Abstract
Background
This cross-sectional study examines parents’ perceptions of their neighborhoods and general and respiratory health among low-income Chicago families. Asthma disproportionately affects non-white, urban, and low socioeconomic status (SES) populations, but Chicago’s burden, and the national epidemic, are not well-explained by known risk factors. Urban dwellers experience acute and chronic stressors that produce psychological distress and are hypothesized to impact health through biological and behavioral pathways. Identifying factors that covary with lower SES and minority-group status -- e.g. stress -- is important for understanding asthma’s social patterning.
Methods
We used survey data from 319 parents of children 5–13 years with asthma/respiratory problems and principal components analysis to create exposure variables representing parents’ perceptions of two aspects of neighborhoods: collective efficacy (“CE”) and physical/social order (“order”). Adjusted binomial regression models estimated risk differences (RD) and 95% confidence intervals (CI) for eight binary outcomes.
Results
Magnitude was generally as expected, i.e., RD for low versus high (most favorable) exposure groups (RDlow v. high) was larger than for the middle vs. high contrast (RDmid v. high). “Parent general health” was strongly associated with “CE” [RDlow v. high=20.8 (95% CI: 7.8, 33.9)] and “order” [RDmid v. high=11.4 (95% CI: 2.1, 20.7)] unlike “child general health” which had nearly null associations. Among respiratory outcomes, only “waking at night” was strongly associated with “CE” [RDlow v. high=16.7 (95% CI: 2.8, 30.6)] and “order” [RDlow v. high=22.2 (95% CI: 8.6, 35.8)]. “Exercise intolerance” [RDlow v. high=15.8 (95% CI: 2.1, 29.5)] and “controllability” [RDmid v. high=12.0 (95% CI: 1.8, 22.3)] were moderately associated with “order” but not with “CE,” while “school absences,” “rescue medication use,” and “unplanned visits” had nearly null associations with both exposures.
Conclusions
More negative perceptions tended to be associated with higher risk of undesirable outcomes, adding to evidence that the social environment contributes to health and supporting research on stress’ health impact among disadvantaged populations. Interventions must address not only traditional “environmental” factors but individuals’ reactions to stress and attempt to mitigate effects of stressors while structural solutions to health inequities are sought.
Introduction
Asthma, one of the most common chronic diseases of childhood in the United States, disproportionately affects non-whites in urban areas and those of low socioeconomic status (SES).1, 2 Chicago’s asthma mortality and hospitalizationrates are among the highest in the nation.3–6 Underdiagnosis, suboptimal care, and dramatic racial/ethnic disparities among Chicagoans have been documented.3, 4, 7–11 Within Chicago, prevalence, morbidity, and mortality aretypically highest in neighborhoods with the lowest SES.3, 7, 12 Despite a decade of extraordinary efforts to increase asthma equity in Chicago, progress has been modest.12
Chicago’s burden, and the national epidemic, are not well-explained by known risk factors. Not all urban communities have excess asthma though they may share low-SES and environmental exposures with high-risk urban areas.3, 13–15 Wright and Subramanian call for attention to “social and physical factors that covary with lower SES and minority-group status (e.g., differential environmental exposures, residential segregation, psychological stress, housing quality, and social capital) that mediate the effects of living in low-SES neighborhoods” to contextualize asthma and understand its social patterning.15
Recent literature has also called for understanding how social environments “get under the skin”16 and become “biologically embedded”17 to influence health.14, 15 Acute and chronic stressors are believed to have psychological effects that in turn influence psychologic and physiologic functioning as well as behavior. Low-SES and other disadvantaged groups may experience increased stressors and be more strongly affected by them due to already-compromised psychological health, social supports, and coping resources.18–20 Studies link psychological stress to asthma, including onset of disease, precedent phenotypes, and disease exacerbation, through hypothesized “dysregulated immunity” mechanisms.14, 21–27 Psychological stress experienced by children or their parents may also have indirect effects on asthma by causing health-compromising behaviors and co-morbidities that compromise disease management.24, 28–31 In general, individual perceptions are important because they are related to psychological distress. Neighborhoods matter because families reside in environments which may impact their physical and mental health.
While an enormous asthma literature exists, the alarming burden of asthma on inner-city populations demands more thoughtful investigation of determinants of risk. This study aims to advance asthma scholarship by incorporating novel exposure variables into a theoretical framework and furthering our understanding of how psychosocial factors become biologically embedded and influence health through psychological stress pathways. We examined associations between parents’ perceptions of neighborhood stressors and parent-reported parent and child general health and child respiratory health., under the hypothesis that less positive perceptions of one’s neighborhood would be associated with increased risk of poor health.
Methods
Study population and design
The Institutional Review Board of the University of North Carolina at Chapel Hill approved this study. We used a cross-sectional study design and survey data collected in 2002–2004 for an observational investigation of childhood asthma disparities among low-income Chicago families with children aged 5–13 years. The study surveyed for respiratory problems with a validated tool32, 33 in 15 public elementary schools that met the following eligibility criteria: more than 75% of enrollment qualified as low-income, no single racial/ethnic group comprised more than two-thirds of enrollment; and only local residents were enrolled. Eligible families participated in a longitudinal study with three data collection phases over 12 months in English or Spanish and comprised three groups: 351 diagnosed asthmatics; 331 undiagnosed (possible) asthmatics; and 562 non-asthmatics (no diagnosis and no respiratory problems). The current study used data from parents of children with diagnosed asthma (n=158) and undiagnosed asthma (n=161) obtained during a home visit (phase 2) since the focus of our hypothesis is exacerbation of respiratory problems, not the development of asthma.. Also, non-asthmatics did not participate after baseline and therefore are not included in the phase 2 dataset (and neighborhood perceptions items were not included in the baseline survey). Descriptive statistics demonstrate that 47% of those who participated at baseline also participated in the home visit and that this subsample is similar to the main study sample for all sociodemographic variables examined, with three exceptions. The current study sample had a higher proportion of parents who were foreign-born (44% vs. 34%), Spanish speakers (34% vs. 23%), and married/cohabiting (64% vs. 57%). The current sample had a lower proportion of children with the unfavorable outcome for three respiratory outcomes: unplanned medical visits (27% vs. 44%); school absences (12% vs. 18%); and rescue medication use (27% vs. 47%).
