Abstract
Postural tachycardia syndrome (POTS) is characterized by increased heart rate (ΔHR) of ≥30 bpm with symptoms related to upright posture. Active stand (STAND) and passive head-up tilt (TILT) produce different physiological responses. We hypothesized these different responses would affect the ability of individuals to achieve the POTS HR increase criterion. Patients with POTS (n=15) and healthy controls (n=15) underwent 30 min of TILT and STAND testing. ΔHR values were analyzed at 5 min intervals. Receiver Operating Characteristics analysis was performed to determine optimal cut point values of ΔHR for both TILT and STAND. TILT produced larger ΔHR than STAND for all 5 min intervals from 5 min (38±3 bpm vs. 33±3 bpm; P=0.03) to 30 min (51±3 bpm vs. 38±3 bpm; P<0.001). Sensitivity (Sn) of the 30 bpm criterion was similar for all tests (TILT-10=93%, STAND-10=87%, TILT30=100%, and STAND30=93%). Specificity (Sp) of the 30 bpm criterion was less at both 10 and 30 min for TILT (TILT10=40%, TILT30=20%) than STAND (STAND10=67%, STAND30=53%). The optimal ΔHR to discriminate POTS at 10 min were 38 bpm (TILT) and 29 bpm (STAND), and at 30 min were 47 bpm (TILT) and 34 bpm (STAND). Orthostatic tachycardia was greater for TILT (with lower specificity for POTS diagnosis) than STAND at 10 and 30 min. The 30 bpm ΔHR criterion is not suitable for 30 min TILT. Diagnosis of POTS should consider orthostatic intolerance criteria and not be based solely on orthostatic tachycardia regardless of test used.
Keywords: orthostatic tachycardia, postural tachycardia syndrome, heart rate, tilt table test, stand
Introduction
Postural Tachycardia Syndrome (POTS) is a chronic disorder of orthostatic intolerance that typically afflicts women of childbearing age (~80% of patients are female).[11] It is associated with significant functional disability and a diminished quality of life.[1] A hallmark hemodynamic criterion of POTS is an excessive orthostatic tachycardia; specifically, an increase in heart rate (HR) by 30 beats per minute (bpm) or a maximum heart rate of 120 bpm within 10 minutes of assumption of upright posture. This must occur without significant orthostatic hypotension.[7;11] The method of achieving upright posture is not specified, and orthostatic tolerance can be assessed with standing (active) or with a head-up tilt table test (passive stand).
With active standing, as opposed to passive standing, the body compresses and releases pressure on the veins of the lower limbs, forcing blood return to the heart, a process which is often called the “skeletal muscle pump”.[8;16;20] This physiological difference can lead to marked differences in the hemodynamic responses of patients undergoing a passive tilt when compared to an active stand.[21] For example, nearly 15% of healthy subjects (no prior clinical faints) experience vasovagal episodes with the passive tilt test (false positives).[13] Despite these data, the differences in physiology between active and passive standing are not accounted for in the current POTS diagnostic criteria.[14] We tested the hypothesis that a passive head-up tilt table test would produce greater increases in HR than an active stand test, and that this difference would cause variability in the number of patients and controls meeting the orthostatic HR criterion for the diagnosis of POTS.
Methods
Subjects
This study was a retrospective analysis of POTS patients referred to the Vanderbilt University Autonomic Dysfunction Center and healthy control research subjects studied between 2002 and 2004. POTS patients had all been diagnosed before the study using the criteria of symptoms of orthostatic intolerance accompanied by a HR increase ≥30 bpm within the first ten minutes of standing without associated orthostatic hypotension (a decrease in blood pressure [BP] greater than 20/10 mmHg). While some centers also include patients with a maximum standing heart rate ≥ 120 bpm in the same timeframe, none of the patients met this criterion who did not otherwise meet the HR increase ≥30 bpm. Patients had symptoms for at least 6 months in the absence of prolonged bed rest or a chronic debilitating condition. Healthy control subjects who did not meet the criteria for POTS were recruited as a part of another study involving both a tilt table test and a stand test. All patients and control subjects were over 18 years. Both patients and controls were free of medications that could impair autonomic tone for at least 5 half-lives, and had not taken fludrocortisone for at least five days before testing. The Vanderbilt University Investigational Review Board approved this study, and written informed consent was obtained from each subject before the study began.
