was obtained from Roche Applied Science. The cDNA synthesis reaction was incubated overnight at 37° C. The cRNA was then purified using spin columns from SuperArray’s cRNA Cleanup Kit. Quality and concentration of cRNA was determined by absorbance of 260nm and 280nm light.
The cRNA was hybridized onto Oligo GEArrays at 60 ° C overnight with continuous agitation. The arrays used were Rat Signal Transduction Pathway FinderTM Microarrays ORN-14, ORN-14.2, and Rat Apoptosis Microarray ORN-12 from SuperArray. Table 1 contains a listing of all the genes on the arrays. After rinsing in wash buffers the arrays were probed using a chemiluminescence method. Arrays were exposed to high performance chemiluminescence film (HyperfilmTM ECL, Amersham, South San Francisco, CA) and developed in a mechanical darkroom developer. Films were scanned at the highest pixel density (1200 dpi output resolution) for analysis.
Statistical analysis of array data
Array scans were analyzed using the internet-based GEArray Expression Analysis Suite provided by SuperArray. All genes were normalized to a series of “housekeeping” gene expression levels and a group of synthetic control sequences included on the array by the manufacturer. For background normalization, a pair of blank spots and local background correction for each tetra spot was employed. Gene expression was considered significant if there was a minimum 1.5-fold increase or decrease compared to the control level.
Acknowledgments
Supported by a grant (RO1 GM 52212) from the USA National Institutes of Health (Washington, D.C.) to PEB.
We thank Pablo Gabatto, BS, Will McKleroy, BS, and Maren Gregerson, BS, Staff Research Associates, Dept. Anesthesia, UCSF, for technical assistance.
Footnotes
Reprints will not be available from the authors
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banasiak KJ, Haddad GG. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Research. 1998;797:295–304. doi: 10.1016/s0006-8993(98)00286-8. [DOI] [PubMed] [Google Scholar]
- Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4:1099–109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- Berglund K, Augustine GJ. Calcium helps neurons identify synaptic targets during development. Neuron. 2008;59:186–7. doi: 10.1016/j.neuron.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Hansen BM. Hypoxia-tolerant neonatal CA1 neurons: Relationship of survival to evoked glutamate release and glutamate receptor-mediated calcium changes in hippocampal slices. Dev Brain Res. 1998;106:57–69. doi: 10.1016/s0165-3806(97)00189-2. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Donohoe PH. Adaptive responses of vertebrate neurons to hypoxia. J Exp Biol. 2002;205:3579–3586. doi: 10.1242/jeb.205.23.3579. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS. Moderate increases in intracellular calcium activate neuroprotective signals in hippocampal neurons. Neuroscience. 2004;127:673–83. doi: 10.1016/j.neuroscience.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS. Expression of signal transduction genes differs following hypoxic or isoflurane preconditioning of rat hippocampal slice cultures. Anesthesiology. 2009 doi: 10.1097/ALN.0b013e3181a8647f. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS, Gray J, McKleroy W. Inositol 1,4,5-triphosphate receptors and NAD(P)H mediate Ca2+ signaling required for hypoxic preconditioning of hippocampal neurons. Neuroscience. 2009;160:51–60. doi: 10.1016/j.neuroscience.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonif M, Meuwis MA, Close P, Benoit V, Heyninck K, Chapelle JP, Bours V, Merville MP, Piette J, Beyaert R, Chariot A. Tnf-α and IKKβ mediated TANK/I-TRAF phosphorylation: implications for interaction with NEMO/IKKγ and NF-κB. Biochem Journal. 2006;394:593–603. doi: 10.1042/BJ20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Chen H, Ho KL, Dereski MO, Brown E, Hetzel FW, Welch KM. Transient hyperthermia protects against subsequent forebrain ischemic cell damage in the rat. Neurology. 1989;39:1396–8. doi: 10.1212/wnl.39.10.1396. [DOI] [PubMed] [Google Scholar]
- Colgan J, Asmal M, Luban J. Isolation, characterization and target disruption of mouse Ppia: cyclophilin A is not essential for mammalian cell viability. Genomics. 2000;68:167–178. doi: 10.1006/geno.2000.6295. [DOI] [PubMed] [Google Scholar]
- da Silva R, Lucchinetti E, Pasch T, Schaub MC, Zaugg M. Ischemic but not pharmacological preconditioning elicits a gene expression profile similar to unprotected myocardium. Physiol Genomics. 2004;20:117–30. doi: 10.1152/physiolgenomics.00166.2004. [DOI] [PubMed] [Google Scholar]
- Dahl NA, Balfour WM. Prolonged Anoxic Survival Due to Anoxia Pre–Exposure: Brain Atp, Lactate, and Pyruvate. Am J Physiol. 1964;207:452–6. doi: 10.1152/ajplegacy.1964.207.2.452. [DOI] [PubMed] [Google Scholar]
- Dang CV. c-Myc Target genes involved in cell growth, apoptosis, and meatbolism. Molecular and Cellular biology. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumours. Molecular Cancer Research. 2005;3:597–605. doi: 10.1158/1541-7786.MCR-05-0221. [DOI] [PubMed] [Google Scholar]
