Abstract
Clostridium perfringens type B and D strains produce epsilon toxin (ETX), which is one of the most potent clostridial toxins and is involved in enteritis and enterotoxemias of domestic animals. ETX is produced initially as an inactive prototoxin that is typically then secreted and processed by intestinal proteases or possibly, for some strains, lambda toxin. During the current work a unique C. perfringens strain was identified that intracellularly processes epsilon prototoxin to an active form capable of killing MDCK cells. This activated toxin is not secreted but instead is apparently released upon lysis of bacterial cells entering stationary phase. These findings broaden understanding of the pathogenesis of type B and D infections by identifying a new mechanism of ETX activation.
Keywords: Clostridium perfringens, epsilon toxin, toxin proteolytic processing
1. Introduction
Clostridium perfringens is a Gram-positive, rod-shaped, spore-forming, anaerobic bacterium. It is a strong toxin producer, capable of producing upwards of seventeen different toxins [1, 2]. However, a single bacterium never produces all of these toxins; thus C. perfringens strains are classified into five types based upon their ability to produce four typing toxins, including alpha, beta, epsilon and iota toxins [2, 3]. All strains are capable of producing alpha toxin, which is the only typing toxin made by type A strains. In addition to alpha toxin, type B strains produce beta and epsilon toxins, type C strains produce beta toxin, type D strains produce epsilon toxin, and type E strains produce iota toxin.
Epsilon toxin (ETX) is produced by both type B and D strains and ranks as the third most potent of all clostridial toxins [2]. This toxin is considered responsible for causing rapidly fatal enterotoxemias or enteritis in several livestock species [2, 4]. When added to Madin-Darby Canine Kidney (MDCK) cells, an epithelial cell line that is sensitive to ETX, the activated toxin forms a large SDS-insensitive heptameric complex [5]. This complex first assembles as a prepore on the plasma membrane surface but, at 37°C, that prepore rapidly inserts into membranes to form an active pore that permeabilizes the host cell [6]. This results in a rapid efflux of potassium ions, along with an influx of chloride and sodium ions [4, 7, 8]. These ion perturbations eventually cause or contribute to cell death [9].
ETX is initially produced and secreted as an inactive prototoxin of approximately 33kDa [10, 11]. The prototoxin can be proteolytically activated by treatment with trypsin or chymotrypsin, with this activation involving cleavage of amino acids from the C-terminus of the prototoxin, although N-terminal amino acids are also removed [12]. Lambda toxin, a secreted ~35 kDa thermolysin-like zinc metalloprotease produced by some type B, D and E strains [13], can also extracellularly activate both epsilon toxin and iota toxin [13–15]. Whether proteolytically-activated by lambda toxin or trypsin, ETX possesses relatively similar lethality when tested in mice [13, 14].
When surveying a collection of type B and D isolates for their processing of epsilon prototoxin, a strain was identified that processes the prototoxin under all tested conditions. This prompted an in-depth characterization, which revealed this as an unusual C. perfringens strain that lacks the lam gene but instead produces an intracellular protease capable of processing the epsilon prototoxin.
2. Materials and Methods
2.1 Bacterial Strains
The C. perfringens strains used in this study were each isolated from diseased animals. Strains with a CN prefix were part of the Burroughs-Wellcome collection and had been originally provided by Dr. Russell Wilkinson, while strains with a JGS prefix were provided by Dr. J. Glenn Songer. The type B strains used included NCTC8533 and CN1795, while the type D strains used were CN2068, JGS4138, JGS1240, JGS1902, CN3842, and CN3718. NCTC6121, which became the main focus of this study, has been classified as a type B strain [11]; however, as described in the Results, the culture maintained for many years in our laboratory now genotypes as type D. Therefore, our culture was redesignated as NCTC6121A.
2.2 Growth of Bacteria
Media used for culturing C. perfringens included FTG medium (fluid thioglycolate medium; Difco Laboratories); TGY medium (3% tryptic soy broth [Becton-Dickinson], 2% glucose [Fisher Scientific], and 1% yeast extract [Becton-Dickinson]) and BHI medium (brain heart infusion broth; Difco Laboratories). Both TGY and BHI broths were supplemented with 0.1% sodium thioglycolate [Sigma Aldrich].
2.3 Mammalian Cell Culture
MDCK cells were grown in a medium composed of a 50/50 mix of DMEM (Sigma) and Nutrient Mixture F12 MAH (Sigma) supplemented with 7% FBS (Mediatech), 1% Pen/Strep (Invitrogen) and 1% glutamine (Invitrogen). The cells were grown in T75 flasks (Corning) and passaged at a 1 to 10 split every 3 or 4 days when they became confluent.
2.4 Genotyping of Strains by PCR
Genomic DNA was prepared from C. perfringens strains using the Epicentre MasterPure Gram Positive DNA Purification Kit. Cultures were grown for 16 h in TGY medium. A 1.5mL aliquot of those cultures was centrifuged and the pellet was resuspended in 150μL of TE buffer. Ready-Lyse Lysozyme (1μL) in the DNA purification kit was added and the sample was incubated overnight at 37°C. The following day, 150μL of Proteinase K/Gram Positive Lysis solution in the kit was added and incubated at 65°C for 15 min with mixing every 5 min. A 175μL aliquot of MPC Protein Precipitation Reagent in the kit was added and the solution was mixed. The samples were then centrifuged for 10 min at 10,000g. A 1μL aliquot of RNase A (5μg/μL) was added to each supernatant. After incubation of these samples at 37°C for 1 h, 500μL of isopropanol was added and the samples were centrifuged for 10 min at 10,000g. The supernatant was removed and the pellet was washed with 70% ethanol and resuspended in 50μL of distilled water.