Analytic strategy
Principal Components Analysis (PCA)
We created summary variables representing neighborhoods by data reduction techniques to take advantage of rich survey data and to capture multi-faceted characteristics of neighborhoods. We identified 27 items representing parent perceptions of their neighborhood in the survey instrument, 14 of which were taken from the Community Survey from the Project on Human Development in Chicago Neighborhoods (PHDCN),34 though no attempt was made to replicate PHDCN’s survey nor was there an expectation that PCA for this study’s sample would yield scales identical to those used in PHDCN research. Thirteen additional survey items were developed by the main study’s investigators and hypothesized to have potential health effects. We dropped 13 variables not correlated with at least one other item at the level of 0.50 from further analysis. We used an iterated PCA of 14 remaining variables for the extraction method in the absence of a priori theoretical knowledge of underlying constructs or of shared variance among variables. Varimax (orthogonal) rotation summarized the co-variation among the variables since the goal was data reduction to a set of uncorrelated measures for subsequent use in multivariate analyses. A three-component solution was supported by scree plot and eigenvalues. Twelve items comprised 3 components which explained 58% of the total variance (table 2). Components1, 2 and 3 accounted for 28%, 16% and 14% of the total variance, respectively. Interpretation of components was limited to theoretically salient variables with loadings >0.60 and no loading >0.30 on the other components. We computed summary scores by obtaining the mean value of these variables, all of which were on the same metric within each component, allowing a maximum of one missing item’s value to be replaced by the mean of the non-missing items. Summary scores were named: 1) physical/social order; 2) collective efficacy; and 3) recent change in neighborhood (hereafter referred to as “order,” “collective efficacy,” and “change”). “Change” was not explored in regression analyses because interpretation of associations would be difficult in the absence of baseline data on the neighborhood at the start of the 5-year period and whether change was desirable. Also, it comprised only 3 items, and allowing substitution of missing data would compromise its validity. Internal consistency, measured by Cronbach’s alpha, was above acceptable35 for both summary scores chosen as main exposures: 0.78 (“collective efficacy”) and 0.83 (“order”).
Table 2.
Neighborhood factors derived from principal components analysis*
| Item Loadings**
|
|||
|---|---|---|---|
| Survey Item | Component 1 Physical/social order | Component 2 Collective efficacy | Component 3 Change in past 5 years |
| I’m going to read a list of things that are problems in some neighborhoods. For each, please tell me how much of a problem it is in your neighborhood. | |||
| How much of a problem is litter, broken glass or trash on the sidewalks and streets? | .811 | −.024 | .087 |
| How much of a problem is graffiti on buildings and walls? | .792 | .064 | .036 |
| How much of a problem are vacant or deserted houses or storefronts? | .784 | .133 | .102 |
| How much of a problem is lack of trust between local businesses and residents? | .730 | .046 | .141 |
| How often does child play in doors instead of outdoors because of the following? | |||
| A hazardous environment, for example, traffic, broken glass, broken playground equipment, the presence of garbage or syringes? | .571 | .171 | −.097 |
| Danger caused by people, for example, violence, crime, gang or drug activity? | .516 | .308 | −.150 |
| Do you strongly disagee, disagree, agree or strongly agree? | |||
| People around here are willing to help their neighbors. | .128 | .754 | .089 |
| This is a close-knit neighborhood. | .135 | .749 | .070 |
| Most of my neighbors vote regularly. | −.013 | .722 | .024 |
| People in this neighborhood can be trusted. | .229 | .693 | .083 |
| People in this neighborhood care about who is elected to local political positions. | .055 | .686 | .061 |
| Now I’m going to ask you about how your neighborhood has changed over the past years (even if you have not lived here the entire time). Please tell me whether you think your neighborhood has gotten better, stayed about the same, or gotten worse over the past five years. | |||
| Personal safety | .041 | .121 | .857 |
| The way the neighborhood looks | .050 | .043 | .853 |
| People living in your neighborhood | .018 | .098 | .845 |
Iterated extraction method and varimax (orthogonal) rotation used to obtain uncorrelated components; 3-component solution accounted for 58% of total variance
Bolded items loading > 0.60 on one component and not crossloading >0.30 on a second component were included in summary scores
Variables
Neighborhood Exposures
Summary scores resulting from PCA were each categorized into 3 levels based on natural cut-points in the distributions and to assure sufficient numbers in each category. Table 1 presents descriptive statistics for the summary scores as well as the categorical exposure variables. “Collective efficacy” was coded as: high (1–1.9); middle (2.0–2.4); and low (2.5–3.8). “Order” was coded as: high (1.0); middle (1.1–1.9); and low (2.0–3.0). Analytic models included 2 indicator variables for each exposure, with the most favorable (hypothesized to be health-protective) category serving as referent (i.e. high collective efficacy and high order), allowing 2 exposure group contrasts: middle versus high and low versus high.
Table I.
