Abstract
In most adult patients, hepatitis B is a self-limiting disease leading to life-long protective immunity, which is the consequence of a robust adaptive immune response occurring weeks after HBV infection. Intriguingly, HBV-specific T cells can be detected shortly after infection but the mechanisms underlying this early immune priming and its consequences for subsequent control of viral replication are poorly understood. Using primary human and murine hepatocytes and mouse models of transgenic and adenoviral HBV expression, we show that HBV-expressing hepatocytes produce endoplasmic reticulum (ER)-associated endogenous antigenic lipids including lysophospholipids that are generated by HBV-induced secretory phospholipases and lead to activation of natural killer T (NKT) cells. The absence of NKT cells, CD1d or a defect in ER-associated transfer of lipids onto CD1d results in diminished HBV-specific T and B cell responses and delayed viral control. NKT cells may therefore contribute to control of HBV infection through sensing of HBV-induced modified self-lipids.
Conventional T and B cells play a crucial role in HBV infection.1–5 In contrast, the contribution of cells at the interface between innate and adaptive immunity such as NKT cells remains controversial.6 NKT cells respond in a T cell receptor (TCR)-restricted manner to lipid antigens presented by CD1d and exhibit pronounced cytokine secretion within hours of cognate antigen recognition, which enables broad effects on activation of other innate (NK) and adaptive immune cells (T and B cells).7,8
The role that NKT cells play in HBV infection is unclear. Analysis of liver gene expression in chimpanzees two weeks after HBV infection has shown evidence for a lack of induction of immune-related genes suggesting that HBV acts a stealth virus that escapes innate immune responses during early infection.9 On the other hand, studies in HBV-infected humans and chimpanzees have demonstrated the presence of HBV-specific T- and B cells within weeks of infection consistent with successful priming of an adaptive immune response.1,10,11 These observations suggest that HBV might be susceptible to recognition by the immune system directly following infection. Accordingly, recent studies in animal models of Hepadnaviridae infection and HBV patients have demonstrated activation of NKT cells at very early time points following infection. Thus, infection with woodchuck hepatitis virus led to hepatic NKT cell infiltration within 48 hours, which correlated with IFN-γ secretion and temporary suppression of viral replication.12 These findings are in accordance with the fact that pharmacological stimulation of invariant (i) NKT cells by administration of the iNKT cell antigen α-galactosylceramide (αGalCer) led to rapid IFN-γ-dependent inhibition of viral replication in HBV transgenic mice.13 Similarly, a study of humans during the incubation phase of HBV infection demonstrated increased levels of peripheral NK cells in accordance with innate immune activation early after HBV infection.11 Most notably, a recent report on two HBV patients demonstrated very early activation of peripheral natural T cells, a population of cells that phenotypically resembles classical NKT cells. Natural T cell activation preceded activation of NK and conventional T cells and was associated with subsequent control of HBV infection.10 These studies demonstrate a correlation between viral control and NKT cell activation. To investigate whether NKT cells are an important checkpoint that contributes to control of HBV infection, we studied various in vitro and in vivo HBV models.
RESULTS
Early activation of NKT cells in response to Ad-HBV
To study NKT cell responses we investigated a mouse model that overcomes non-permissiveness of murine hepatocytes to HBV through adenoviral delivery of a replication-competent HBV genome under the control of its endogenous promoters.14–16 Injection of 1×109 HBV-expressing adenoviral particles (Ad-HBV), a dose shown by us (Suppl. Fig. 1a) and others16 to induce an immune response against HBV but not the adenoviral carrier, led to HBV replication (Suppl. Fig. 1b) followed by a rapid drop in hepatic HBV DNA and serum HBsAg that preceded hepatitis (Suppl. Fig. 1b–c). A control adenovirus expressing β-galactosidase (Ad-LacZ) did not lead to hepatitis, confirming HBV-dependent inflammation (Suppl. Fig. 1a). 14–16 Since Ad-HBV infection in mice resembles the course of natural HBV infection in humans in several aspects17, we further studied NKT cell responses in this model.
Interestingly, the entire population of liver but not splenic iNKT cells exhibited activation and IFN-γ secretion as early as one day after Ad-HBV but not Ad-LacZ administration and thus before histological inflammation and serum ALT elevation (Fig. 1a–c, Suppl. Fig. 2). Similar to iNKT cells, hepatic but not splenic non-invariant NKT cells, an NKT cell population expressing a more diverse set of TCRs that were detected by a novel reporter model (see Suppl. Results and Suppl. Fig. 3), displayed pronounced activation and IFN-γ secretion in response to Ad-HBV (Fig. 1d–f, Suppl. Fig. 4). Thus, invariant and non-invariant NKT cells exhibited broad and rapid activation upon Ad-HBV exposure and directly contributed to secretion of IFN-γ. In contrast, activation of NK and T cells was not observed until three days after Ad-HBV challenge (Figure 1g–i and data not shown) and thus followed activation of NKT cells, similar to observations in HBV patients.10
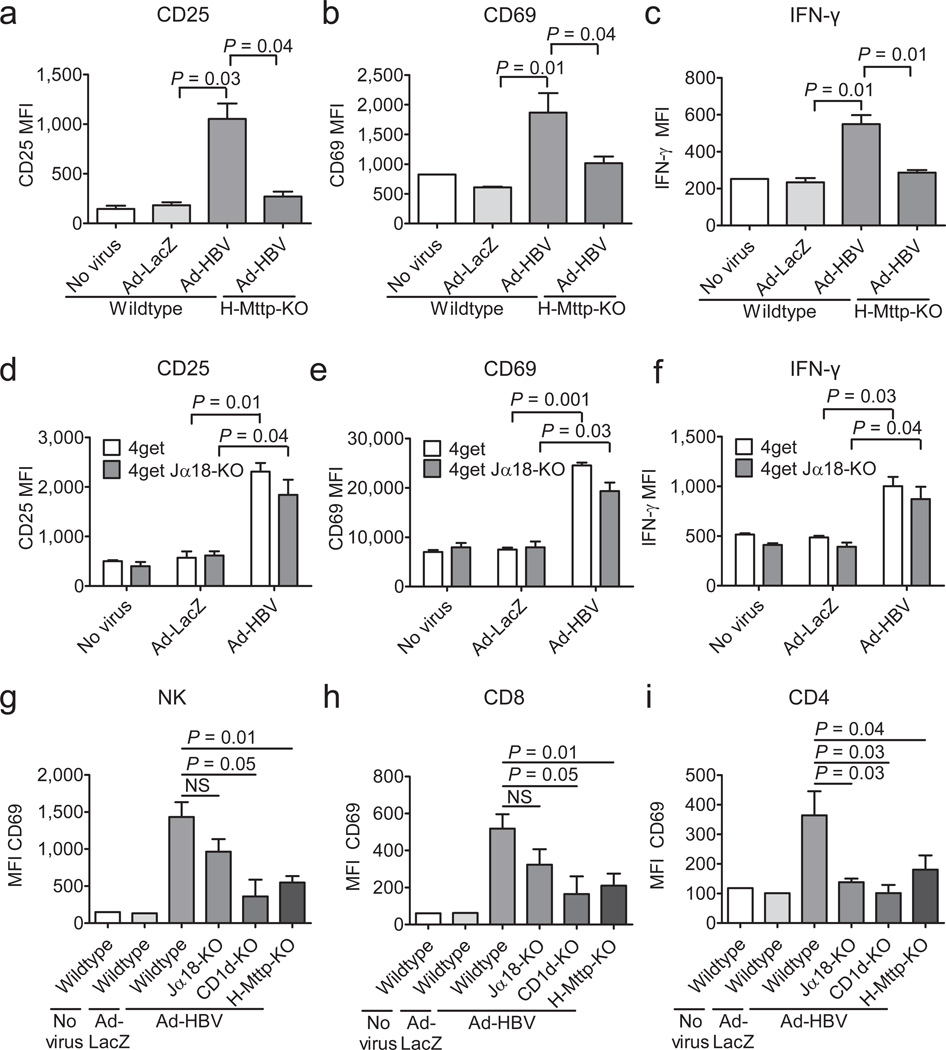
Figure 1.