Protocol
The investigation was performed at the Elliot V. Newman Clinical Research Center at Vanderbilt University. All subjects consumed a caffeine-free research diet containing 150 mEq/day of sodium and 70 mEq/day of potassium.
Active Stand Test
HR and BP were measured between 8am–9am in a fasting state. All subjects were supine for at least 1 hour, and many for the entire night. Standing HR and BP measurements were taken at 1, 3, 5, 10, 20, and 30 minutes using automated oscillometric sphygmomanometer (Dinamap, GE Medical Systems Information Technologies). Patients were discouraged from walking around or running in place, but were permitted to move their legs. No instructions were given on how quickly to arise or how to direct their eyes. They were not provided any physical support.
Passive HUT Test
Tilt data from a volume study for both POTS patients and healthy volunteers were used for this analysis.[4] All data were gathered between 8am–9am with subjects in a fasting state. ECG, HR, oscillometric BP (Vital-Guard 450C, Ivy Biomedical Systems, Branford, CT), and finger BP (Finapres 2300, Ohmeda, Madison, WI) were continuously recorded and digitized at a sampling rate of 500 Hz with DI-720USB and Windaq Pro+ software (DATAQ Instruments, Akron, OH). This data was then processed with user software written in PV-Wave (Visual Numerics, Houston, TX). Heart rate was calculated from ECG, and blood pressures from the finger BP measurements were used in analysis. Hemodynamic values from the penultimate minute before tilt were used as the baseline to eliminate any artifact from anticipation and activity in the room immediately prior to the tilt. Healthy subjects (n=15) and POTS patients (n=15) were tilted directly to 60° for 30 minutes as tolerated. Time was measured (in minutes) from the start of the upright tilt. Patients were told not to move their legs or close their eyes during the tilt. The subjects’ left arms were supported in a sling to keep finger cuff at heart level. The subjects’ feet were supported with a footboard, and subjects were strapped into the table at 3 points to prevent falling if syncope did occur. Subjects were tilted quickly and informed in advance about the procedures.
The maximum increase in HR up to that time during tilt was used at each time point. This is the clinically relevant endpoint when assessing “orthostatic tachycardia”, and avoids artificially low HR due to an intervening vasovagal reaction or artificially high values due to syncope with data drop out later during the tilt. Patients who experienced syncope or pre-syncope terminated the test, and their maximum ΔHR achieved up to the point of terminating the test was carried forward for the remaining time points in analysis.
Statistical Analysis
The primary outcome measures were maximum changes in HR (ΔHR) at 10 minutes of upright posture from baseline. The secondary outcomes included the maximum ΔHR at up to 30 minutes of upright posture, as well as the maximum ΔHR from baseline measured at 5 minutes intervals. Increases in heart rate over time were analyzed by a repeated measures analysis of variance (ANOVA) that assumed an exchangeable correlation structure for repeated measures on the same subject. We used Fisher’s protected least significant differences approach to assessing differences in ΔHR at individual time points.[3] That is, these differences, which were assessed by t-tests, were only declared significant if the ANOVA test of ΔHR was significant at all time points.