Parent-reported sociodemographic characteristics, main exposures and health outcomes for parents* and their diagnosed or undiagnosed asthmatic children, n=319
| SOCIODEMOGRAPHICS | NUMBER (PERCENT) |
|---|---|
| Child race/ethnicity** | |
| Non-Hispanic white | 37 (11.6) |
| Non-Hispanic black | 106 (33.2) |
| Hispanic | 176 (55.2) |
| Foreign born | |
| Child | 31 (9.7) |
| Parent | 140 (43.9) |
| Spanish language survey | 110 (34.5 |
| Female | |
| Child | 178 (55.8) |
| Parent | 298 (93.4) |
| Less than high school parent education | 89 (27.9) |
| Parent married/cohabiting | 204 (64.2) |
| Homeownership | 108 (33.9) |
| Health insurance | |
| Child | |
| Public | 156 (49.1) |
| Private | 124 (39.0) |
| Uninsured | 38 (11.9) |
| Parent | |
| Public | 72 (22.7) |
| Private | 155 (48.9) |
| Uninsured | 90 (28.4) |
| Asthma diagnosis | |
| Child | 158 (49.5) |
| Parent | 50 (15.7) |
| MEAN + STANDARD DEVIATION (MINIMUM-MAXIMUM) | |
| Age (years)* | |
| Child | 10 + 2 (5–13) |
| Parent | 36 + 8 (22–74) |
| Months at current address | 55 + 55 (1–457) |
| Parent Center for Epidemiological Studies-Depression (CES-D) score | 12 + 11 (0–54) |
| NEIGHBORHOOD EXPOSURES | |
| PCA summary scores | |
| Collective efficacy | 2.2 + 0.6 (1.0–3.8) |
| Physical/social order | 1.6 + 0.6 (1.0–3.0) |
| NUMBER (PERCENT) | |
| Categorical exposure*** | |
| Collective efficacy | |
| High (referent) (1.0–1.9) | 77 (24.6) |
| Middle (2.0–2.4) | 141 (45.0) |
| Low (2.5–3.8) | 95 (30.4) |
| Physical/social order | |
| High (referent) (1.0) | 96 (30.6) |
| Middle (1.1–1.9) | 136 (43.3) |
| Low (2.0–3.0) | 82 (26.1) |
| BINARY HEALTH OUTCOMES | |
| General health (fair/poor)† | |
| Parent-reported child health | 47 (14.7) |
| Self-reported parent health | 68 (21.4) |
| Parent-reported child respiratory health (any vs, none)†† | |
| Unplanned medical visits | 78 (24.6) |
| Waking at night | 135 (42.5) |
| School absences | 35 (11.8) |
| Rescue medication use | 42 (26.8) |
| Exercise intolerance | 111 (35.1) |
| Controllability (not at all/somewhat) | 62 (19.6) |
9 (2.8%) grandmothers and 3 (0.9%) aunts among survey respondents
Child race/ethnicity is an imperfect proxy for parent race/ethnicity, which was not ascertained in the main study; n=5 non-Hispanic, non-Hispanic black “other” cases were categorized as white
3-level categorical exposure variables were created from summary scores and used in analytical models with the high category serving as referent
General health items recoded as fair or poor versus excellent or very good or good (referent); no reference time period specified
Child respiratory outcomes were number of days in previous 2 weeks recoded as any versus none (referent) except for unplanned medical visits (6 months) and controllability (not at all/somewhat; no reference time period specified); rescue medication use includes 158 diagnosed asthmatics only
Health Outcomes
We recoded 8 parent-reported health outcomes as binary variables with the absence of symptoms/healthcare or medication utilization/school absences or the most favorable outcome (for general health and controllability) serving as the referent category, i.e., outcomes were modeled as any versus none or unfavorable versus favorable. We used the global/general health items from the Child Health Questionnaire36 and the Short Form-12,37 to measure parent-reported child health and self-reported parent health, respectively. We recoded the 5-response scales as poor or fair vs. good or very good or excellent. Respiratory outcomes were developed by the main study’s investigators, including a pediatric allergist, and based on their clinical and research expertise and review of the literature. A number of measures of asthma symptoms, therapies and control for children and adults exist, though time reference periods differ.38–40 We used the following four commonly-utilized respiratory outcomes with a two-week reporting period to minimize recall bias: 1) waking at night; 2) school absences; 3) rescue medication use, and 4) exercise intolerance, recoded as any versus none. Analyses using the rescue medication outcome included only diagnosed asthmatics since undiagnosed children might not have access to prescribed asthma medications. The number of unplanned visits to an emergency department, physician’s office or clinic for child’s asthma or breathing problems was obtained for a six-month period since urgent care is not typically a common occurrence and coded as any vs. none. Controllability was intended to be a subjective measure of a parent’s sense of whether her child’s asthma is controllable; this 4-response variable was recoded as not at all or somewhat vs. quite or extremely controllable.
Individual-level sociodemographic variables
Covariates included: parenteducation (less than high school versus. high school or beyond (referent)); parent marital status (unmarried versu. married or cohabiting partner (referent)); parent nativity (foreign-born versus. U.S,-born (referent); parent age (20–29, 30–39 (referent), >=40 years); and child race/ethnicity (Hispanic (referent), non-Hispanic black, non-Hispanic white). Parent race/ethnicity was not ascertained in the main study; child race/ethnicity is considered a proxy. Five non-Hispanic, non-Hispanic black, “other” cases were categorized as white.
Binomial Regression Analyses
Univariate analyses demonstrated high prevalence of all outcomes (12–43%; table 1); therefore, odds ratios would overestimate relative risk. We preferred an absolute measure of effect, risk difference (RD). Binomial regression (SAS GENMOD specifying identity link function and binomial distribution) estimated RDs and 95% confidence intervals (CI) as the measures of association between the summary neighborhood scores and binary health outcomes for cases with complete data. Bivariate analyses first estimated crude RDs. We assessed effect measure modification (EMM) by Mantel-Haenszel chi-squared tests of homogeneity and did not observe consistent evidence of modification for variables including child asthma diagnosis, race, age and sex and parent age, nativity, marital status, education and depressive symptoms score.
With the same variables assessed as potential modifiers, we next identified potential confounders in directed acyclic graph (DAG) analyses;41 therefore, we did not quantitatively assess variables on causal pathways, variables that were not associated with both outcome and exposure, or variables that had hypothesized bi-directional associations with other variables as confounders. We then assessed all potential confounders with a change-in-estimate strategy42 and adjusted for them in multivariate models created by backward elimination, with removal from the full model of covariates that changedthe magnitude of association by <10%. Each model therefore had a potentially unique set of adjustment variables. We evaluated the predictive importance of each exposure by the magnitude of the RDs and the width of the CIs. We used SPSS 16.0 (SPSS Inc., Chicago, Illinois) for data reduction by PCA, SAS 9.1 (SAS Institute Inc., Cary, North Carolina) for estimation of risk differences by binomial regression, STATA 10.1 (StataCorp, College Station, Texas) for graphical data displays, and the Microsoft Excel program “Episheet” (version of June 11, 2008) written by Ken J. Rothman for additional tabular analyses.