NKT cells become activated in response to Ad-HBV and contribute to adaptive immune responses. Expression of the indicated markers on hepatic iNKT cells (a–c, αGalCer/CD1d+CD3+) and non-invariant NKT cells (d–f, 4Get X Jα18-KO) two days after i.v. administration of PBS (no virus), Ad-HBV or Ad-LacZ as determined by flow cytometry. In a–c, H-Mttp-KO and wildtype mice are shown. (g–i) NK (CD3− NK1.1+) and conventional (αGalCer/CD1d−) CD8+ and CD4+ T cell activation five days after virus injection as described above. Shown is the mean fluorescence intensity (MFI). Shown are mean ± s.e.m of 4–6 mice per group. Results are representative of three independent experiments.
NKT activation contributes to innate and adaptive immune responses against HBV
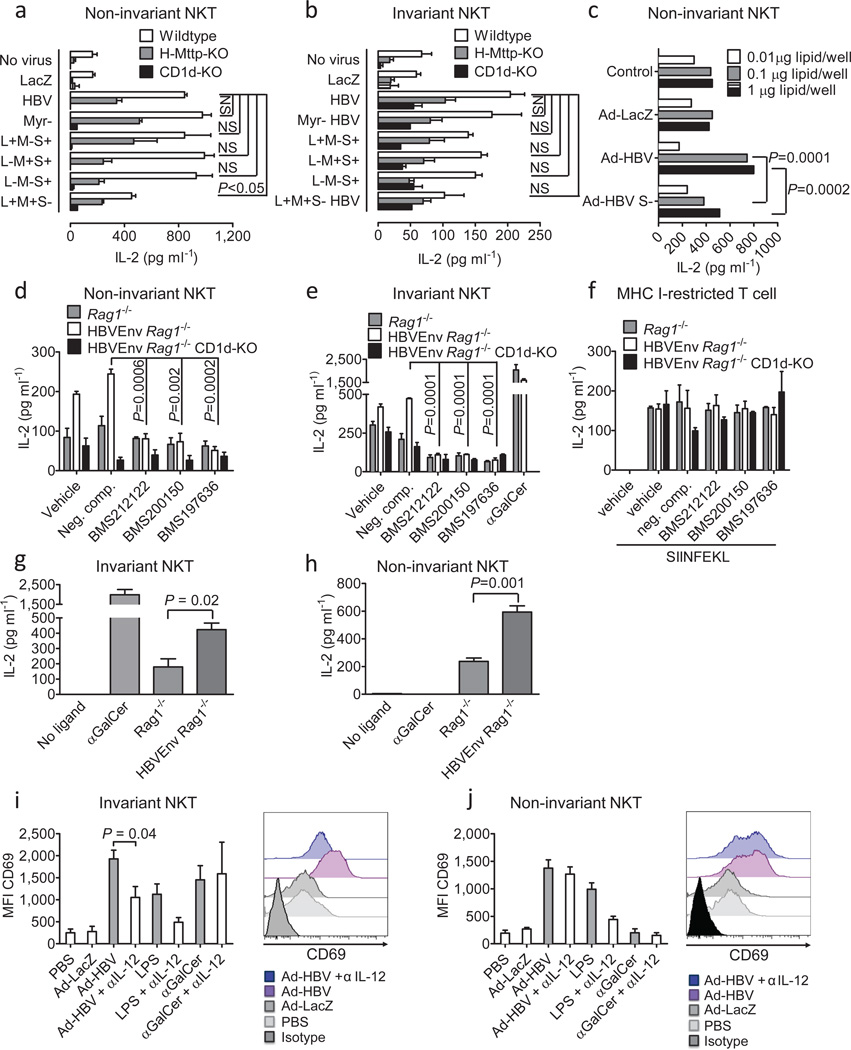
To investigate whether NKT cells contribute to innate and adaptive immune responses against HBV, we analyzed activation of liver mononuclear cells (LMNCs) in response to Ad-HBV in mice lacking invariant (Jα18−/−) or all NKT cells (CD1d−/−). In wild-type mice, hepatic NK, CD4+, and CD8+ T cells exhibited strong activation and IFN-γ secretion in response to Ad-HBV, but not Ad-LacZ. In contrast, CD1d−/− mice exhibited significantly diminished NK, CD4+, and CD8+ T cell activation, whereas Jα18-deficiency predominantly affected CD4+ T cell activation (Fig. 1g–i). Viral transduction of hepatocytes was similar in all mouse strains (Suppl. Fig. 5).
NKT-dependent activation was also observed for HBV-specific CD8+ T cell responses against envelope- (S190–197) and core-antigens (C93–100) but not in response to phytohemagglutinin (Fig. 2a, Suppl. Fig. 6a–b). Analysis of LMNC responses to pools of peptides spanning the entire HBV envelope18 revealed pronounced defects in the magnitude but not diversity of HBV-specific CD8+ T cell responses in HBV envelope transgenic (Tg) × Rag1−/− × CD1d−/− (HBVEnv Rag1−/− CD1d−/−) mice that received, by adoptive transfer, wild-type splenocytes (Fig. 2b, Suppl. Fig. 6c). Adaptive immune defects extended to B cells since levels of anti-HBsAg antibodies were lower in Ad-HBV-challenged CD1d−/− and Jα18−/− mice (Fig. 2c) and in HBVEnv Rag1−/− CD1d−/− mice (Fig. 2d), resulting in lack of sustained HBsAg clearance from serum (Fig. 2e). These data show that NKT cells contribute to activation of NK, T and B cells in different HBV mouse models.
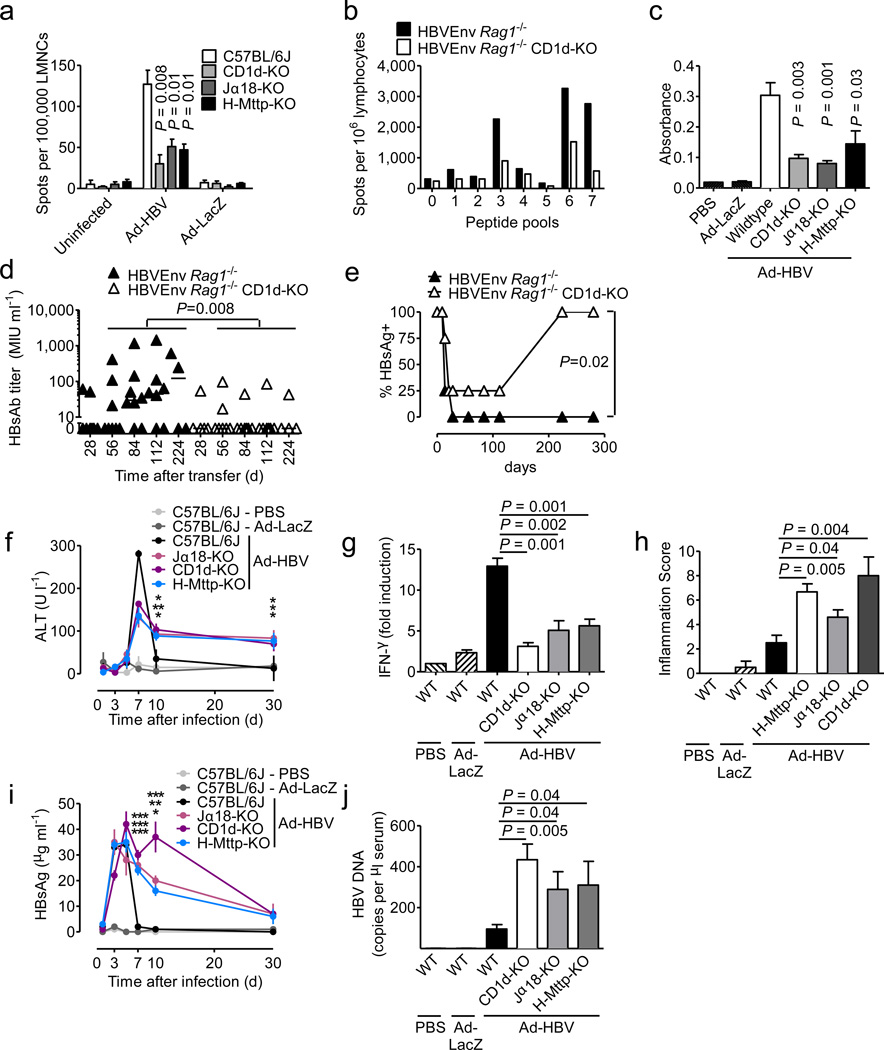
Figure 2.