In the initial screening of strains, PCR was used to determine which strains carried epsilon toxin gene (etx) ORF sequences and lambda toxin gene (lam) ORF sequences. Primers used for amplification of lam ORF sequences were Lambda-F (5′-GTTATGCTGGCGTAGTGTATG-3′) and Lambda-R (5′-CCTGAATTAATATGAA-CACCACC-3′). Primers used for amplification of internal etx ORF sequences were Etx-F (5′-GCGGTGATATCCATCTATTC-3′) and Etx-R (5′-CCACTTACTTGTCCTAC-TAAC-3′).
Each reaction was comprised of 10μL deionized water, 10μL 2x Taq Mix (New England BioLabs), 0.1μL 100μM primer (IDT), and 0.5μL genomic of DNA. The amplification conditions used were: 2 min at 95°C followed by 35 cycles of 30 sec at 95°C, 40 sec at 55°C, and 1 min at 68°C, and a final extension at 68°C for 10 min. The products were electrophoresed on a 1.5% agarose gel. The gel was then stained with ethidium bromide, washed with distilled water, and photographed using a ChemiDoc (BioRad).
In addition to assessing carriage of etx and lam ORF sequences, strains were also checked for the presence of the cpb ORF using PCR. The cpb ORF primer sequences used were CpbF (5′-GCAGGATCCATGAAGAAAAAATTTAT-3′) and CpbR (5′-ATACTCGAGCTAAATA-GCTGTTACTTT-3′). The reaction was performed and visualized using the same conditions as above.
2.5 Southern Blots
Toxin gene Southern blots [16] were performed to verify the results obtained by PCR (see Results). Genomic DNA (3 μg) from C. perfringens strains NCTC6121A, JGS1240, or CN3842 was diluted into a total volume of 10μL. A reaction mixture for XbaI digestion was made using this genomic DNA sample, 2.5μL of NEB buffer 4, 10.5μL of deionized water, and 2μL of XbaI. The digestion was run overnight at 37°C. The following morning, the products were run on a 1% agarose gel. The gel was stained with ethidium bromide, washed with distilled water, and photographed.
Digoxigenin (DIG)-labeled cpb, lam, and etx ORF probes were constructed, as described previously [17], with a PCR DIG probe synthesis kit (Roche, New Jersey). After hybridization of the probes, performed as described previously, the Southern blots were developed using reagents from the DIG labeling and detection kit (Roche) [18].
2.6 Sequencing of the etx ORF
The etx ORF was PCR-sequenced in two fragments. Primer pairs used to amplify the two etx ORF fragments were, F1 (5′-TATAGAAAAATATATTAATGAAAGG-3′) and R1 (5′-TTAAGAGAGCTTTTCCAACA-3′) or F2 (5′-CCTAATGCTATGGCATATTT-3′) and R2 (5′-GAGGAAAATTAGATTACATACA-3′). The same PCR conditions used in the etx and lam ORF detection PCR were used for amplification of these etx ORF fragments for sequencing. The PCR products were run on a 1.5% agarose gel and stained with ethidium bromide. The PCR products were then gel-purified and sent for sequencing (University of Pittsburgh Genomics and Proteomics Core Laboratory) using the same primer pairs listed above. The sequence is deposited in GenBank (Accession number JX010451).
2.7 Detection of Extracellular Prototoxin or Processed Prototoxin by Western Blotting
Cultures were grown for 16 h in TGY or BHI medium at 37°C and centrifuged the following morning. A 20μL aliquot of the supernatants was subjected to SDS-PAGE and then transferred to a nitrocellulose membrane for ETX Western blotting [19]. The membrane was blocked and reacted overnight with mouse anti-ETX monoclonal antibody 5B7 [19]. The following day, the membrane was washed twice for 5 min with TBS and rabbit anti-mouse HRP antibodies (Sigma) were then applied for 1 h. The membrane was washed twice for 15 min with TBS buffer. SuperSignal West Pico Chemiluminescent Substrate (Pierce) was added and incubated for 10 min. Signal on the membrane was visualized by autoradiography.
2.8 Protease Detection
A QuantiCleave Protease Assay Kit (Thermo Fisher) was used to detect the activity of proteases released into the media. Briefly, 100μL of BHI culture supernatant was incubated with 50μL of casein substrate for 1 h. A 50μL aliquot of TNBSA (2,4,6 trinitrobenzene sulfonic acid 5% (w/v) in methanol) working solution was added and incubated at room temperature for 30 min. The plate was then read at 450nm. Since proteins in the media also reacted with the substrate, duplicate samples were tested, one at 4°C and the other at 37°C. The difference was taken as indicative of protease activity and the values were normalized to a trypsin standard.