Results
Sample characteristics
The following descriptive statistics are presented in table 1. More of than half of children were identified as Hispanic while, about one-third were non-Hispanic black and 12% were non-Hispanic white. Only 10% of children but 44% of parents were foreign-born, and about 1/3 spoke Spanish for the survey. Approximately equal numbers of boys and girls participated, but the majority of parent respondents were female. Twenty-eight percent of parents had not earned a high school diploma; 64% were married or cohabiting, and 1/3 of families owned their homes. Most children (88%) and parents (72%) had health insurance. The mean Center for Epidemiologic Studies-Depression (CES-D) score was 12 (SD=11), range=0–54. Validation studies support a score of 16 to discriminate individuals with depressive symptomatology from those without.43, 44 Parent-reported general health was fair or poor for 21% of parents and 15% of children. Prevalence of undesirable child respiratory outcomes was high. One quarter had at least 1 unplanned medical visit. Night disturbance had the highest prevalence (43%) and school absences had the lowest (12%) while rescue medication use and exercise intolerance were experienced by 27% and 35%, respectively. Twenty percent of parents reported their child’s asthma/breathing problems as not at all or somewhat controllable.
Risk differences
Adjustment did not change the magnitude of the RDs dramatically from the crude RDs (tables 3 and 4); adjusted RDs are discussed below and graphically represented in figures 1 and 2. Adjustment caused about half of the RDs to move closer to the null value of 0 and about half to move away. Generally, the magnitude of the RDs for each outcome was as expected; that is, the RD for the middle versus high contrast (RDmid v. high) was smaller than for the low vs. high contrast (RDlow v. high).
Table 3.
Crude and adjusted risk differences (RD) and 95% confidence intervals (CI) for the associations of neighborhood factors and fair/poor parent and child general health
| General Health Outcome* by Neighborhood Exposure Level** | Excess Number of Persons per 100 at Risk of Unfavorable Outcome | |
|---|---|---|
| Crude RD (95% CI) | Adjusted RD (95% CI) | |
| COLLECTIVE EFFICACY
|
||
| Self-reported parent general health | ||
| middle vs. high | 14.9 (5.8, 24.1) | 13.5 (2.3, 24.6)*** |
| low vs. high | 22.7 (11.7–33.8) | 20.8 (7.8, 33.9)*** |
| Parent-reported child general health | ||
| middle vs. high | 2.5 (−6.7, 11.7) | 2.5 (−6.1, 11.1)† |
| low vs. high | 6.2 (−4.3, 16.7) | 7.5 (−2.5, 17.6) † |
| PHYSICAL/SOCIAL ORDER
|
||
| Self-reported parent general health | ||
| middle vs. high | 8.3 (−2.1, 18.8) | 11.4 (2.1, 20.7)†† |
| low vs. high | 5.3 (−6.4, 16.9) | 4.4 (−6.7, 15.5)†† |
| Parent-reported child general health | ||
| middle vs. high | −2.8 (−11.7, 6.1) | −0.8 (−10.2, 8.6)††† |
| low vs. high | 6.2 (−5.1, 17.4) | 6.3 (−5.0, 17.5)††† |
General health recoded as fair or poor vs. excellent or very good or good (referent); no reference time period specified
3-level neighborhood exposures yielded 2 RDs; high (most favorable) level= referent
Adjusted for child race/ethnicity, parent education, and parent marital status
Adjusted for parent education
Adjusted for parent education, parent marital status, and parent nativity
Adjusted for child race/ethnicity, parent education, parent marital status, and parent age
Table 4.
Crude and adjusted risk differences (RD) and 95% confidence intervals (CI) for the associations of neighborhood factors and poorer parent-reported child respiratory health
| Child Respiratory Health Outcome* by Neighborhood Exposure Level** | Excess Number of Persons per 100 at Risk of Unfavorable Outcome | |
|---|---|---|
| Crude RD (95% CI) | Adjusted RD (95% CI) | |
| COLLECTIVE EFFICACY
|
||
| Waking at night | ||
| middle vs. high | 12.3 (−0.9, 25.5) | 8.1 (−5.0, 21.1)*** |
| low vs. high | 21.3 (6.9, 35.7) | 16.7 (2.8, 30.6)*** |
| Exercise intolerance | ||
| middle vs. high | 4.9 (−8.3, 18.1) | 3.9 (−9.2, 17.1)† |
| low vs. high | 3.7 (−10.6, 18.0) | 1.1 (−12.9, 15.1)† |
| School absences | ||
| middle vs. high | 1.2 (−8.4, 10.9) | −0.4 (−11.1, 8.0)†† |
| low vs. high | −3.3 (−13.1, 6.5) | −5.0 (−17.6, 6.5)†† |
| Rescue medication use | ||
| middle vs. high | −6.0 (−24.3, 12.4) | −9.8 (−27.9, 8.3)††† |