NKT cells contribute to control of Ad-HBV and prevent chronic hepatitis. (a) IFN-γ secretion of LMNCs after in vitro stimulation with HBsAg S190–197 peptide 14 days after injection of the indicated viruses. (b) LMNCs obtained eight days after wildtype splenocyte transfer into HBV envelope × Rag1−/− (HBVEnv Rag1−/−) and HBV envelope × Rag1−/− × CD1d−/− (HBVEnv Rag1−/− CD1d-KO) mice (C57BL/6 background) were restimulated in the presence of the indicated HBV envelope peptide pools and IFN-γ-secreting cells were detected by ELISpot. (c) Anti-HBsAg level in wildtype mice 30 days after injection of the indicated viruses. d–e) Anti-HBsAg antibody titers (d) and HBsAg-positive mice (e) at the indicated days after transfer of wildtype splenocytes into the indicated HBsAg transgenic mouse strains. Serum alanine aminotransferase (ALT) (f), IFN-γ mRNA in liver tissue as determined by qPCR (g, day 5 after virus injection), histological grading of liver inflammation (Ishak et al.41) (h, day 30 after virus injection), serum HBsAg (i) and serum HBV DNA (j, day 7 after virus injection). In f and i significance levels as indicated by asterisks reflect from top to bottom Jα18-KO, CD1d-KO, H-Mttp-KO vs. control. Mean ± s.e.m. is shown (6–8 mice per group). Results are representative of two independent experiments.
NKT cells contribute to early antiviral action in response to Ad-HBV
In accordance with a central role of NKT cells in HBV-induced hepatitis19,20, CD1d−/− and Jα18−/− mice exhibited reduced peak levels of serum ALT (Fig. 2f), liver IFN-α (Fig. 2g), and hepatic immune cell infiltration (data not shown). However, CD1d−/− and Jα18−/− mice developed chronic low-grade inflammation that persisted at least to day 30 (Fig. 2f, h), the end of the observation period. This was associated with delayed clearance of serum HBsAg (Fig. 2i) and serum and liver HBV DNA (Fig. 2j, Suppl. Fig. 7d–e), consistent with defects in viral control leading to chronic inflammation. In accordance with a critical role of CD8+ T cells in the antiviral response against HBV1, NKT cells were necessary but not sufficient for viral control since Tap1−/− mice lacking CD8+ T cells also exhibited severe defects in HBV clearance (Suppl. Fig. 7). These results show that NKT cells contribute to the initiation of antiviral immune responses against HBV and to viral control and prevention of chronic viral replication in the Ad-HBV model.
HBV- and Ad-HBV-infected human and murine hepatocytes activate NKT cells
To identify the cell type responsible for NKT cell activation upon Ad-HBV challenge, we studied activation of NKT cell hybridomas in response to liver and spleen mononuclear cells and primary hepatocytes obtained from Ad-HBV-infected mice. Only Ad-HBVtransduced hepatocytes but not T cell-depleted mononuclear cells induced activation of NKT cells (Fig. 3a, Suppl. Fig. 8a and data not shown). Similar observations were made upon in vitro exposure of primary hepatocytes to Ad-HBV (Suppl. Fig. 8b; for purity and transduction of primary hepatocytes see Suppl. Fig. 9). NKT activation was CD1drestricted (Fig. 3b) and limited to NKT cells, as demonstrated by unaffected MHC class I presentation (Fig. 3c). Activation was specific for a subset of invariant (24.7 cell line) and non-invariant (14S.6 cell line) murine NKT cell hybridomas (Fig. 3a) and could also be demonstrated with human primary hepatocytes infected with Ad-HBV or a primary (non-adenoviral) HBV isolate (Fig. 3d–e). These data reveal an unanticipated role of hepatocytes in HBV-dependent NKT cell activation.
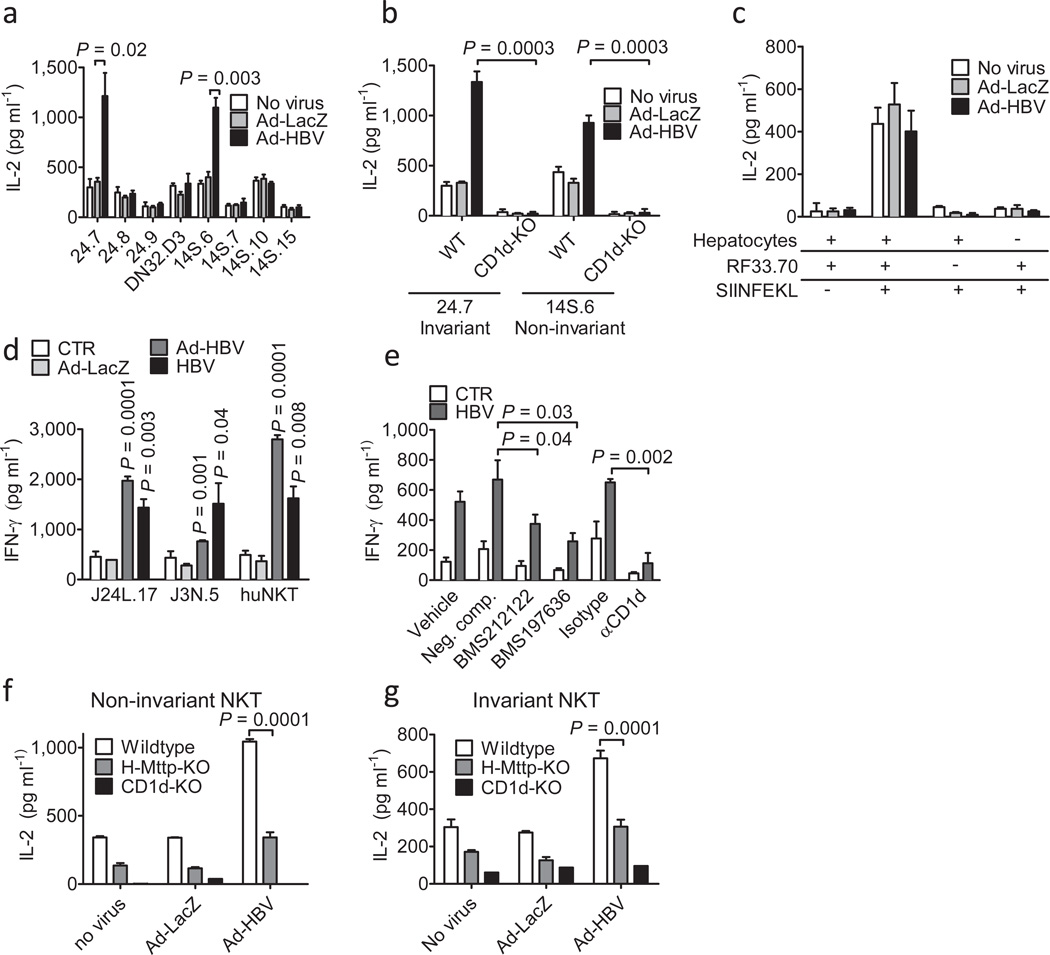
Figure 3.
NKT activation in response to HBV is mediated by hepatocytes and dependent on hepatocyte CD1d and MTP. (a) Activation of invariant (24.7, 24.8, 24.9, DN32.D3) and non-invariant (14S.6, 14S.7, 14S.10, 14S.15) NKT hybridomas in response to coculture with wildtype primary mouse hepatocytes 48h after Ad-HBV transduction. (b) Activation of invariant 24.7 and non-invariant 14S.6 in response to primary hepatocytes of wildtype and CD1d-KO mice 48h after virus administration. Viral transduction was similar in hepatocytes from both strains (see Suppl. Fig. 9) (c) Activation of the MHC class I-restricted T cell hybridoma RF33.70 in response to SIINFEKL (1 µg/ml) presented by primary hepatocytes 48h post infection. (d) Activation of human iNKT cell clones J24L.17, J3N.5, and Jα24Vβ11-positive human iNKT cells expanded from peripheral blood in response to human hepatocytes infected with Ad-LacZ, Ad-HBV, and a primary HBV isolate. (e) Primary human hepatocytes were infected with a primary HBV isolate and were treated with blocking CD1d antibodies and MTP-inhibitors (BMS212122, BMS197636) or a structurally related negative compound. IFN-γ secretion of J24L.17 NKT cells was determined 72h after infection and 48h after MTP inhibitor treatment. (f–g) Activation of non-invariant 14S.6 (f) and invariant 24.7 (g) by primary hepatocytes of the indicated mouse strains 48h after infection with the indicated viruses or vehicle. Cytokine secretion in (a–g) was determined by ELISA 24h after coculture. Mean ± s.e.m. of triplicate cultures are shown. Results are representative of three independent experiments.