2.9 Time Course Study of the Appearance of Extracellular Prototoxin or Processed Prototoxin
A time course study was performed to determine when prototoxin or processed prototoxin appears in culture supernatants. This study was performed using strains JGS1240, NCTC6121A, and CN3842. For this experiment, cultures of each strain were grown for 16 h in BHI medium. A 0.1mL aliquot of each starter culture was then used to inoculate 10mL of pre-warmed BHI medium. The OD600 was measured at 0, 2, 4, 6, and 8 h to measure the growth kinetics of the strains. At 2, 4, 6, and 8 h, the cultures were centrifuged and their supernatants were collected. The supernatants were then assayed for protease expression and for the presence of prototoxin or processed prototoxin, as described above.
2.10 Time Course Analysis of Intracellular Processing of Prototoxin by NCTC6121A
A starter culture of strain NCTC6121A was grown for 16 h in BHI and then used to inoculate fresh cultures in BHI broth, which were then incubated at 37°C. At 2, 4, and 6 h, the BHI cultures were centrifuged and the supernatants were discarded. A 0.1g aliquot of pellet was then resuspended in 1mL of HBSS buffer. The pellets were sonicated on ice for 8 min (2 min of sonication followed by 2 min of rest, with this process repeated once). After centrifugation, the supernatants were collected, subjected to SDS-PAGE and assayed for intracellular prototoxin processing by Western blot.
2.11 Cytotoxicity Assay
MDCK cells were inoculated into 6 well plates two days before assay. The same day, fresh 16 h supernatants of NCTC6121A and JSG1240 were concentrated 20X using Millipore Centrifugal Filter Units and then buffer-exchanged using the GE Healthcare PD-10 Desalting Column. On the day of the assay, the cells were washed 3 times with HBSS buffer. The supernatants were, i) left untreated, ii) treated with trypsin (100μL sample + 32μL trypsin, incubated for 1 h at 37 °C, at which time 132μL trypsin inhibitor was added for 30 min at room temperature) or iii) the tryspin-activated toxin was treated with trypsin inhibitor (as above), followed by the addition of anti-ETX or anti-alpha toxin (PLC) monoclonal antibody (100μL sample + 20μL of monoclonal antibody, which was then incubated for 1 h at room temperature). For a negative control, some cells were treated with trypsin and trypsin inhibitor alone (no supernatants). The supernatants were added to HBSS buffer for a total volume of 1mL. That mixture was added to the washed cells, which were then incubated for 1 (for cytotoxicity assay) or 2 (for microscopy) hours at 37 °C. The cells were then photographed.
To quantify cellular damage, the Roche LDH Cytotoxicity Detection Kit was used. Briefly, the supernatants were collected from the cells and centrifuged to pellet residual cell debris. A 100μL aliquot of supernatant was mixed with 100μL of prepared reaction mixture. The samples were incubated at room temperature for 5 min and then read at 490nm.
3. Results
3.1 Genotype Characterization of Type B and D Strains
Before assessing their ability to process epsilon prototoxin, several type B and D strains were genotyped to confirm that they carry etx sequences (Fig 1A); these strains included type B strains NCTC8533 and CN795, as well as type D isolates CN2068, JGS4138, JGS1240, JGS1902, CN3842, and CN3718. Each strain tested PCR-positive for the presence of an etx ORF, as expected. Additionally, as seen in Fig 1B, the type B strains NCTC8533 and CN1795 also carried the cpb ORF, while none of the type D strains tested positive for possession of a cpb ORF, as expected. These PCR results were verified by Southern blot analysis using probes specific for the etx or cpb ORF (results not shown).
Figure 1. Genotypic screening of type B and D strains.
Panel A, PCR showing that all surveyed type B and D strains carry etx sequences since their DNA supports amplification of a PCR product of approximately 650bp using internal etx ORF primers. Panel B, PCR showing that only some strains possess lam sequences; all surveyed strains except NCTC6121, CN3842, and CN3718 tested positive for lam sequences as DNA from these strains supported amplification of a 500 bp product using internal lam ORF primers. Panel C, PCR showing that, amongst the surveyed strains, cpb sequences are possessed only by type B strains NCTC8533 and CN1795. Note that NCTC6121 (now re-named NCTC6121A) also tested negative, indicating that this strain, originally classified as type B [25], has lost the cpb gene.
Initial isolation many years ago had characterized NCTC6121 as a type B isolate and a later study by another laboratory using modern molecular techniques confirmed that this strain carries both cpb and etx genes [11]. However, as demonstrated in Figures 1A and C, and confirmed by Southern blotting (data not shown), the NCTC6121 culture maintained for many years in our laboratory still possesses the etx ORF but no longer carries the cpb ORF. This result indicated that our culture of NCTC6121, which we are redesignating as NCTC6121A, is now genotypically a type D strain.
PCR results indicated that this NCTC6121A culture also lacked lambda toxin gene (lam) sequences (Fig. 1C). When lam ORF carriage amongst the other surveyed isolates in Fig. 1A was tested by PCR (Fig. 1C), both type B strains were lam-positive, as were 4 of the 6 type D strains. However, type D strains CN3842 and CN3719 were lam-negative by PCR. These results matched previous genotyping results for these strains [20] and were confirmed by Southern blot analyses using a lam ORF-specific probe (data not shown).