| low vs. high | −8.4 (−27.6, 10.7) | −15.7 (−36.1, 4.8)††† |
| Unplanned medical visits | ||
| middle vs. high | −4.9 (−17.1, 7.3) | −4.7 (−16.6, 7.2)# |
| low vs. high | −1.0 (−14.5, 12.4) | −3.0 (−16.3, 10.3)# |
| Controllability | ||
| middle vs. high | 4.6 (−6.1, 15.2) | 4.8 (−5.7, 15.5)## |
| low vs. high | 4.0 (−7.6, 15.6) | 7.5 (−4.0, 19.0)## |
| PHYSICAL/SOCIAL ORDER
|
||
| Waking at night | ||
| middle vs. high | 12.9 (0.4, 25.3) | 11.4 (−0.5, 23.3)### |
| low vs. high | 23.1 (8.9, 37.4) | 22.2 (8.6, 35.8) ### |
| Exercise intolerance | ||
| middle vs. high | 11.5 (−0.6, 23.5) | 11.6 (−0.3, 23.6)@ |
| low vs. high | 14.4 (0.5, 28.3) | 15.8 (2.1, 29.5)@ |
| School absences | ||
| middle vs. high | 0.5 (−8.3, 9.2) | −2.7 (−13.0, 7.7)@@ |
| low vs. high | −1.3 (−10.8, 8.2) | −1.5 (−10.9, 7.9)@@ |
| Rescue medication use | ||
| middle vs. high | 5.2 (−10.1, 20.5) | 5.0 (−11.3, 21.2)@@@ |
| low vs. high | 11.5 (−8.0, 31.0) | 13.9 (−7.3, 35.2)@@@ |
| Unplanned medical visits | ||
| middle vs. high | 1.4 (−9.5, 12.4) | −0.8 (−12.5, 10.8)^ |
| low vs. high | 6.3 (−6.6, 19.2) | 4.5 (−8.5, 17.5)^ |
| Controllability | ||
| middle vs. high | 7.9 (−2.2, 18.0) | 12.0 (1.8, 22.3)^^ |
| low vs. high | 4.6 (−6.6, 15.8) | 8.1 (−3.2, 19.4)^^ |
Child respiratory outcomes were number of days in previous 2 weeks recoded as any versus none (referent) except for unplanned medical visits (6 months) and controllability (not at all/somewhat vs. quite or extremely controllable (referent); no reference time period specified); rescue medication use includes 158 diagnosed asthmatics only
3-level neighborhood exposures yielded 2 RDs; high (most favorable) level= referent
Adjusted for child race/ethnicity, parent education, and parent marital status
Adjusted for child race/ethnicity, parent marital status, parent nativity, and parent age
Adjusted for child race/ethnicity, parent education, parent marital status, parent nativity, and parent age
Adjusted for child race/ethnicity, parent marital status, parent nativity, and parent age
Adjusted for parent nativity
Adjusted for parent education, parent marital status, parent nativity, and parent age
Adjusted for child race/ethnicity, parent education, and parent marital status
Adjusted for parent marital status
Adjusted for child race/ethnicity, parent education, parent marital status, and parent nativity
Adjusted for child race/ethnicity, parent education, parent marital status, parent nativity and parent age
Adjusted for child race/ethnicity and parent nativity
Adjusted for child race/ethnicity, parent marital status, parent nativity and parent age
Figure 1. Association of parent perceptions of neighborhood collective efficacy and health (n=319).
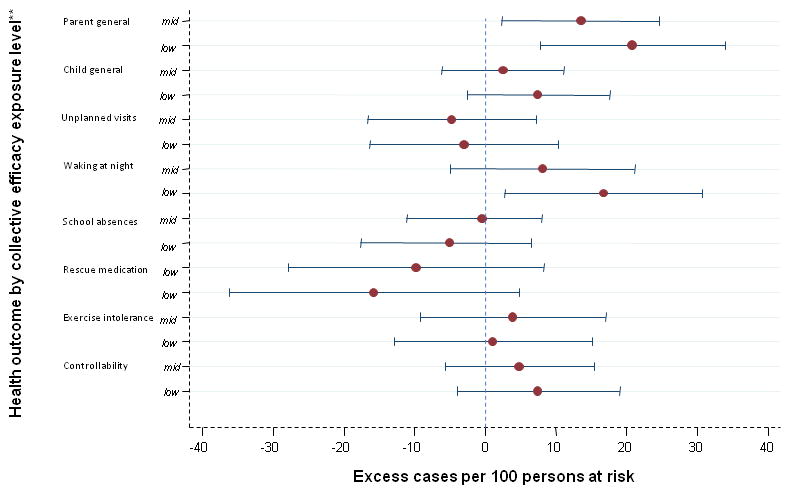
*Adjusted for parent race, age, education, marital status, and/or nativity
**Each RD contrasts the middle (mid) or the low exposure level with the high level
Figure 2. Association of parent perceptions of neighborhood physical/social order and health (n=319).
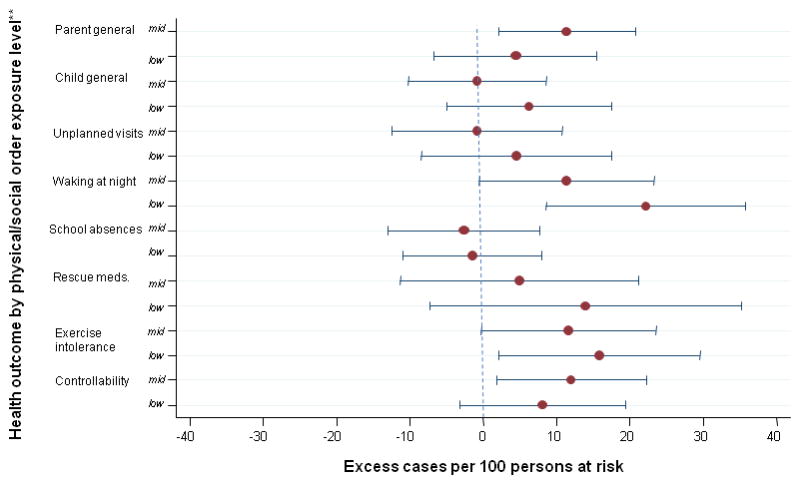
*Adjusted for parent race, age, education, marital status, and/or nativity
**Each RD contrasts the middle (mid) or the low exposure level with the high level
Collective efficacy
The association of general health and “collective efficacy” was strong for parents [RDmid v. high =13.5 (95% CI: 2.3, 24.6); RDlow v. high =20.8 (95% CI: 7.8, 33.9)] but not for children. (table 3). “Waking at night” was strongly associated with “collective efficacy” yielding RDlow v. high approximately twice as large as RDmid v. high: [RDmid v. high =8.1 (95% CI: −5.0, 21.1); RDlow v. high =16.7 (95% CI: 2.8, 30.6)] (table 4).
Physical/social order
For the association of “order” with parent health, RDmid v. high was unexpectedly larger [11.4 (95% CI: 2.1, 20.7)] than RDlow vs. high [4.4 (95% CI: −6.7, 15.5)], while for child health, estimates for both contrasts were nearly null (table 3). “Waking at night” had a strong association with “order,” again yielding RDlow v. high approximately twice as large as RDmid v. high: [RDmid v. high =11.4 (95% CI: −0.5, 23.3); RDlow v. high =22.2 (95% CI: 8.6, 35.8)] (table 4). Exercise intolerance [RDlow v. high =15.8 (95% CI: 2.1, 29.5)] and controllability [RDmid v. high (12.0 (95% CI: 1.8, 22.3)] had moderately strong associations with “order” (table 4).