HBV-induced NKT cell activation is dependent on hepatocyte microsomal triglyceride transfer protein (MTP)
MTP is an ER-resident protein that transfers endogenous phospholipids onto CD1d and is critical for CD1d function.21–25 To delineate the role of hepatocyte MTP and CD1d in Ad-HBV infection, we generated mice with hepatocyte-specific deletion of Mttp encoding for MTP (H-Mttp−/− 26, Suppl. Fig. 10a). MTP-deficiency led to severe defects in the presentation of endogenous and exogenous lipid antigens that were specific for hepatocytes and limited to CD1d (Suppl. Fig. 10b–e).
Consistent with a critical role of MTP in HBV-induced and CD1d-restricted antigen presentation, primary hepatocytes from H-Mttp−/− mice exhibited impaired activation of NKT cells in response to Ad-HBV in vitro (Fig. 3f–g, Suppl. Fig. 10). Similar observations were made upon chemical inhibition of MTP (Suppl. Fig. 11) and using human hepatocytes infected with a primary HBV isolate (Fig. 3e). These results were confirmed by in vivo experiments, in which NKT cells from Ad-HBV-challenged H-Mttp−/− mice exhibited impaired activation and IFN-γ secretion (Fig. 1a–c). Accordingly, NKT cell-dependent activation of NK cells and HBV-specific T and B cells was impaired in HMttp−/− mice (Fig. 1g–i, Fig. 2a, c, Suppl. Fig. 6a). H-Mttp−/− mice exhibited diminished acute hepatitis upon Ad-HBV challenge, impaired viral control and chronic low-grade inflammation (Fig. 2, Supp. Fig. 7). These data confirm that hepatocytes are the antigen presenting cells responsible for NKT cell activation and show that this effect is dependent upon hepatocyte expression of MTP and CD1d.
Ad-HBV expression is associated with alterations in endogenous hepatocyte lipids
Ad-HBV-induced NKT cell activation was not the consequence of altered CD1d expression or trafficking (Suppl. Fig. 12a, b). Accordingly, iNKT cell activation by hepatocytes loaded with the exogenous model lipid was unaltered by Ad-HBV (Suppl. Fig. 12c).
Since HBV buds from the ER and selectively recruits ER lipids for its envelope27, we investigated whether HBV-induced NKT activation is the consequence of alterations in endogenous ER-acquired CD1d lipids and studied activation of NKT hybridomas in response to plate-bound CD1d loaded with lipids from Ad-HBV-infected primary murine hepatocytes. Even uninfected primary hepatocytes contained antigenic CD1d lipids that were enriched in microsomal ER preparations (Suppl. Fig. 13a–e). Importantly, microsomes from Ad-HBV- but not Ad-LacZ-transduced hepatocytes exhibited significantly increased activation of invariant and non-invariant NKT cell hybridomas (Fig. 4a–b). Similar alterations in microsomal lipids were found in CD1d−/− and H-Mttp−/− hepatocytes (Suppl. Fig. 13f and data not shown), demonstrating that these antigenic lipids were unable to be loaded (H-Mttp−/−) or presented by CD1d (CD1d−/−). Accordingly, purified MTP enhanced presentation of immunogenic Ad-HBV-dependent lipids (Fig. 4c).
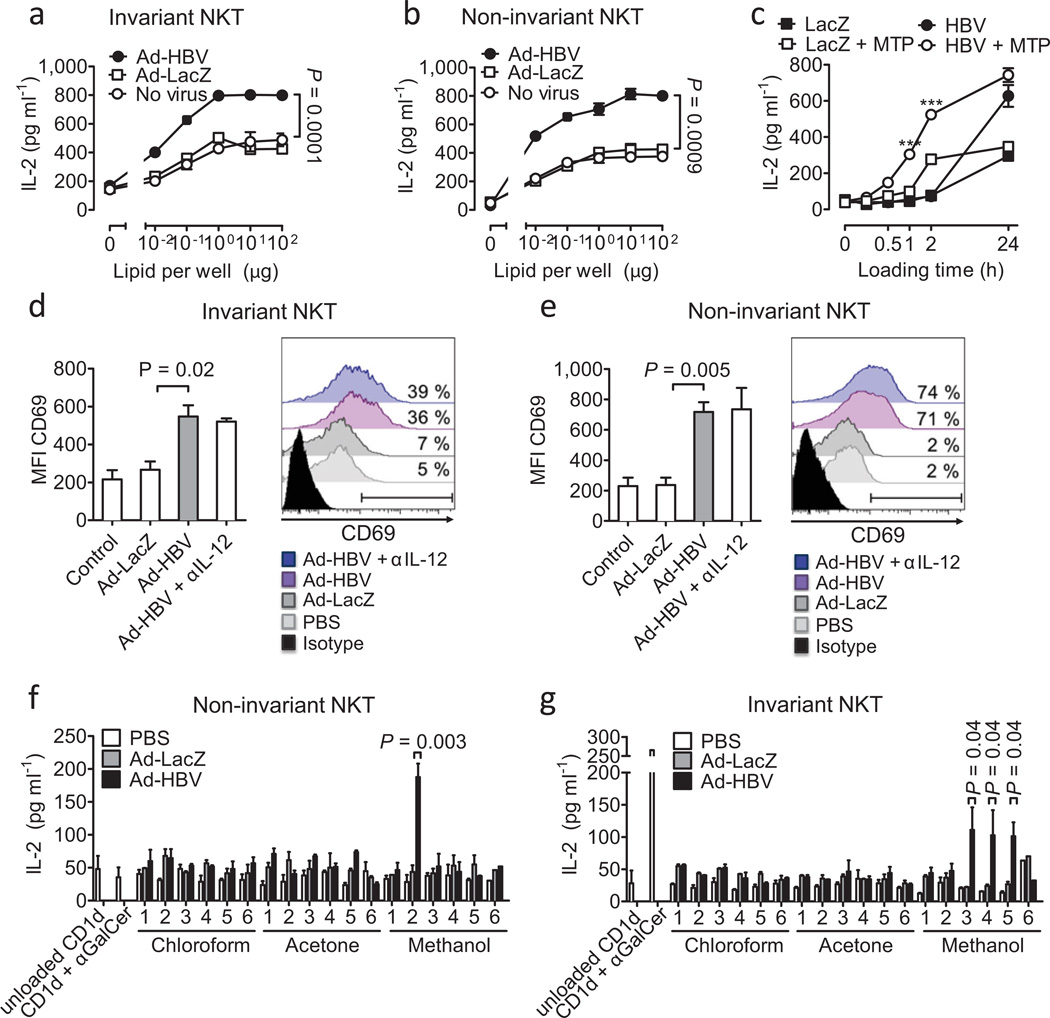
Figure 4.