3.2 NCTC6121A Proteolytically Processes Epsilon Prototoxin
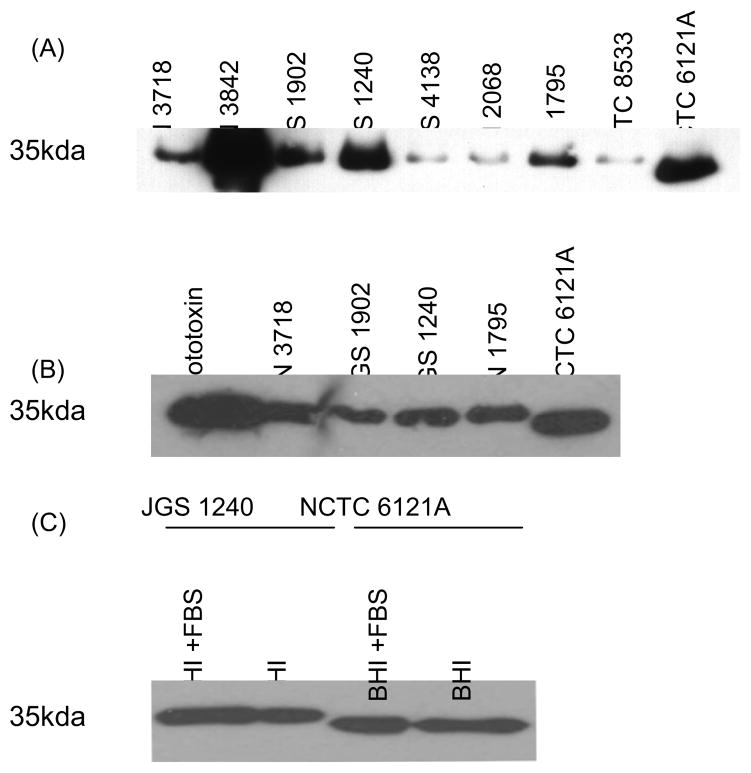
The strains surveyed in Fig 1A were next assayed by Western blot for their ability to produce and process epsilon prototoxin (Fig. 2). All isolates showed some production of ETX antibody-reactive protein in both BHI and TGY media, although the levels of this protein production varied considerably amongst different strains. Interestingly, only NCTC6121A showed processing of its prototoxin, as evidenced by the lower molecular weight band shown in the Fig 2A and 2B Western blots.
Figure 2. Western blot analysis of prototoxin processing.
Panel A, Cultures of the surveyed strains were grown 16 h in TGY medium and the supernatants were then harvested and electrophoresed on a 12% acrylamide gel containing SDS. Note that only strain NCTC6121A proteolytically processed the prototoxin. Panel B, Supernatants from 16 h BHI cultures of the specified strains were Western blotted with an ETX monoclonal antibody. The prototoxin was run as a control in lane 1 to show the size of the unprocessed prototoxin. Again, note that only strain NCTC6121A supernatant contained processed prototoxin. Panel C, To assess whether more prototoxin processing occurs when strains are grown in rich medium with a high serum content, BHI medium was fortified with 2% FBS. When JGS1240, which is lam-positive, was grown in this medium it did not promote processing of the prototoxin (note the presence of a similar-sized prototoxin band in the presence or absence of serum). Strain NCTC6121A processed the prototoxin similarly under both growth conditions.
Since other groups had used a medium with a high serum content to purify lambda toxin [13], it was conceivable that prototoxin processing by lam-positive strains might require the presence of serum. To test this possibility, the BHI medium was fortified with 2% FBS and lam-positive isolate JGS1240 was then retested for prototoxin processing. As can be seen in Fig 2C, no difference was noted in prototoxin processing by JGS1240 in the presence or absence of serum.
3.3 Kinetics of Prototoxin or Processed Prototoxin Appearance in Culture Supernatants
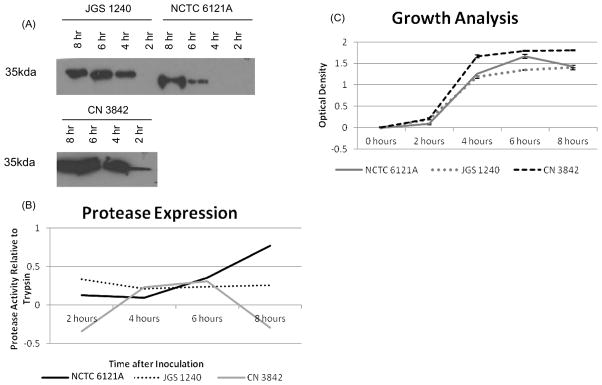
When the presence and size of ETX in culture supernatants of three type D strains was compared by Western blotting (Fig 3A), variations were noted. Whereas prototoxin appeared in the culture supernatants of JGS1240 and CN3842 within 2 h of culture, no prototoxin or processed prototoxin was detectable in culture supernatants of NCTC6121A until 6 h of culture. These studies also confirmed the Fig. 2 results indicating that CN3842 is a strong prototoxin producer, while NCTC6121A and JGS1240 eventually produced about the same amounts of extracellular protein that reacts with an ETX-specific monoclonal antibody. Also in agreement with Fig. 2, the culture supernatants of NCTC6121A contained only processed prototoxin, while the culture supernatants of the other two strains contained only intact prototoxin.