Discussion
Overall, results supported the study’s hypothesis; exposure levels reflecting more negative neighborhood perceptions tended to be associated with higher risk of undesirable general and respiratory health outcomes. While perceptions are subjective and do not necessarily reflect actual neighborhood characteristics, they may be important proxies of the psychological burden of stressors on individuals.45 This study adds to growing evidence that the social environment, in addition to the physical environment, contributes to asthma burden in urban areas. Specifically, this study furthers the conceptualization of psychological stress as a “social pollutant” that may be “breathed” into the body.27 Acute and chronic stressors experienced by low-income, urban dwellers may impact health through psychological stress pathways, and the experience of psychological distress may be influenced by individuals’ perceptions. Implications of these findings are different than for “conventional” risk factors, that is, interventions must address not only “bricks and mortar” but individuals’ reactions to stress and attempt to mitigate the effects of stressors while structural solutions to health inequities are sought.
Low-SES populations, such as in the current study, may have increased vulnerability to respiratory disease because of increased exposure to acute and chronic stressors which cause psychological stress and its sequelae. 18–20, 29, 46 Single parenting may add to the burden of such circumstances.47 Exposure to violence, problematic family relationships, parenting difficulties, caregiver stress, critical attitudes of one’s mother, and negative life events have been related to wheeze, asthma onset and/or adverse asthma outcomes among infants and youths. 22–25, 48–52 Psychological stress experienced by parents of children with asthma may lead to impaired problem solving, influence reporting of symptoms, quality of life, and perceptions of asthma outcomes, and allow suboptimal disease management and healthcare utilization. 28, 31, 48, 49
A study of neighborhood-level variation in asthma and respiratory diseases in Chicago found that collective efficacy, but not disorder (observable physical and social decay), was protective.13 The authors hypothesized that collective efficacy may protect against respiratory diseases through: 1) social control of health-compromising behaviors; 2) access to health services; 3) management of physical hazards; and 4) promotion of psychosocial health by minimizing fear of being outside and engaging with community. The study differs from the current study by investigating neighborhood- rather than individual-level exposures; however, parents’ perceptions may indeed be correlated with contextual factors such as collective efficacy and work through similar mechanisms to affect health.
Not all associations were strong, and results differed somewhat for “collective efficacy” and “order.” Associations of parents’ perceptions with their own general health were moderate to strong while associations with their children’s general health were nearly null. The influence of psychological stress through physiologic mechanisms (directly affecting a parent’s health) may be stronger than through behavioral mechanisms (indirectly impacting disease management for their child). It is also possible that parents reported their children’s health less accurately than their own, obscuring associations.
Whereas general health outcomes were more strongly associated with “collective efficacy,” child respiratory outcomes tended to be more strongly associated with “order.” The more material nature of the variables comprising “order” compared to the more interpersonal variables in “collective efficacy” may be correlated with asthma triggers (i.e. cockroaches, mold) that make disease management less predictable. Such triggers may be most common outside where exercise intolerance is likely to occur or rescue medication is needed because children are more active outdoors. The hypothesized direct effect of “collective efficacy” through physiologic pathways may be weak for children, who are typically less concerned with neighborhood affairs than adults.
“Waking at night” was the only respiratory outcome strongly associated with both exposures, and for both, the contrast of the least favorable exposure group and the most favorable group produced RDs twice as big as for the middle group. “Waking at night” is a relatively objective outcome, often used in surveys of asthma control and quality of life, (though waking from factors other than breathing difficulties, such as noise, may have been reported). Misclassification may have biased RDs toward the null. Other outcomes are more subjective, involve health behaviors and attitudes, and may not accurately reflect symptom frequency/severity. It is unlikely that all children consistently reported symptoms that occurred while away from their parents. Associations of “school absences,” “rescue medication use” and “unplanned medical visits” with both neighborhood exposures were nearly null. The current sample compared to the main study’s sample had a lower proportion of children with the unfavorable outcome for all three of these outcomes. Further, these outcomes depend not only on disease activity but on resources and health behaviors. The current sample had a higher proportion of foreign-born and Spanish-speaking parents; access to care and cultural health beliefs and practices may have caused underestimates in outcomes. Undiagnosed children were excluded from analyses with “rescue medication use” as the outcome, leading to reduced power. Bias might have resulted from the self-report nature of both exposures and outcomes. Coding outcomes as binary rather than ordinal variables should have minimizedbias.
The study sample was low-income by definition given the recruitment strategy of the main study, thereby minimizing confounding by SES. Parent education was included as a covariate to further control for confounding by SES. The main study’s respiratory survey captured 90% of the schools’ enrollment (n=12,699), thereby adding to the generalizability of results. The baseline survey achieved a 64% response rate, and survey 2, which provided the data for the current analysis, had a 47% response rate. Selection bias may have influenced results.
We used exploratory factor analysis with unique data not collected in large-scale health studies to create multidimensional measures of perceptions of neighborhoods robust to problems with single variables, for example, influences of secular, geographic or seasonal trends. This strategy summarized the relationships within a collection of public policy-relevant variables among a low-income, urban, racially/ethnically heterogeneous sample of parents of young children., allowing us to address the complex circumstances of low-income, urban families that impact onset and expression of asthma, Summary scores, rather than PCA factor loadings, were used to represent exposures since the component variables were untested and exploratory, with no evidence of reliability or validity; summary scores also preserved the variation in the data, beneficial for their subsequent use in multivariate analyses.53
Assessment of EMM informed the decision not to stratify analyses based on asthma diagnosis status (though undiagnosed children were excluded a priori from analyses of rescue medication use). RDs tended to be imprecise due to small sample size; stratification or interaction terms in models would have further compromised precision. Parents of undiagnosed children may have been less aware of symptoms, resulting in outcome misclassification. Nonetheless, interventions to address health-harming neighborhood stressors would likely be targeted to low-income families generally and not only to those with diagnosed asthma, especially since underdiagnosis is a well-documented problem.9, 10 The average effect for all children with respiratory problems was desired since diagnosis is a sociological process based not only on underlying disease but on family and community resources and health attitudes and practices.