Microsomal lipids of Ad-HBV-infected hepatocytes contain NKT cellactivating lipid antigens. (a–c) Platebound CD1d was loaded with microsomal lipids of hepatocytes infected with Ad-LacZ and Ad-HBV and lipids were presented to invariant 24.7 (a) and non-invariant 14S.6 (b) NKT cells. In (c), microsomal lipids were loaded in the presence or absence of purified MTP. ***p=0.0001 of HBV vs. HBV + MTP. (d–e) Activation of primary invariant (d) and non-invariant (e) NKT cells in response to platebound CD1d presenting microsomal lipids obtained from hepatocytes transduced with the indicated viruses. Invariant and non-invariant NKT cells were obtained as sorted GFP+ αGalCer/CD1d-tetramer+ 4Get (d) and GFP+ 4Get X Jα18-KO liver mononuclear cells (e), respectively. Flow cytometric analysis of CD69 (MFI, left; histogram, right) gated on GFP+ CD3+ cells is shown. Numbers in histograms indicate the percentage of cells that shift in expression of CD69 compared to microsomal lipids of uninfected cells. IL-12 neutralization demonstrates absence of indirect cytokine-mediated NKT cell activation as expected in an APC-free assay. (f–g) Activation of non-invariant 14S.6 (f) and invariant 24.7 (g) in response to plate-bound CD1d presenting chloroform-, acetone-, and methanol-soluble lipids obtained two days after infection with Ad-LacZ and Ad-HBV. IL-2 secretion was determined by ELISA. Mean ± s.e.m. of triplicate cultures is shown. Results are representative of two (d–e) or three (a–c, f–g) independent experiments.
Consistently, a significant population of sorted primary non-invariant liver NKT cells recognized Ad-HBV-induced alterations in microsomal lipids as determined by upregulation of CD69 expression (Fig. 4e). CD69 upregulation was less pronounced for sorted iNKT cells suggesting that iNKT cell responses to Ad-HBV are either restricted to a subpopulation of these cells or that iNKT cells are broadly but less strongly activated by Ad-HBV (Fig. 4d). In conclusion, exposure to Ad-HBV is associated with increased antigenicity of ER lipids, which are recognized by NKT cells.
Ad-HBV leads to increased abundance of antigenic lysophosphatidylethanolamine
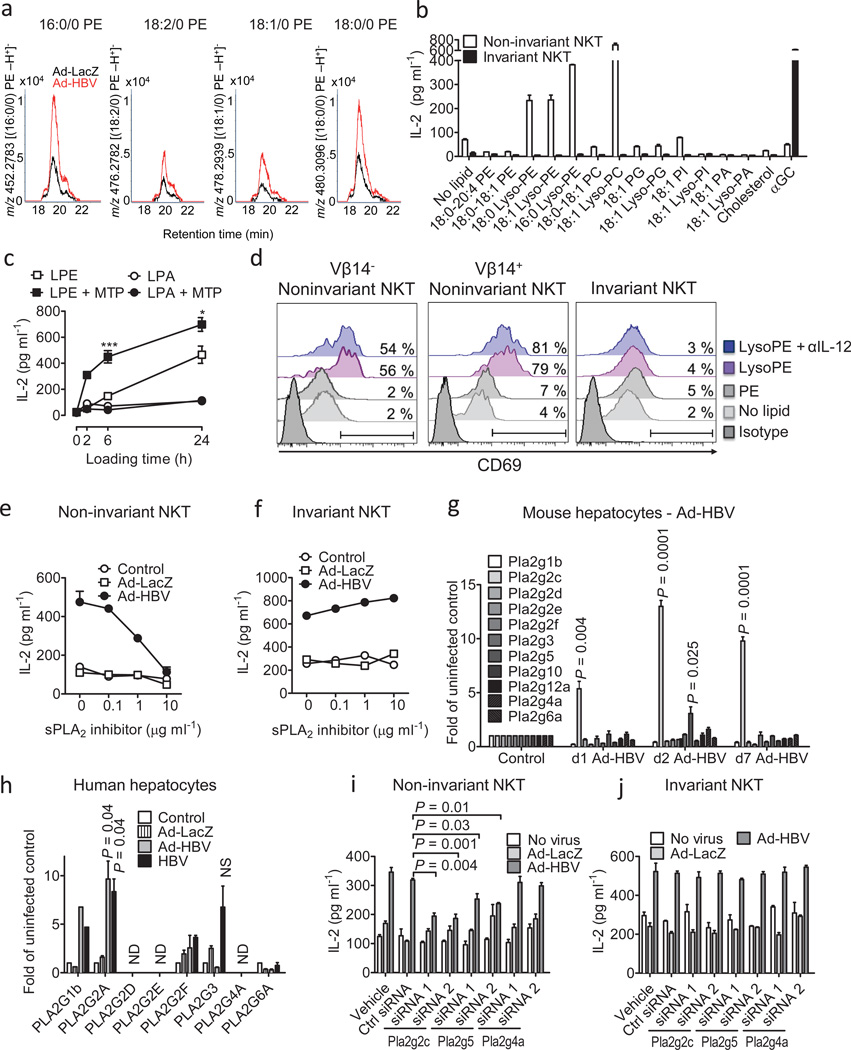
To characterize Ad-HBV-dependent lipid alterations, microsomal lipids were separated according to solubility.28 Ad-HBV-induced stimulatory fractions for non-invariant and invariant NKT cells were both recovered in phospholipid-enriched methanol eluents, but subfractionation indicated that the two NKT cell subtypes were activated by different lipids (Fig. 4f–g). To characterize the particular lipids induced by Ad-HBV and stimulatory for NKT cells, we screened methanol fractions by high performance liquid chromatography mass-spectrometry (HPLC-MS). Previous reports demonstrated selective recruitment of ER lipids by HBV and underrepresentation of PE species in the HBV envelope.27 Comparison of chromatograms corresponding to the mass and collisional spectra of diacyl phosphatidylethanolamine (PE) showed strong augmentation of PE and lysophosphatidylethanolamine (lysoPE) after Ad-HBV exposure (Suppl. Fig. 14). Lysophospholipids have recently been shown to be antigenic endogenous CD1d ligands in humans in vitro.29,30 Therefore, we rescreened stimulatory fractions at masses corresponding to monoacyl PE species with C16, C18:2, C18:1 and C18 monacyl forms, and found in all cases that these lipids were upregulated after Ad-HBV administration in vivo (Fig. 5a). There is thus an overlap among the types of lipids upregulated in response to Ad-HBV and lysophosopholipids that can stimulate NKT cells.
Figure 5.
HBV-mediated activation of non-invariant NKT cells is dependent on lysophospholipids and sPLA2. (a) Methanol fraction 2 of lipid extracts of microsomes obtained from Ad-HBV and Ad-LacZ-infected hepatocytes was analyzed by MS, and ion chromatograms corresponding in mass to the indicated lyso-PE species are shown. (b) Phospholipids were loaded onto plate-bound CD1d. Presentation to non-invariant 14S.6 and invariant 24.7 NKT cells is shown. PA, phosphatidic acid; PC, phosphatidylcholine; PG, phosphatidylglycerol; PI, phosphatidylinositol. (c) Lysophosphatidylethanolamine (LPE) and lysophosphatidic acid (LPA) were loaded onto plate-bound CD1d in the presence or absence of purified MTP before addition of 14S.6 NKT cells. ***p=0.005, *p=0.04 of LPE vs. LPE + MTP. (d) Activation of primary non-invariant (left, middle) and iNKT (right) cells in response to plate-bound CD1d presenting the indicated lipids. NKT cells were obtained as sorted invariant GFP+ αGalCer/CD1d-tetramer+ 4Get (right) and non-invariant GFP+ 4Get X Jα18-KO liver NKT cells (left, middle). Flow cytometric analysis of CD69 gated on GFP+ CD3+ cells is shown. (e–f) Primary hepatocytes were infected as indicated and treated with the sPLA2 inhibitor c(2NapA)LS(2NapA)R before addition of NKT cells. (g) Expression of PLA2 transcripts in murine livers following in vivo exposure to Ad-HBV. (h) Analysis of PLA2 RNA expression in primary human hepatocytes 24h after in vitro infection. Mean ± s.e.m. of triplicate cultures are shown. (i–j) Primary hepatocytes were infected with the indicated viruses before transfection with siRNAs as indicated and coculture with NKT cells. Results are representative of two (a, d) or three (b–c, e–j) independent experiments.