Figure 3. Comparative kinetics of the appearance of prototoxin or processed prototoxin in culture supernatants, culture exoprotease activity, and growth by selected strains.
Panel A, Culture supernatant was Western blotted to detect the presence of extracellular prototoxin or processed prototoxin over time. Note that all of the extracellular material made by NCTC6121A that reacted with ETX monoclonal antibody was in the form of processed prototoxin and the appearance of this material was delayed for NCTC6121A, with detection only after 6 hours in culture. JGS1240 cultures contained detectable levels of extracellular prototoxin at 4 h, while supernatant prototoxin was detectable at 2 h for CN3842. Note also that only NCTC6121A supernatants contained processed prototoxin. Panel B, At 2 h intervals, supernatants from these cultures were assayed for the secretion of proteases using the QuantiCleave Kit. Note that the levels of exoprotease in the NCTC6121A strain steadily increased over time, whereas the exoprotease activity of the other two strains either remained constant or decreased. Panel C, NCTC6121A, JGS1240, and CN3842 were inoculated into prewarmed BHI medium. The culture OD600 was then measured at 0, 2, 4, 6, and 8 hours. Note that CN3842 grows slightly faster than the other two strains.
The protease activity in culture supernatants of these three type D strains was then compared to see if overall exoprotease activity differences amongst strains could explain the observed strain-related differences in the supernatant appearance of processed prototoxin (Fig. 3B). The surveyed strains showed considerable variation in their expression of overall supernatant protease activity. NCTC6121A had a steady increase in exoprotease activity over time, whereas the other two type D strains showed level or decreasing supernatant protease activity over time. However, the culture supernatants from all three strains contained approximately equal amounts of protease activity at 6 h, a time-point where prototoxin was processed by NCTC6121A but not by the other two type D strains.
Lastly, the growth of the three strains was compared (Fig. 3C) to see if growth difference might explain the variations in prototoxin processing noted in Fig. 3A. When cultures were similarly inoculated, CN3842 exhibited faster growth kinetics than strains JGS1240 and NCTC6121A, which exhibited similar growth characteristics to one another.
3.4 Intracellular Prototoxin Processing
Most type B or D strains secrete full-length prototoxin, as supported by the Fig. 2 results and previous results [19–21]. Since no extracellular epsilon prototoxin was observed for NCTC6121A after 2 h of growth (Fig. 3A), and this strain is lam-negative, we hypothesized that NCTC6121A might process its prototoxin intracellularly using a novel protease. If true, this effect could involve cleavage of N-terminal secretion sequences during processing, which could interfere with secretion and thus help explain why extracellular processed prototoxin was only detected after 6 h culture for this strain, i.e., a time by which the culture is in stationary phase and some bacterial cells would have lysed.
To test this hypothesis, ETX Western blot analyses examined whether the intracellular prototoxin present in NCTC6121A cells had been processed. When sonicates of 4 h cultures were evaluated (Fig 4), the prototoxin had been processed since the cytoplasmic protein reacting with ETX monoclonal antibody was smaller than the prototoxin made by JGS1240. These data indicated that NCTC6121A processes prototoxin intracellularly, i.e., this strain possesses an intracellular protease capable of cleaving the toxin.
Figure 4. Time course analysis of intracellular processing of prototoxin by NCTC6121A.
A 16 h starter culture of strain NCTC6121A was inoculated into fresh BHI medium. Cells were harvested from those cultures after 2, 4 or 6 h growth at 37°C and then lysed. The lysate was electrophoresed on a 12% acrylamide gel containing SDS; lysate of a JGS1240 culture was included as a control to show the migration of prototoxin. Note that processed intracellular prototoxin can be observed in NCTC6121A lysates as early as 4 h, a timepoint before extracellular toxin appears (Fig. 3), indicating that the prototoxin is processed intracellularly by this strain.
3.5 Cytotoxicity of Type D Culture Supernatants
To determine if the processed prototoxin produced by strain NCTC6121A was active and capable of killing cells, supernatants from 16 h cultures of this strain were concentrated and applied to MDCK cells, which are sensitive to active ETX [5, 6]. Concentrated 16 h supernatant from strain JGS1240 was used as a control since 16 h supernatants from this strain contained similar amounts of protein reactive with ETX monoclonal antibody as was present in 16 h supernatants from NCTC6121A, yet this immunoreactive protein in JGS1240 cultures remained in the prototoxin form (Fig. 3A). The two type D 16 h culture supernatants were then, i) treated with trypsin to process the toxin, followed by incubation with trypsin inhibitor, ii) treated with trypsin, followed by incubation with tryspin inhibitor and then antibody to block active ETX or alpha toxin, or iii) left untreated.
A Western blot of the supernatants was performed before and after trypsin treatment (Fig 5A) to assess whether the prototoxin had or had not been processed in these samples. Prior to trypsin treatment, the JGS1240 supernatant contained only full-length prototoxin as evidenced by the presence of a 32.5 kDa protein reactive with ETX monoclonal antibody. After trypsin treatment, the JGS1240 protein reactive with ETX monoclonal antibody was reduced in size to ~30 kDa, indicating the expected proteolytic processing of the prototoxin [12]. In contrast, non-trypsin treated supernatant of strain NCTC6121A contained a smaller protein reactive with ETX antibody due to processing of the prototoxin by this bacterium, as already shown in Fig. 2. This protein became slightly smaller upon trypsin treatment, indicating that the intracellular bacterial protease of NCTC6121A and trypsin cut the prototoxin differently.