- Fischer MG, Heeger S, Hacker U, Lehner CF. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Curr Biol. 2004;14:2019–24. doi: 10.1016/j.cub.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994;168:221–4. doi: 10.1016/0304-3940(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behaviuor. Annual Rev Cell Devel Biology. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen–activated protein kinases. Anesthesiology. 2005;102:606–15. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- Hamaya Y, Takeda T, Dohi S, Nakashima S, Nozawa Y. The effects of pentobarbital, isoflurane, and propofol on immediate-early gene expression in the vital organs of the rat. Anesth Analg. 2000;90:1177–83. doi: 10.1097/00000539-200005000-00034. [DOI] [PubMed] [Google Scholar]
- Hess KR, Zhang W, Baggerly KA, Stivers DN, Coombes KR. Microarrays: handling the deluge of data and extracting reliable information. Trends Biotechnol. 2001;19:463–8. doi: 10.1016/s0167-7799(01)01792-9. [DOI] [PubMed] [Google Scholar]
- Hyrc K, Handran DS, Rothman SM, Goldberg MP. Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low affinity fluorescent calcium indicators. J Neurosci. 1997;17:6669–6677. doi: 10.1523/JNEUROSCI.17-17-06669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Drummond JC, Davis DP, Cole DJ, Patel PM. Combination of isoflurane and caspase inhibition reduces cerebral injury in rats subjected to focal cerebral ischemia. Anesthesiology. 2004;101:75–81. doi: 10.1097/00000542-200407000-00013. [DOI] [PubMed] [Google Scholar]
- Inoue S, Davis DP, Drummond JC, Cole DJ, Patel PM. The combination of isoflurane and caspase 8 inhibition results in sustained neuroprotection in rats subject to focal cerebral ischemia. Anesth Analg. 2006;102:1548–55. doi: 10.1213/01.ane.0000202381.40516.8d. [DOI] [PubMed] [Google Scholar]
- Laake JH, Haug F–M, Weiloch T, Ottersen OP. A simple in vitro model of ischemia based on hippocampal slice cultures and propidium iodide fluorescence. Brain Research Protocols. 1999;4:173–84. doi: 10.1016/s1385-299x(99)00021-5. [DOI] [PubMed] [Google Scholar]
- Lamb JA, Ventura JJ, Hess P, Flavelli RA, Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell. 2003;11:1479–1489. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]
- Landoni G, Fochi O, Torri G. Cardiac protection by volatile anaesthetics: a review. Curr Vasc Pharmacol. 2008;6:108–11. doi: 10.2174/157016108783955284. [DOI] [PubMed] [Google Scholar]
- Li L, Peng L, Zuo Z. Isoflurane preconditioning increases B-cell lymphoma-2 expression and reduces cytochrome c release from the mitochondria in the ischemic penumbra of rat brain. Eur J Pharmacol. 2008;586:106–13. doi: 10.1016/j.ejphar.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Jiang X, Wu X, Pan H, Guo Z, Mattson Blondeau MPN, Novelli A, Lipksy RH. Pre-conditioning and neurotrophins: a model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids. 2007;32:299–304. doi: 10.1007/s00726-006-0414-y. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, Rogatsky G. Mitochondrial Function In Vivo Evaluated by NADH Fluorescence: From Animal Models to Human Studies. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00249.2006. [DOI] [PubMed] [Google Scholar]
- Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–46. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- Newell DW, Barth A, Papermaster V, Malouf AT. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J Neuroscience. 1995;15:7702–11. doi: 10.1523/JNEUROSCI.15-11-07702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JZ, Wei H, Hecker JG, Tobias JW, Eckenhoff RG, Eckenhoff MF. Rat brain DNA transcript profile of halothane and isoflurane exposure. Pharmacogenet Genomics. 2006;16:171–82. doi: 10.1097/01.fpc.0000189795.21770.08. [DOI] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D. NF-kB activation by a signaling complex containing TRAF2, TANK, and TBK1, a novel IKK-related kinase. The EMBO Journal. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampil IJ, Moller DH, Bell AH. Isoflurane modulates genomic expression in rat amygdala. Anesth Analg. 2006;102:1431–8. doi: 10.1213/01.ane.0000202384.96269.51. [DOI] [PubMed] [Google Scholar]
- Roizen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, N.J: 2000. pp. 365–366. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Imai J, Nishikawa A, Honma R, Ito E, Yanagisawa Y, Kawamura M, Ogawa R, Watanabe S. Influence of inhalation anesthesia assessed by comprehensive gene expression profiling. Gene. 2005;356:39–48. doi: 10.1016/j.gene.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Shpargel KB, Jalabi W, Jin Y, Dadabayev A, Penn MS, Trapp BD. Preconditioning paradigms and pathways in the brain. Cleve Clin J Med. 2008;75(Suppl 2):S77–82. doi: 10.3949/ccjm.75.suppl_2.s77. [DOI] [PubMed] [Google Scholar]
- Simon R, Henshall D, Stoehr S, Meller R. Endogenous mechanisms of neuroprotection. Epilepsia. 2007;48(Suppl 8):72–3. doi: 10.1111/j.1528-1167.2007.01356.x. [DOI] [PubMed] [Google Scholar]
- Stapels M, Piper C, Yang T, Li M, Stowell C, Xiong ZG, Saugstad J, Simon RP, Geromanos S, Langridge J, Lan JQ, Zhou A. Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance. Sci Signal. 2010;3:ra15. doi: 10.1126/scisignal.2000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–37. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sullivan BS, Leu D, Taylor DM, Fahlman CS, Bickler PE. Isoflurane prevents delayed cell death in an organotypic slice culture model of cerebral ischemia. Anesthesiology. 2002;96:189–95. doi: 10.1097/00000542-200201000-00033. [DOI] [PubMed] [Google Scholar]
- Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–50. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]