To further test initial conclusions derived from complex cellular lipid mixtures generated in vivo, we tested activation of murine NKT hybridomas in response to various purified phospholipids in vitro. 14S.6, a non-invariant NKT cell hybridoma that responded to Ad-HBV (Fig. 3a), also responded to 16:0/0 and 18:0/0 lysoPE and lysoPC (Fig. 5b). Since lysoPE but not lysoPC was increased in response to Ad-HBV (Suppl. Fig. 14), only lysoPE is implicated in the response to Ad-HBV. NKT cell activation by lysoPE was, among hybridomas, specific for 14S.6 and not observed with other iNKT (24.7, 24.8, 24.9, DN32.D3) or non-invariant NKT hybridomas (14S.7, 14S.10, 14S.15; Fig. 5b and data not shown). Lyso-PE-induced NKT activation required plate-bound CD1d (data not shown) and purified MTP facilitated lysoPE presentation (Fig. 5c). These results extended to primary NKT cells since more than fifty percent of sorted non-invariant 4Get X Jα18−/− liver NKT cells exhibited upregulation of CD69 in response to lysoPE but not PE (Fig. 5d). Non-invariant NKT cell activation was not limited to cells expressing Vβ14, the TCRβ chain shared by the non-invariant NKT hybridoma (14S.6) that recognized lysoPE (Fig. 5d) suggesting that lysoPE broadly activates non-invariant NKT cells in the liver. In contrast, iNKT cells did not exhibit activation in response to lysoPE (Fig. 5d).
HBV-induced activation of non-invariant NKT cells is dependent on secretory phospholipases (sPLA2)
sPLA2 enzymes contribute to the generation of lysophospholipids recognized by NKT cells and are active within secretory compartments.30,31 We therefore investigated the role of sPLA2 in Ad-HBV-dependent NKT cell activation. Chemical inhibitors of sPLA2 but not cytoplasmic PLA2 (cPLA2) prevented HBV-dependent activation of non-invariant NKT cells without affecting MHC class I presentation (Figure 5e, Suppl. Fig 15a–g). sPLA2 inhibitors also did not affect iNKT cells in accordance with the lack of lysoPE-dependent iNKT activation (Fig. 5f).
Since Ad-HBV-transduced hepatocytes exhibited increased abundance of PE (Suppl. Fig. 14b), the substrate for PLA2-mediated production of lysoPE, we investigated hepatocyte expression of sPLA2, cPLA2 (Pla2g4a), and calcium-independent PLA2 (iPLA2, Pla2g6a). Ad-HBV led to rapid and selective upregulation of sPLA2 Pla2g2c and Pla2g5 but not cPLA2, iPLA2, and other sPLA2 genes in vivo (Fig. 5g, Suppl. Fig. 16a). These results are in accordance with increased hepatic PLA2 group II and V expression in patients with viral hepatitis.32,33 Primary murine hepatocytes also exhibited Ad-HBV-induced Pla2g2c upregulation confirming hepatocyte-specific effects and demonstrating that sPLA2 regulation is not secondary to hepatic inflammation but a direct consequence of exposure to Ad-HBV (Suppl. Fig. 16b). Primary human hepatocytes exposed to Ad-HBV or a primary HBV isolate also exhibited upregulation of sPLA2, specifically of PLA2G2A (Fig. 5h). Since lysoPE activates transcription of PLA2G2A34, it likely supports its production through a positive feedback loop involving sPLA2. Thus, HBV leads to upregulation of sPLA2 group II in murine and human hepatocytes. Specific subgroups of sPLA2 differ between humans and mice in accordance with differences in genomic organization of sPLA2 loci (PLA2G2C is a pseudogene in humans35, Pla2g2a is inactivated by a frameshift in C57BL/6J mice).
Consistent with a critical role of sPLA2 in HBV-dependent generation of lysophospholipids as major antigens recognized by non-invariant NKT cells, small interfering RNA (siRNA) directed against Pla2g2c and Pla2g5 (Suppl. Fig. 17a) but not other sPLA2, cPLA2, and iPLA2 enzymes, prevented Ad-HBV-induced activation of non-invariant NKT but not invariant NKT cells or MHC class I-restricted T cells (Fig. 5i–j, Suppl. Fig. 17b, and data not shown).
The small HBsAg contributes to the activation of non-invariant NKT cells
To investigate the requisite HBV element for NKT cell activation, we inactivated individual HBV open reading frames and studied the effects on NKT activation. While all viral mutants showed similar transduction (Suppl. Fig. 5, 9), only deletion of the small HBsAg (S-), which is required for viral secretion, led to impaired activation of non-invariant NKT cells (Fig. 6a). A similar but non-significant trend was observed for iNKT cells (Fig. 6b). Consistently, Ad-HBV S- led to impaired hepatocyte sPLA2 upregulation and activation of non-invariant NKT cells by microsomal hepatocyte lipids (Fig. 6c, Suppl. Fig. 16c). To extend these findings, we examined mice with transgenic HBsAg expression.19 HBsAg-transgenic hepatocytes led to CD1d-dependent activation of noninvariant and invariant NKT but not MHC class I-restricted T cell hybridomas (Fig. 6d–f). In addition, microsomal lipids of HBsAg-transgenic hepatocytes activated NKT cells (Fig. 6g–h). Consistently, in vivo administration of Ad-HBV S- resulted in prolonged chronic necroinflammation and delayed viral control in association with sustained viral replication and reduced hepatic IFN-γ levels (Suppl. Fig. 18). These studies show that the small HBsAg contributes to Ad-HBV-mediated activation of NKT cells, which is in accordance with a temporal association between HBsAg expression and early NKT cell activation in human HBV.10
Figure 6.
Expression of HBsAg and cytokines contribute to NKT cell activation. (a–b) Primary hepatocytes were infected in vitro with the indicated Ad-HBV mutants and were cocultured 24h after infection with noninvariant (14S.6, a) and invariant (24.7, b) NKT cell hybridomas. (c) Microsomal lipids of primary hepatocytes obtained 48h after infection with the indicated viruses. Presentation by plate-bound CD1d to the non-invariant NKT cell hybridoma 14S.6 is shown. (d–f) Primary hepatocytes of the indicated mouse strains were treated with MTP inhibitors or 9-fluorenyl carboxylic acid as negative compound for 48h. Hepatocytes were then cocultured with non-invariant 14S.6 NKT cells, invariant 24.7 NKT cells, and MHC-class I-restricted RF33.70 T cells. (g–h) Microsomal lipids of the indicated mouse strains were loaded onto plate-bound CD1d. Presentation to invariant 24.7 and non-invariant 14S.6 is shown. IL-2 secretion by T cell hybridomas in a–h was determined by ELISA. (i–j) 4Get mice were injected with the indicated viruses, LPS (indirect NKT cell activation) and αGalCer (direct iNKT cell activation) in the presence or absence of antibody-mediated IL-12 neutralization. Liver mononuclear cells were obtained 6 hours (αGalCer) and 24 hours (viruses, LPS) after stimulation and expression of CD69 was determined by flow cytometric analysis of GFP+ αGalCer/CD1d-tetramer+ CD3+ invariant NKT cells (i) and GFP+ αGalCer/CD1d21 tetramer− CD3+ non-invariant NKT cells (j). Representative histograms and dot plots are shown. Bar graphs show mean ± s.e.m. Results are representative of two (d–j) or three (a–c) independent experiments.