Figure 5. Cytotoxicity assay indicates that the supernatant of NCTC6121A, which contains processed prototoxin, is toxic to MDCK cells.
Panel A, ETX Western blot analysis of supernatant from a 16 h culture of strains NCTC6121A or JGS1240. Shown are JGS1240 supernatants that had (lane 1) or had not (lane 2) been treated with trypsin. Lanes 3 and 4 show the supernatant from NCTC6121A that had not (lane 3) or had (lane 4) been treated with trypsin. Panel B, The supernatants from 16 h cultures of JGS1240 and NCTC6121A were concentrated, desalted and applied to MDCK cells for 2 hours. Effects of the supernatants without trypsin activation are shown in the first column, the effects of trypsin-treated supernatants, after trypsin inhibition, are shown in the second column, the effects of the trypsin-treated supernatant, after trypsin inhibition and neutralization with ETX monoclonal antibody are shown in the third column and the effects of trypsin and trypsin inhibitor (no supernatant) are shown in column four. An anti-PLC neutralizing monoclonal antibody failed to neutralize supernatant-induced damage (not shown). Panel C, LDH release assay to quantify cytotoxicity caused by 1 h treatment with various supernatants (note that results are expressed as percent of release caused by treating cells for 1 h with a 2% Triton solution). MDCK cytotoxicity results shown are effects of treatment with NCTC6121A or JGS1240 supernatants alone (first two groups), NCTC6121A or JGS1240 supernatants after trypsin treatment and addition of trypsin inhibitor (next two groups), or trypsin and trypsin inhibitor (no supernatant). Also shown for each group are the effects of preincubating supernatants with ETX or PLC monoclonal antibodies.
As shown in Fig 5B, the 16 h NCTC6121A culture supernatant caused some damage to MDCK cells even without trypsin activation. In contrast, without trypsin treatment, the 16 h JGS1240 culture supernatant failed to damage MDCK cells, as might be expected since that sample contained only inactive prototoxin. After the two 16 h type D culture supernatants had been treated with trypsin, both caused a significant amount of MDCK cell damage (Fig. 5B). This damage was not due to trypsin since a control containing trypsin and trypsin inhibitor (but no culture supernatant) failed to damage MDCK cells. In addition, when the trypsin-treated supernatants were pretreated with a neutralizing ETX-specific monoclonal antibody prior to administration of that sample to MDCK cells (Fig. 5B), no damage developed in the MDCK cells. This protection was specific since an anti-PLC monoclonal antibody did not inhibit the development of damage (data not shown). These results further supported the damage present in the middle panel of Fig. 5B as being caused by active ETX.
To quantify the cellular damage shown in Fig. 5B, an LDH release assay was performed (Fig. 5C). Without trypsin treatment, 16 h culture supernatant from JGS1240 was unable to cause substantial cytotoxicity in MDCK cells, whereas the corresponding non-trypsin-treated 16 h culture supernatant of NCTC6121A killed roughly 20% of those cells. Interestingly, trypsin treatment did not have the same effect on both supernatants. Trypsin-treated culture supernatants of NCTC6121A caused much higher cytotoxicity than did the comparably trypsin-treated culture supernatants of JGS1240. No significant cytotoxicity was detected when cells were treated with trypsin and trypsin inhibitor (no culture supernatant). Finally, the addition of ETX monoclonal antibody substantially reduced the cytotoxic activity of both trypsin-activated culture supernatants (Fig. 5C). In contrast, preincubation with an anti-PLC monoclonal antibody did not prevent cytotoxicity (Fig. 5C). Collectively, these results supported the involvement of active ETX in these effects.
4. Discussion
ETX is initially produced by C. perfringens type B and D strains as a prototoxin that must be proteolytically-activated to form the heptameric pores causing or contributing to cytotoxicity and lethality in mice [5, 6, 12, 22]. It has been well established that intestinal proteases such as trypsin and chymotrypsin can perform this ETX activation. Trypsin removes 14 amino acids from the N-terminus, and 22 amino acids from the C-terminus, of the prototoxin [12]. Chymotrypsin also removes 14 amino acids from the N-terminus of the prototoxin, but cleaves 29 amino acids from the C-terminus of the prototoxin [12]. Studies have shown that C-terminal peptide cleavage is necessary for prototoxin activation [12]. Consistent with that observation, both trypsin-activated and chymotrypsin-activated ETX are cytotoxic and lethal for mice [12].
Some C. perfringens type B, D and E strains can produce λ toxin, a ~35 kDa thermolysin-like metalloprotease [13, 14]. By itself, λ toxin can increase vascular permeability and cause hemorrhagic edema in mice. However, studies with purified λ toxin showed that this protease can also activate both ETX and iota toxin, in vitro [13–15]. With respect to ETX activation, λ toxin resembles trypsin by removing 14 amino acids from the N-terminus of the prototoxin [14]. However, this C. perfringens protease resembles chymotrypsin in removing 29 amino acids from the C-terminus of the prototoxin [14]. Consistent with those findings, λ toxin-activated ETX has an LD50 for mice that is similar to that of ETX activated by trypsin or chymotrypsin [13, 14].