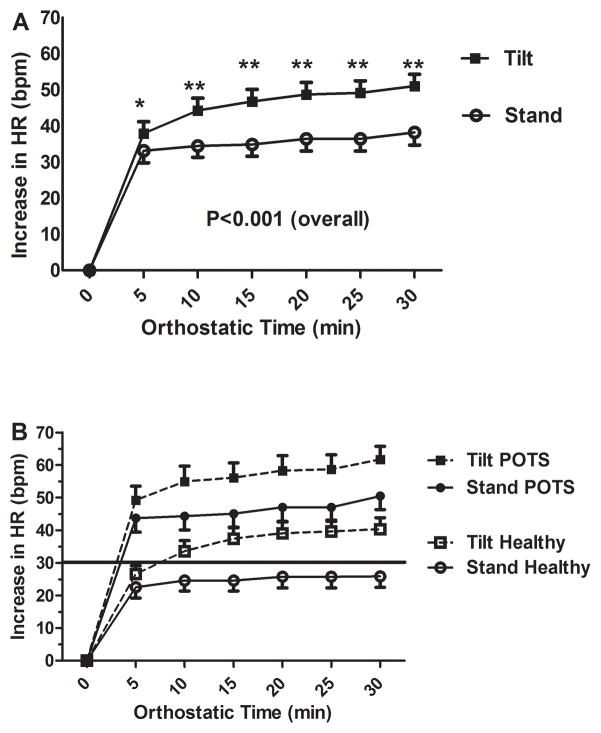
In Figure 1, error bars denote standard errors.
Figure 1.
Figure 1a- Orthostatic Increase in Heart Rate over Time (Overall Group)
Mean orthostatic change in heart rate is shown over time. Results are divided into tilted subjects (solid rectangles) and active standing patients (outlined circles).
Figure 1b- Orthostatic Increase in Heart Rate over Time (By Diagnosis)
Mean orthostatic change in heart rate is shown over time. Results are divided into tilted POTS patients (solid rectangles), standing POTS patients (solid circles), tilted healthy controls (outlined rectangles), and standing healthy controls (outlined circles). The solid horizontal line is the 30 bpm delta heart rate criterion currently in use for the diagnosis of POTS.
Sensitivity was defined as the proportion of true positives (POTS patients diagnosed as POTS) within the overall group of patients with POTS. Specificity was defined as the proportion of true negatives (control subjects diagnosed as not having POTS) with the overall group of control subjects. An ideal diagnostic test would have both sensitivity and a specificity that approaches 1, although this rarely happens. The sensitivity and specificity will vary inversely (e.g. specificity increases while sensitivity decreases) as the “threshold” (e.g. HR cut-point) for a given test is altered. Receiver Operating Characteristics (ROC) curves plots sensitivity as a function of 1-specificity, thereby allowing for a visual representation of test characteristics. Sensitivity and specificity of the 30 bpm ΔHR criterion and optimal HR cut-points for stand and tilt at 10 and 30 min were determined using these curves. Optimal HR cut-points were determined by finding the point on the ROC curve that maximized the distance to the line of identity (i.e. the straight ROC curve with 45° slope associated with a test that has no ability to distinguish between POTS and normal patients). Data are presented as mean±SEM. Analysis was performed with IBM SPSS Statistics Version 19 (IBM Corporation) and GraphPad Prism 5.02 (GraphPad Software, San Diego, CA).
Results
Demographics
Demographic data for the subjects of this trial are shown in Table 1. Tilt and stand data for 30 subjects (27 female; age 35±2 years) were analyzed, and included 15 POTS patients (13 female; age 36±3 years) and 15 healthy volunteers (14 female; age 33±2 years).
Table 1.
Demographic Information
| POTS | Healthy | Overall | |
|---|---|---|---|
| Number | 15 | 15 | 30 |
| Female | 13 | 14 | 27 |
| Mean Age (years) | 36±3 | 33±2 | 35±2 |
| Age Range (years) | 18–53 | 24–49 | 18–53 |
| Height (cm) | 165±2 | 168±2 | 166±2 |
| Weight (kg) | 64±5 | 74±3 | 69±3 |
| BMI (kg/m2) | 24±2 | 26±1 | 25±1 |
Orthostatic HR Responses to Passive Tilt and Active Stand
The orthostatic increases in HR for the combined groups (patients and control subjects) are shown in Figure 1a. An ANOVA found a significant difference in ΔHR between passive tilt and active stand (p<0.001) for all patients combined, and for POTS and control patients (p=0.009 and 0.005, respectively). For all patients combined, these differences were larger for passive tilt than active stand for all 5 min intervals from 5 min (38±3 bpm vs. 33±3 bpm; P=0.03) to 30 min (51±3 bpm vs. 38±3 bpm; P<0.001). HR response to active stand and passive head up tilt are shown by diagnosis in Figure 1b. For the POTS group, the orthostatic changes in HR were not significantly different at 5 min for passive tilt and active stand (49±4 bpm vs. 44±4 bpm; P=0.147). The change in HR was greater with tilt than stand for all 5 min intervals from 10 min (55±5 bpm vs. 44±4 bpm; P=0.01) through 30 min (62±4 bpm vs. 50±4 bpm; P=0.009). For the control group, HR response for passive tilt and active stand were not significantly different at 5 min (27±3 bpm vs. 23±3 bpm; P=0.127), but tilt had a greater response than stand for all values from 10 min (34±3 bpm vs. 25±3 bpm; P=0.01) through 30 min (40±4 bpm vs. 26±3 bpm; P=0.002). At ten minutes, for subjects who reached the 30 bpm criterion, 19 of 23 developed symptoms or orthostatic intolerance. Of those, all 14 patients with POTS developed symptoms, while 5 of 9 healthy subjects developed mild symptoms.