Asthma etiology is complex. This study, cross-sectional in design and limited to one city, did not incorporate all known risk factors or test causation. “Traditional,” “environmental” risk factors (e.g., mold and cockroaches), have been well-documented in the biomedical literature and were not the focus of this study. Biological (e.g., cytokines and cortisol) and behavioral (e.g., smoking and allergen reduction in homes) measures are hypothesized to be on causal pathways and were not included as covariates. Future research must address psychological factors over the lifecourse, the possibility that the asthma phenotype is programmed before birth, and reverse causality, since stress and consequent problems may be caused or aggravated by having asthma or caring for someone with asthma.54 In addition to an ecological perspective, longitudinal data and a multi-level approach are required to understand structural forces that influence the distribution of neighborhood stressors -- and as a result, psychological stress.
These findings emphasize the importance of addressing not only neighborhood-level mediators of the effects of low-SES neighborhoods but residents’ sense of their neighborhoods. Mediators, in fact, are not expected to explain additional variance in asthma outcomes. However, all relevant risk factors must be identified if we are to understand causal mechanisms. Psychological stress may be a crucial determinant of the burden of asthma and other illnesses experienced by urban populations. Sociodemographic factors and health outcomes are not necessarily easy targets of interventions, but recognizing which stressors are associated with asthma and which groups are most vulnerable to stress is necessary for effective public health and social policies and reduction of health disparities.
Acknowledgments
This analysis is based on data collected for the Social Factors and the Environment in Pediatric Asthma Study, which was funded by the National Institute of Environmental Health Sciences of the National Institutes of Health (grant 1 R01 ES10908). Carolyn A. Berry, PhD, Center for Healthcare Strategies, Inc. and Steven Wing, PhD, Department of Epidemiology, University of North Carolina Gillings School of Global Public Health, provided guidance with this study and this manuscript.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Akinbami LJ. The state of childhood asthma, united states, 1980–2005. advance data from vital and health statistics. Hyattsville, MD: National Center for Health Statistics; 2006. p. 381. [PubMed] [Google Scholar]
- 2.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- 3.Marder D, Targonski P, Orris P, Persky V, Addington W. Effect of racial and socioeconomic factors on asthma mortality in chicago. Chest. 1992;101(6 Suppl):426S–429S. doi: 10.1378/chest.101.6_supplement.426s. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SD, Whitman S. Asthma hospitalizations and mortality in chicago: An epidemiologic overview. Chest. 1999;116(4 Suppl 1):135S–141S. doi: 10.1378/chest.116.suppl_2.135s. [DOI] [PubMed] [Google Scholar]
- 5.Weiss KB, Wagener DK. Geographic variations in US asthma mortality: Small-area analyses of excess mortality, 1981–1985. Am J Epidemiol. 1990;132(1 Suppl):S107–15. doi: 10.1093/oxfordjournals.aje.a115771. [DOI] [PubMed] [Google Scholar]
- 6.Weiss KB, Wagener DK. Changing patterns of asthma mortality. identifying target populations at high risk. JAMA. 1990;264(13):1683–1687. [PubMed] [Google Scholar]
- 7.Persky VW, Slezak J, Contreras A, et al. Relationships of race and socioeconomic status with prevalence, severity, and symptoms of asthma in chicago school children. Ann Allergy Asthma Immunol. 1998;81(3):266–271. doi: 10.1016/S1081-1206(10)62824-4. [DOI] [PubMed] [Google Scholar]
- 8.Lenhardt RO, Catrambone CD, McDermott MF, Walter J, Williams SG, Weiss KB. Improving pediatric asthma care through surveillance: The illinois emergency department asthma collaborative. Pediatrics. 2006;117(4 Pt 2):S96–105. doi: 10.1542/peds.2005-2000G. [DOI] [PubMed] [Google Scholar]
- 9.Quinn K, Shalowitz MU, Berry CA, Mijanovich T, Wolf RL. Racial and ethnic disparities in diagnosed and possible undiagnosed asthma among public-school children in chicago. Am J Public Health. 2006;96(9):1599–1603. doi: 10.2105/AJPH.2005.071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shalowitz MU, Sadowski LM, Kumar R, Weiss KB, Shannon JJ. Asthma burden in a citywide, diverse sample of elementary schoolchildren in chicago. Ambul Pediatr. 2007;7(4):271–277. doi: 10.1016/j.ambp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Shields AE, Comstock C, Weiss KB. Variations in asthma care by race/ethnicity among children enrolled in a state medicaid program. Pediatrics. 2004;113(3 Pt 1):496–504. doi: 10.1542/peds.113.3.496. [DOI] [PubMed] [Google Scholar]
- 12.Weiss KB. Eliminating asthma disparities: Introduction to the chicago story. Chest. 2007;132(5 Suppl):856S–857S. doi: 10.1378/chest.07-1929. [DOI] [PubMed] [Google Scholar]
- 13.Cagney KA, Browning CR. Exploring neighborhood-level variation in asthma and other respiratory diseases: The contribution of neighborhood social context. J Gen Intern Med. 2004;19(3):229–236. doi: 10.1111/j.1525-1497.2004.30359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandel M, Wright RJ. When home is where the stress is: Expanding the dimensions of housing that influence asthma morbidity. Arch Dis Child. 2006;91(11):942–948. doi: 10.1136/adc.2006.098376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132(5 Suppl):757S–769S. doi: 10.1378/chest.07-1904. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SE, Repetti RL, Seeman T. Health psychology: What is an unhealthy environment and how does it get under the skin? Annu Rev Psychol. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- 17.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 18.McLeod JD, Kessler RC. Socioeconomic status differences in vulnerability to undesirable life events. J Health Soc Behav. 1990;31(2):162–172. [PubMed] [Google Scholar]
- 19.Gee GC, Payne-Sturges DC. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler RC. Stress, social status, and psychological distress. J Health Soc Behav. 1979;20(3):259–272. [PubMed] [Google Scholar]