Ad-HBV-induced activation iNKT cells is cytokine-dependent in vivo
Primary liver iNKT cells were not activated by lysoPE (Fig. 5d) and exhibited less robust activation to Ad-HBV microsomal lipids (Fig. 4d). We therefore investigated whether cytokine-mediated indirect activation contributes to Ad-HBV-induced iNKT cell activation in vivo. Indeed, neutralization of IL-12, a cytokine critical for indirect NKT cell activation36–38, largely prevented CD69 upregulation and IFN-γ secretion by iNKT cells but had negligible effects on non-invariant NKT cells (Fig. 6i–j, Suppl. Fig. 19). These results suggest that non-invariant NKT cells are directly activated upon Ad-HBV exposure by CD1d-restricted presentation of lysoPE and other uncharacterized hepatocyte antigens. This in turn leads to cytokine-dependent activation iNKT cells, presumably through activation of liver dendritic cells and macrophages.36,37 Importantly, IL-12-mediated indirect activation of liver iNKT cells requires hepatocyte MTP (Fig. 1a–c), suggesting that cytokine-dependent iNKT activation is downstream of CD1d-restricted activation of non-invariant NKT cells and NKT-dependent DC maturation.39,40
DISCUSSION
The findings presented here show that immune responses provided by NKT cells not only contribute to HBV-induced hepatitis19 but also to viral control. Although HBV-induced NKT cell activation is associated with acute hepatitis, NKT cells contribute significantly to the emergence of anti-viral B and T cell immunity. In the absence of this NKT cell response, not only is adaptive immunity and viral control diminished but chronic, low-grade hepatitis ensues. We show that the cell type responsible for orchestrating these HBV-induced responses is unanticipated and importantly the infected hepatocyte itself. Within the hepatocyte, HBV induces ER lipids including lysophospholipids derived from PE through the action of HBV-induced secretory phospholipases. CD1d-restricted presentation of lysophospholipids leads to direct activation of non-invariant NKT cells and subsequent IL-12-mediated indirect iNKT cell activation, confirming that both direct and indirect mechanisms contribute to NKT cell activation in vivo.36,37
The generation of antigenic lipids is partially dependent on the presence of the HBV surface antigen, the principal HBV structural component responsible for assembling PC-rich particles in the ER, and possibly results from an imbalanced lipid milieu within the ER.27 As such, the hepatocyte utilizes secretory phospholipases, MTP and CD1d to sense the presence of HBV and alert NKT cells through the display of modified self-lipids on the cell surface of the hepatocyte to trigger an adaptive immune response. Our findings of an NKT cell response soon after HBV exposure are in accordance with other recent observations in humans and animal models of HBV10–12 and suggest that NKT cells are part of an early, important sensing system that activates the immune response leading to effective priming of HBV-specific adaptive immune cells that are required for viral clearance. Our data suggest that although HBV acts as a stealth virus during a prolonged phase preceding viral control9, it is susceptible to a distinct type of immune recognition directly following infection which is important for subsequent immune clearance. Further studies in HBV patients are required to confirm a central role of NKT cells in human HBV infection.
METHODS
Mice
CD1d−/−, Jα18−/−, and Tap1−/− mice have been described before.42–44 H-Mttp−/− mice exhibiting an hepatocyte-specific knockout of the gene (Mttp) encoding for microsomal triglyceride transfer protein (MTP) were generated by crossing Alb-Cre (B6.Cg-Tg(Alb-cre) 21Mgn/J) mice45 that express Cre recombinase under control of the hepatocyte-specific albumin promoter with Mttpfl/fl mice that contain loxP sites flanking Mttp exon 1.46 To generate mice that allow for specific detection of invariant and noninvariant NKT cells, 4Get mice expressing GFP via an internal ribosome entry site (IRES) in the IL-4 transcript (IL-4/GFP-enhanced transcript (4Get) mice)47,48 were crossed with CD1d−/− and Jα18−/− mice (see Suppl. Results for further information). All mice were maintained on C57BL/6J background and were backcrossed for at least eight generations. HBV-Env mice expressing the entire HBV envelope (subtype ayw) under control of the albumin promoter49 were crossed with Rag1−/− and CD1d−/− mice as described previously.19
Primary cells and cell lines
Primary human hepatocytes were obtained from Invitrogen (Carlsbad, CA). Primary murine hepatocytes were extracted as described before.50 Purity and transduction rates of primary hepatocytes are shown in Suppl. Fig. 9. For a description of all cell lines please refer to the supplementary material.
Lipids and chemical inhibitors
Please refer to the supplementary material.
Viral constructs and virus administration
The parental plasmid for HBV constructs was pGEM7-HBV1.3 containing a 1.3-fold-overlength genome of HBV, subtype ayw, with a 5′ terminal redundancy encompassing enhancers I and II, the origin of replication (direct repeats DR1 and DR2), the X- and pregenomic/core promoter regions, the transcription initiation site of the pregenomic RNA, the unique polyadenylation site, and the entire X open reading frame. To generate HBV mutants, point mutations were introduced using the QuickChange II site-directed mutagenesis kit and protocol (Agilent Technologies, Santa Clara, CA) at the 19th codon of preS1 gene from TTG to TAG (L-) or at the initiation codon of pre-S2 and S from ATG to GTG (M- and S-). The PreS1 myristylation-defective (myr-) mutation was generated by changing the second codon of the preS1 gene from GGG (glycine) to GCG (alanine).
Please refer to supplementary material for additional information on virus generation and application. Viral transduction rates in vivo and in vitro are shown in Suppl. Fig. 5 and 9.
Determination of ALT, HBsAg, HBV DNA levels and liver histology
Please refer to the supplementary material.
Flow cytometry
Flow cytometry was performed as described before.25 For a detailed description please refer to the supplementary material.
Preparation and separation of cellular and microsomal lipids
For extraction of microsomal lipids, primary hepatocytes were obtained as described above, microsomes were extracted according to Ernster et al.51 and lipids were extracted following the Folch protocol.52 Total hepatocyte lipids were extracted in a similar manner. Separation of lipids according to solubility was done as described in 28. For a detailed description please refer to the supplementary material.
Liquid chromatography-mass spectrometry
Lipid extracts were dried down, weighed and then re-suspended in 95% 60:40 hexanes:isopropanol and 5% methanol (V: V) prior to mass spectrometry analyses. Twenty µg of lipid from each fraction was injected onto a 150 mm × 2.0 mm monochrom 3 diol column (Varian), and eluted with a gradient program in which solvent A consisted of methanol (0.1% formic acid and 0.05% ammonium hydroxide, M:V) and solvent B consisted of 60% hexanes and 40% isopropanol (V: V) (0.1% formic acid and 0.05% ammonium hydroxide M:V). The gradient was run from 95% to 85% solvent B from 0 to 6.6 minutes, to 0% solvent B until 16.2 minutes, then increased back to 95% solvent B from 22.8 to 26 minutes. The HPLC system was an Agilent 1200 series with a 6520 quadrupole accurate mass time of flight (Q-TOF) mass spectrometer (Agilent Technologies, Santa Clara, CA), with the voltage 3.5 kV, source temperature 325 °C, drying gas 5L/min, nebulizer pressure 30 psi, running in the negative ion mode. Collision experiments were carried out by subjecting target ions to 25eV in the collision cell. Data analysis was performed using Agilent Qualitative Analysis Mass Hunter Software Version B.03.01.
Antigen presentation
Antigen presentation assays were performed in 96 well flat bottom plates using 2×104 hepatocytes, 5×104 RMA-S/d cells, 1×105 splenocytes or hepatic mononuclear cells and, as responders, 1×105 NKT cells or NKT cell hybridoma cells. Cytokine secretion was determined by ELISA after 16 hours of coculture (BD Biosciences). In some experiments, MTP inhibitors were added at a final concentration of 10 µM (BMS212122, BMS200150) and 100 nM (BMS197636), respectively. Alternatively, purified monoclonal CD1d blocking antibodies (1B1 clone, 19G11 clone) were used at a final concentration of 10 µg/ml.
For cell-free antigen presentation assays, monomeric mouse CD1d (NIH Tetramer Core Facility) was loaded onto 96 well flat bottom plates (0.25 µg/well), unbound CD1d was washed off, and lipids were added at a molar ratio of 40 :1. Unbound lipids were then washed off, NKT cells were added and cytokine secretion was determined by ELISA as described above. In some experiments, MTP purified from bovine liver (M.M.H.) was added at a final concentration of 500 ng/ml. In addition, in some experiments, GFP+ αGalCer/CD1d-tetramer+ 4Get invariant NKT cells and GFP+ 4Get X Jα18−/− noninvariant NKT cells were sorted from liver mononuclear cells using a BD Biosciences FACSAria II cell sorter and were used as responders in antigen presentation assays. Flow cytometric determination of cell surface CD69 and intracellular IFN-γ expression on GFP+CD3+ NKT cells was studied as readout. For in vitro neutralization of IL-12, a monoclonal anti-IL-12 p40 antibody (clone C17.8, eBioscience) was used at a final concentration of 10 µg/ml.
For ELISpot assays please refer to supplementary material.
Protein extraction, western blotting
Protein extraction and western blotting were performed as described previously.25 For a detailed description please refer to the supplementary material.
Real-time PCR
Real-time PCR was performed as described before.25 For a detailed description please refer to the supplementary material.