Diagnostic Characteristics of the 30 bpm Criterion
Table 2 contains the sensitivities and specificities for the 30 bpm cutoff criterion for the 2 tests at 10 minutes and at 30 minutes. For 10 min of testing, sensitivity (Sn) was similar, but specificity (Sp) was much lower for a passive tilt (Sn=93% & Sp=40%) compared to active stand (Sn=87% & Sp=67%). At 30 min, the Sp was lower while Sn was similar for passive tilt (Sn=100% & Sp=20%) compared to active stand (Sn=93% & Sp=53%). Using only the current 30 bpm criterion, 80% of control subjects would have been diagnosed with orthostatic tachycardia (false positive rate) using a 30 minute tilt table test, compared to only 47% with a 30 minute stand. The false positive rate for a 10 minute tilt was 60%, compared to 33% during a 10 minute stand. Of note, 1 patient previously diagnosed with POTS did not meet the 30 bpm HR criterion during the stand test nor with the 10 minute tilt, thus the sensitivities were less than 100%.
Table 2.
Sensitivity and Specificity of 30 bpm criterion
| 10 min stand | 10 min tilt | 30 min stand | 30 min tilt | |
|---|---|---|---|---|
| Sn | 87% | 93% | 93% | 100% |
| Sp | 67% | 40% | 53% | 20% |
| AUC | 0.918 | 0.891 | 0.945 | 0.921 |
Optimal HR Criteria Based on Test Type and Duration
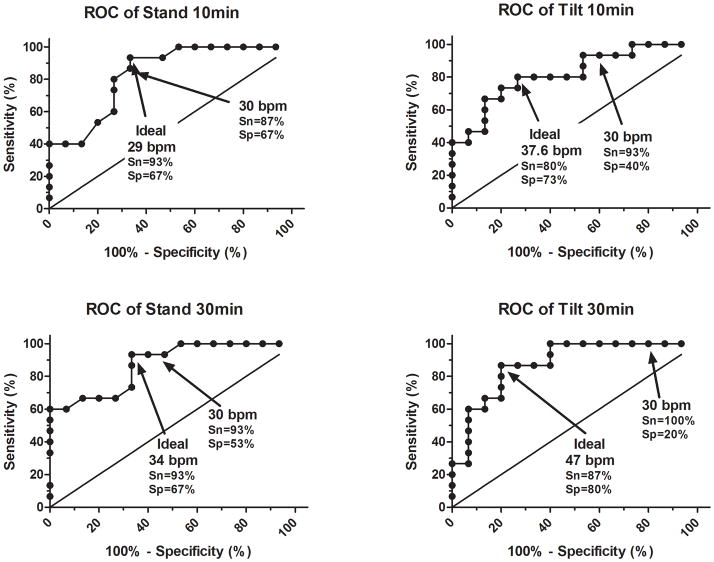
Table 3 contains the most accurate cut points for diagnosing orthostatic tachycardia for the various tests. At 10 min, the optimal orthostatic tachycardia threshold to diagnose POTS after passive tilt was 38 bpm (area under curve [AUC] = 0.918; Sn=80% & Sp=73%) compared with 29 bpm (AUC=0.891; Sn=93% & Sp=67%) after active stand. After 30 min, the optimal cut-points were 47 bpm (AUC=0.945; Sn=87% & Sp=80%) with passive tilt, compared with 34 bpm (AUC=0.921; Sn=93% & Sp=67%) for active stand. Figure 2 shows the 30 bpm criterion and ideal criterion for a 10 min stand, 10 min tilt, 30 min stand, and 30 min tilt on their respective ROC curves.