- 21.Wright RJ. Stress and atopic disorders. J Allergy Clin Immunol. 2005;116(6):1301–1306. doi: 10.1016/j.jaci.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Sandberg S, Jarvenpaa S, Penttinen A, Paton JY, McCann DC. Asthma exacerbations in children immediately following stressful life events: A cox’s hierarchical regression. Thorax. 2004;59(12):1046–1051. doi: 10.1136/thx.2004.024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandberg S, Paton JY, Ahola S, et al. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356(9234):982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- 24.Kozyrskyj AL, Mai XM, McGrath P, Hayglass KT, Becker AB, Macneil B. Continued exposure to maternal distress in early life is associated with an increased risk of childhood asthma. Am J Respir Crit Care Med. 2008;177(2):142–147. doi: 10.1164/rccm.200703-381OC. [DOI] [PubMed] [Google Scholar]
- 25.Chen E, Chim LS, Strunk RC, Miller GE. The role of the social environment in children and adolescents with asthma. Am J Respir Crit Care Med. 2007;176(7):644–649. doi: 10.1164/rccm.200610-1473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ippoliti F, De Santis W, Volterrani A, et al. Psychological stress affects response to sublingual immunotherapy in asthmatic children allergic to house dust mite. Pediatr Allergy Immunol. 2006;17(5):337–345. doi: 10.1111/j.1399-3038.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 27.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5(1):23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Annett RD, Bender BG, DuHamel TR, Lapidus J. Factors influencing parent reports on quality of life for children with asthma. J Asthma. 2003;40(5):577–587. doi: 10.1081/jas-120019030. [DOI] [PubMed] [Google Scholar]
- 29.Archea C, Yen IH, Chen H, et al. Negative life events and quality of life in adults with asthma. Thorax. 2007;62(2):139–146. doi: 10.1136/thx.2006.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booker CL, Gallaher P, Unger JB, Ritt-Olson A, Johnson CA. Stressful life events, smoking behavior, and intentions to smoke among and multiethnic sample of sixth graders. Ethn Health. 2004;9(4):369–397. doi: 10.1080/1355785042000285384. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett SJ, Kolodner K, Butz AM, Eggleston P, Malveaux FJ, Rand CS. Maternal depressive symptoms and emergency department use among inner-city children with asthma. Arch Pediatr Adolesc Med. 2001;155(3):347–353. doi: 10.1001/archpedi.155.3.347. [DOI] [PubMed] [Google Scholar]
- 32.Berry CA, Quinn K, Wolf R, Mosnaim G, Shalowitz M. Validation of the spanish and english versions of the asthma portion of the brief pediatric asthma screen plus among hispanics. Ann Allergy Asthma Immunol. 2005;95(1):53–60. doi: 10.1016/S1081-1206(10)61188-X. [DOI] [PubMed] [Google Scholar]
- 33.Wolf RL, Berry CA, Quinn K. Development and validation of a brief pediatric screen for asthma and allergies among children. Ann Allergy Asthma Immunol. 2003;90(5):500–507. doi: 10.1016/S1081-1206(10)61843-1. [DOI] [PubMed] [Google Scholar]
- 34.Earls FJ, Brooks-Gunn J, Raudenbush SW, Sampson RJ. Project on human development in chicago neighborhoods: Community survey 1994–1995 [Google Scholar]
- 35.DeVellis RF. Scale Development: Theory and Applications. Thousand Oaks, CA: Sage Publications; 2003. [Google Scholar]
- 36.Landgraf JM, Abetz L, Ware JEJ. Child Health Questionnaire (CHQ): A User’s Manual. Boston, MA: HealthAct; 1999. [Google Scholar]
- 37.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Lara M, Sherbourne C, Duan N, Morales L, Gergen P, Brook RH. An english and spanish pediatric asthma symptom scale. Med Care. 2000;38(3):342–350. doi: 10.1097/00005650-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Murphy KR, Zeiger RS, Kosinski M, et al. Test for respiratory and asthma control in kids (TRACK): A caregiver-completed questionnaire for preschool-aged children. J Allergy Clin Immunol. 2009;123(4):833–9.e9. doi: 10.1016/j.jaci.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 40.Schatz M, Sorkness CA, Li JT, et al. Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 42.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 43.Masten WG, Caldwell-Colbert AT, Alcala SJ, Mijares BE. Confiabilidad y validez de la escala de depresion del centro de estudios epidemiologicos. Hispanic Journal of Behavioral Sciences. 1986;8:77–84. [Google Scholar]
- 44.Radloff LS. The CES-D depression scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 45.Cohen S, Williamson G. Perceived stress in a probability sample of the united states. In: Spacapam S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 46.Wright RJ. Further evidence that the wealthier are healthier: Negative life events and asthma-specific quality of life. Thorax. 2007;62(2):106–108. doi: 10.1136/thx.2006.067926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ceballo R, McLoyd VC. Social support and parenting in poor, dangerous neighborhoods. Child Dev. 2002;73(4):1310–1321. doi: 10.1111/1467-8624.00473. [DOI] [PubMed] [Google Scholar]
- 48.Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104(6):1274–1280. doi: 10.1542/peds.104.6.1274. [DOI] [PubMed] [Google Scholar]
- 49.Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: The inner-city asthma study. Am J Public Health. 2004;94(4):625–632. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turyk ME, Hernandez E, Wright RJ, et al. Stressful life events and asthma in adolescents. Pediatr Allergy Immunol. 2008;19(3):255–263. doi: 10.1111/j.1399-3038.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 51.Shalowitz MU, Berry CA, Quinn KA, Wolf RL. The relationship of life stressors and maternal depression to pediatric asthma morbidity in a subspecialty practice. Ambul Pediatr. 2001;1(4):185–193. doi: 10.1367/1539-4409(2001)001<0185:trolsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Kilpelainen M, Koskenvuo M, Helenius H, Terho EO. Stressful life events promote the manifestation of asthma and atopic diseases. Clin Exp Allergy. 2002;32(2):256–263. doi: 10.1046/j.1365-2222.2002.01282.x. [DOI] [PubMed] [Google Scholar]
- 53.Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate Data Analysis. 3. New York: Macmillan; 1992. [Google Scholar]
- 54.Wright RJ. Exploring biopsychosocial influences on asthma expression in both the family and community context. Am J Respir Crit Care Med. 2008;177(2):129–130. doi: 10.1164/rccm.200710-1526ED. [DOI] [PubMed] [Google Scholar]