RNA interference
Inhibition of phospholipase expression was achieved by FlexiTube siRNA (Qiagen Inc., Hilden, Germany). siRNA was transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. 48 and 72 hours after transfection, downregulation of PLA2 expression was investigated by SYBR green qPCR. For antigen presentation assays, primary hepatocytes were infected with Ad-HBV and control viruses and were transfected with siRNA 24 hours after infection. 48 hours after siRNA transfection, NKT cells were added and cytokine secretion was determined after 16 hours of coculture.
Statistical analysis
Data are expressed as means ± s.e.m. Statistical testing was done using the unpaired Student’s t-test. For comparisons of more than two groups, one-way analysis of variance was performed and was followed by Dunnett’s correction. Statistical analyses were done using GraphPad Prism (GraphPad Software, Inc.).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grants DK51362, DK44319, DK53056, DK88199, the Harvard Digestive Diseases Center DK034854 (to R.S.B.); the Deutsche Forschungsgemeinschaft (Ze 814/1-1, Ze 814/4-1), the FP7-PEOPLE program of the European Commission (Marie Curie International Reintegration Grant 256363), and the Crohn’s and Colitis Foundation of America (to S.Z.); the Crohn’s and Colitis Foundation of America, Austrian Science Fund, and Max Kade Foundation (to A.K.); NIH AR048632, AI049313, and the Burroughs Wellcome Fund for Translational Research (to D. B. M.); DK46900 (to M.M.H.); the NIH Intramural Research Program (K.M, Z.H, T.J.L); NIH grants AI068090, DK026743, and the Burroughs Wellcome Fund (to J.L.B.); and the A.P. Gianinni Foundation (to J.P.) We thank David E. Cohen and Stephanie K. Dougan for insightful discussions.
Footnotes
AUTHOR CONTRIBUTIONS
S.Z. designed, performed, and analyzed experiments and prepared the manuscript with R.S.B. and T.J.L; K.M., Z.H. generated adenoviruses and adenoviral mutants and contributed to Ad-HBV studies; L.S. and D.B.M. designed, performed, and analyzed LC-MS experiments together with S.Z.; J.P. and J.L.B. designed, performed and analyzed studies with HBV-Env mice; A.K. generated H-Mttp−/− mice and contributed to their characterization; K.B. and C.R. performed histopathological analyses; M.M.H. obtained purified MTP; E.B. performed PLA2 inhibitor and siRNA studies; R.G. obtained primary HBV isolates; A.A. and J.H. contributed to human hepatocyte studies; S.S. contributed to supervision of the studies; T.J.L. and R.S.B. supervised the studies.
There are no competing financial interests.
REFERENCES
- 1.Thimme R, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo W, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 3.Esteve M, et al. Chronic hepatitis B reactivation following infliximab therapy in Crohn's disease patients: need for primary prophylaxis. Gut. 2004;53:1363–1365. doi: 10.1136/gut.2004.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese LH, Zein NN, Vassilopoulos D. Hepatitis B virus (HBV) reactivation with immunosuppressive therapy in rheumatic diseases: assessment and preventive strategies. Ann Rheum Dis. 2006;65:983–989. doi: 10.1136/ard.2005.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thio CL, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 7.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 8.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 9.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisicaro P, et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut. 2009;58:974–982. doi: 10.1136/gut.2008.163600. [DOI] [PubMed] [Google Scholar]
- 11.Webster GJ, et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117–1124. doi: 10.1053/jhep.2000.19324. [DOI] [PubMed] [Google Scholar]
- 12.Guy CS, Mulrooney-Cousins PM, Churchill ND, Michalak TI. Intrahepatic expression of genes affiliated with innate and adaptive immune responses immediately after invasion and during acute infection with woodchuck hepadnavirus. J Virol. 2008;82:8579–8591. doi: 10.1128/JVI.01022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isogawa M, Kakimi K, Kamamoto H, Protzer U, Chisari FV. Differential dynamics of the peripheral and intrahepatic cytotoxic T lymphocyte response to hepatitis B surface antigen. Virology. 2005;333:293–300. doi: 10.1016/j.virol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Sprinzl MF, Oberwinkler H, Schaller H, Protzer U. Transfer of hepatitis B virus genome by adenovirus vectors into cultured cells and mice: crossing the species barrier. J Virol. 2001;75:5108–5118. doi: 10.1128/JVI.75.11.5108-5118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Freyend MJ, et al. Sequential control of hepatitis B virus in a mouse model of acute, self-resolving hepatitis B. J Viral Hepat. 2011;18:216–226. doi: 10.1111/j.1365-2893.2010.01302.x. [DOI] [PubMed] [Google Scholar]
- 17.Guidotti LG, et al. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 18.Publicover J, et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest. 2011;121:1154–1162. doi: 10.1172/JCI44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baron JL, et al. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 20.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brozovic S, et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- 22.Dougan SK, Rava P, Hussain MM, Blumberg RS. MTP regulated by an alternate promoter is essential for NKT cell development. J Exp Med. 2007;204:533–545. doi: 10.1084/jem.20062006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougan SK, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaser A, et al. Microsomal triglyceride transfer protein regulates endogenous and exogenous antigen presentation by group 1 CD1 molecules. Eur J Immunol. 2008;38:2351–2359. doi: 10.1002/eji.200738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeissig S, et al. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J Clin Invest. 2010;120:2889–2899. doi: 10.1172/JCI42703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khatun I, et al. Phospholipid transfer activity of microsomal triglyceride transfer protein produces apolipoprotein B and reduces hepatosteatosis while maintaining low plasma lipids in mice. Hepatology. 2011 doi: 10.1002/hep.25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoh O, Umeda M, Imai H, Tunoo H, Inoue K. Lipid composition of hepatitis B virus surface antigen particles and the particle-producing human hepatoma cell lines. J Lipid Res. 1990;31:1293–1300. [PubMed] [Google Scholar]
- 28.Gumperz JE, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 29.Cox D, et al. Determination of cellular lipids bound to human CD1d molecules. PLoS One. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox LM, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000228. e1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni Z, Okeley NM, Smart BP, Gelb MH. Intracellular actions of group IIA secreted phospholipase A2 and group IVA cytosolic phospholipase A2 contribute to arachidonic acid release and prostaglandin production in rat gastric mucosal cells and transfected human embryonic kidney cells. J Biol Chem. 2006;281:16245–16255. doi: 10.1074/jbc.M513874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito M, et al. Distribution of type V secretory phospholipase A2 expression in human hepatocytes damaged by liver disease. J Gastroenterol Hepatol. 2004;19:1140–1149. doi: 10.1111/j.1440-1746.2004.03435.x. [DOI] [PubMed] [Google Scholar]
- 33.Masuda S, Murakami M, Ishikawa Y, Ishii T, Kudo I. Diverse cellular localizations of secretory phospholipase A2 enzymes in several human tissues. Biochim Biophys Acta. 2005;1736:200–210. doi: 10.1016/j.bbalip.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Kuwata H, Yamamoto S, Takekura A, Murakami M, Kudo I. Group IIA secretory phospholipase A2 is a unique 12/15-lipoxygenase-regulated gene in cytokine-stimulated rat fibroblastic 3Y1 cells. Biochim Biophys Acta. 2004;1686:15–23. doi: 10.1016/j.bbalip.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Tischfield JA, et al. Low-molecular-weight, calcium-dependent phospholipase A2 genes are linked and map to homologous chromosome regions in mouse and human. Genomics. 1996;32:328–333. doi: 10.1006/geno.1996.0126. [DOI] [PubMed] [Google Scholar]
- 36.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 37.Brigl M, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 39.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishak K, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 42.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 43.Cui J, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 44.Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4–8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 45.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 46.Raabe M, et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 48.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chisari FV, et al. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc Natl Acad Sci U S A. 1987;84:6909–6913. doi: 10.1073/pnas.84.19.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scapa EF, et al. Regulation of energy substrate utilization and hepatic insulin sensitivity by phosphatidylcholine transfer protein/StarD2. FASEB J. 2008;22:2579–2590. doi: 10.1096/fj.07-105395. [DOI] [PubMed] [Google Scholar]
- 51.Ernster L, Siekevitz P, Palade GE. ENZYME-STRUCTURE RELATIONSHIPS IN THE ENDOPLASMIC RETICULUM OF RAT LIVER : A Morphological and Biochemical Study. J Cell Biol. 1962;15:541–562. doi: 10.1083/jcb.15.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.