Because of the previously-reported ability of purified λ toxin to activate ETX [14], it has been proposed that λ toxin plays an important role in the pathogenesis of type B and D infections. However, in previous studies, we had determined that only 9 of 38 (~25%) type D isolates and 13 of 17 (~75%) type B animal disease isolates even carry lam gene sequences encoding lambda toxin [17, 23]. These findings regarding variability in lam carriage amongst type B and D strains were confirmed in the present study. Furthermore, this and previous studies [17, 23] showed that relatively few lam-positive strains consistently self-process their prototoxin, at least under the experimental conditions tested. Collectively, these findings with animal disease isolates could suggest that λ toxin cleavage of prototoxin is not necessary for type B and D disease.
However, since the lam gene is carried on a different plasmid from the etx gene, some of these lam-negative strains could have lost their lam plasmid upon storage. The possibility of lam plasmid loss is indirectly supported by the absence of the plasmid-borne cpb gene from NCTC6121A, as detected in the current study. Since the presence of cpb sequences in NCTC6121 was conclusively shown by another laboratory [24], this strain was not incorrectly typed. Instead, it appears that the NCTC6121 strain maintained for many years in our laboratory has undergone “type degradation”, possibly caused by cpb plasmid loss, which is a commonly reported phenomenon for C. perfringens [25].
It is also conceivable that, when produced, λ toxin could activate prototoxin during disease caused by some lam-positive type B or D isolates if those strains express much higher levels of lambda toxin in vivo than during in vitro culture. In addition, λ toxin might contribute to disease in other ways [15]. For example, when absorbed from the intestines into the circulation, λ toxin might contribute to internal organ damage during enterotoxemias. Therefore, the tentative suggestion that lambda toxin may not be important for type B and D pathogenesis should be tested further in vivo using fresh disease isolates and isogenic lam null mutants.
Of perhaps greater interest, this study identified a new mechanism of proteolytic activation of prototoxin beyond intestinal proteases and lambda toxin. Specifically, we characterized a lam-negative strain, i.e., NCTC6121A, that consistently produces a proteolytically processed ETX. Interestingly, ETX processing by this strain was shown to be mediated by an intracellular protease since processed prototoxin was detectable by Western blotting of cell lysates. This prototoxin processing by NCTC6121A apparently involves cleavage of N-terminal signal sequences, given the observed delay in secretion of the processed prototoxin until the culture reached stationary phase, a time when some bacterial cells would begin lysing. However, since i) removal of some C-terminal sequences are needed to activate the prototoxin [12] and ii) the processed toxin made by NCTC6121A exhibits some cytotoxicity, the intracellular protease of NCTC6121A must also remove some C-terminal sequences from the prototoxin. Interestingly, trypsin treatment caused a further activation of the prototoxin made by NCTC6121A, indicating that the unidentified NCTC6121A protease removes less than 22 amino acids from the C-terminus of the prototoxin.
The identity of the intracellular NCTC6121A protease that processes the prototoxin remains to be determined. However, this protease appears to be expressed in relatively few type B and D strains based upon surveys indicating that most type B and D strains cannot self-process prototoxin [this study; [17, 23]]. In theory, an alternative explanation for our NCTC6121A observations would be that most type B and D isolates produce this protease but NCTC6121A produces a prototoxin with a variant sequence that renders it uniquely susceptible to this protease. However, sequencing results (data not shown) argue against this possibility since the ETX encoded by NCTC6121A was found to be 100% identical at the amino acid level (data not shown) to that of NCTC8533 [26], a strain that does not process the prototoxin (Fig. 1). If the NCTC6121A protease is produced by only a few strains, it could possibly be encoded by a rare plasmid, but this requires further study. Once the protease is identified, it would also be of interest to see whether any type E strains express this protease to proteolytically activate their iota toxin components.
Highlights.
Type B and D strains with a lambda toxin gene often cannot process epsilon prototoxin
Strain NCTC6121A lacks the lambda toxin gene but processes epsilon prototoxin
The NCTC6121A processed epsilon toxin accumulates intracellularly until cell lysis
NCTC6121A epsilon toxin is less cytotoxic than epsilon toxin activated by trypsin
Trypsin further actives NCTC6121A toxin, so the intracellular processing is partial
Acknowledgments
This research was generously supported by NIAID through MARCE grant 2U54AI057168-08 (M. Levine, PI) and R01 AI056177-08 (B. McClane, PI). We thank Dr. Paul Hauer for supplying ETX monoclonal antibody 5D7 and PLC monoclonal antibody N6H71F3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9(2):216–34. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClane BA, et al. The enterotoxic clostridia. In: Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E, editors. The Prokaryotes: a handbook on the biology of bacteria. New York, NY: Springer-Verlag; 2006. pp. 698–752. [Google Scholar]
- 3.Daube G, et al. Typing of Clostridium perfringens by in vitro amplification of toxin genes. J Appl Bacteriol. 1994;77(6):650–5. doi: 10.1111/j.1365-2672.1994.tb02815.x. [DOI] [PubMed] [Google Scholar]
- 4.Uzal FA, et al. The pathology of peracute experimental Clostridium perfringens type D enterotoxemia in sheep. J Vet Diagn Invest. 2004;16(5):403–11. doi: 10.1177/104063870401600506. [DOI] [PubMed] [Google Scholar]
- 5.Petit L, et al. Clostridium perfringens epsilon-toxin acts on MDCK cells by forming a large membrane complex. J Bacteriol. 1997;179(20):6480–7. doi: 10.1128/jb.179.20.6480-6487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson SL, et al. Evidence for a prepore stage in the action of Clostridium perfringens epsilon toxin. PLoS One. 2011;6(7):e22053. doi: 10.1371/journal.pone.0022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagahama M, Ochi S, Sakurai J. Assembly of Clostridium perfringens epsilon-toxin on MDCK cell membrane. J Nat Toxins. 1998;7(3):291–302. [PubMed] [Google Scholar]
- 8.Petit L, et al. Clostridium perfringens epsilon toxin induces a rapid change of cell membrane permeability to ions and forms channels in artificial lipid bilayers. J Biol Chem. 2001;276(19):15736–40. doi: 10.1074/jbc.M010412200. [DOI] [PubMed] [Google Scholar]
- 9.Chassin C, et al. Pore-forming epsilon toxin causes membrane permeabilization and rapid ATP depletion-mediated cell death in renal collecting duct cells. Am J Physiol Renal Physiol. 2007;293(3):F927–37. doi: 10.1152/ajprenal.00199.2007. [DOI] [PubMed] [Google Scholar]
- 10.Habeeb AF. Studies on epsilon-prototoxin of Clostridium perfringens type D. Physicochemical and chemical properties of epsilon-prototoxin. Biochim Biophys Acta. 1975;412(1):62–9. doi: 10.1016/0005-2795(75)90339-6. [DOI] [PubMed] [Google Scholar]
- 11.Hunter SE, et al. Cloning and nucleotide sequencing of the Clostridium perfringens epsilon-toxin gene and its expression in Escherichia coli. Infect Immun. 1992;60(1):102–10. doi: 10.1128/iai.60.1.102-110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyata S, et al. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J Biol Chem. 2001;276(17):13778–83. doi: 10.1074/jbc.M011527200. [DOI] [PubMed] [Google Scholar]
- 13.Jin F, et al. Purification, characterization, and primary structure of Clostridium perfringens lambda-toxin, a thermolysin-like metalloprotease. Infect Immun. 1996;64(1):230–7. doi: 10.1128/iai.64.1.230-237.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minami J, et al. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol Immunol. 1997;41(7):527–35. doi: 10.1111/j.1348-0421.1997.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 15.Willis A. Clostridia of Wound Infections. London: Butterworth and Company; 1969. [Google Scholar]
- 16.Hughes ML, et al. Epsilon-toxin plasmids of Clostridium perfringens type D are conjugative. J Bacteriol. 2007;189(21):7531–8. doi: 10.1128/JB.00767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayeed S, Li JH, McClane BA. Characterization of virulence plasmid diversity among Clostridium perfringens type B isolates. Infect Immun. 2010;78(1):495–504. doi: 10.1128/IAI.00838-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JH, Miyamoto K, McClane BA. Comparison of virulence plasmids among Clostridium perfringens type E isolates. Infect Immun. 2007;75(4):1811–1819. doi: 10.1128/IAI.01981-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayeed S, et al. Epsilon-toxin is required for most Clostridium perfringens type D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect Immun. 2005;73(11):7413–21. doi: 10.1128/IAI.73.11.7413-7421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayeed S, Li J, McClane BA. Characterization of virulence plasmid diversity among Clostridium perfringens type B isolates. Infect Immun. 2010;78(1):495–504. doi: 10.1128/IAI.00838-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayeed S, Li J, McClane BA. Virulence plasmid diversity in Clostridium perfringens type D isolates. Infect Immun. 2007;75(5):2391–8. doi: 10.1128/IAI.02014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein J, et al. Clostridium perfringens epsilon toxin increases the small intestinal permeability in mice and rats. PLoS One. 2009;4(9):e7065. doi: 10.1371/journal.pone.0007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayeed S, Li J, McClane BA. Virulence plasmid diversity in Clostridium perfringens type D isolates. Infect Immun. 2007;75(5):2391–2398. doi: 10.1128/IAI.02014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter SE, et al. Molecular genetic analysis of beta-toxin of Clostridium perfringens reveals sequence homology with alpha-toxin, gamma-toxin, and leukocidin of Staphylococcus aureus. Infect Immun. 1993;61(9):3958–65. doi: 10.1128/iai.61.9.3958-3965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonel JL. Toxins of Clostridium perfringens (types A, B, C, D and E) In: Dorner F, Drews J, editors. Pharmacology of bacterial toxins. Pergamon Press; Oxford: 1986. pp. 477–528. [Google Scholar]
- 26.Miyamoto K, et al. Sequencing and diversity analyses reveal extensive similarities between some epsilon-toxin-encoding plasmids and the pCPF5603 Clostridium perfringens enterotoxin plasmid. J Bacteriol. 2008;190(21):7178–88. doi: 10.1128/JB.00939-08. [DOI] [PMC free article] [PubMed] [Google Scholar]