Table 3.
Optimal ΔHR cutoffs for diagnosis of POTS
| 10 min stand | 10 min tilt | 30 min stand | 30 min tilt | |
|---|---|---|---|---|
| ΔHR | 29 | 37.6 | 34 | 47 |
| Sn | 93% | 80% | 93% | 87% |
| Sp | 67% | 73% | 67% | 80% |
Figure 2. ROC Analysis for Tilt and Stand.
ROC analysis (sensitivity vs. 1-specificity) is shown with sensitivity and specificity for points of interest (ideal cutoff and 30 bpm cutoff) marked for a 10 minute stand (Panel A), 10 min tilt (Panel B), 30 min stand (Panel C), and 30 min tilt (Panel D). The solid angled straight line is the line of identity, indicating a test with no diagnostic value.
Discussion
Current POTS diagnostic criteria require a 30 bpm increase in HR or an increase to a maximum HR of 120 bpm with assumption of an upright posture with concurrent orthostatic symptoms without hypotension.[11] These criteria do not, however, differentiate between active and passive assumption of upright posture, and these different postures have very different physiological responses in both healthy individuals and patients with POTS.[11;13–15;20;21] Active standing activates a series of responses in the body that help to minimize blood pooling in the lower limbs, such as the “skeletal muscle pump”, increases abdominal pressure, causes rhythmic swaying, decreases vagal tone, and increases cardiac and vascular sympathetic activity.[8;9;15;16;19;20] The increased pressure and skeletal muscle contraction increase venous return to the heart, and the activation of skeletal muscles leads to a transient increase in heart rate, the so called “exercise reflex.”[2;20] These work to counteract some of the physiological effects of both initial and prolonged standing and are either absent, in the case of the exercise reflex, or present but significantly less, in the case of the skeletal muscle pump, during passive tilt.[2] It has already been shown that standing for 3 minutes correlates poorly with a full 30-minute tilt for both change in heart rate and blood pressure.[5;10] We aimed to carry out this analysis to a full 30 minutes of tilt and standing for change in heart rate given the recent description of “delayed POTS.”[12]
As expected, the physiological response varied over prolonged periods of standing. Without the aid of the skeletal muscle pump, venous return to the heart can decrease over time. Increased microvascular filtration of plasma into interstitial space on assumption of upright posture also contributes to decreased venous return by causing decreased blood volume, but, while it has been shown to occur in both tilt and stand, the differences between the two tests have not been elucidated.[6;9;17] Regardless, higher heart rates were required to maintain an adequate cardiac output when both patients and controls were passively tilted. While the heart rate response to 10-minute stand and 30-minute stand were both similar, the 10-minute tilt and 30-minute tilt were not nearly as accurate when using 30 bpm as a cutoff. The 10-minute stand was the most accurate test, with highest specificity while maintaining good sensitivity, as expected by the definition of POTS. However, while the 10-minute tilt correctly identified 93% of the POTS patients, it also identified 60% of the normal control subjects as having orthostatic tachycardia (false positives). This suggests that at 10 min for tilt, the 30 bpm criterion is highly sensitive, but has poor specificity. By increasing the HR cutoff to 37 bpm, the test was made much more specific (40% to 73%) while maintaining good sensitivity. The 30-minute tilt had even poorer specificity, correctly identifying all the patients but falsely identifying 80% of the healthy controls with orthostatic tachycardia. By increasing the heart rate threshold to 47 bpm, the specificity was increased to 80% while maintaining similar sensitivity.
The increase in HR is not the only criterion for diagnosing POTS. Patients must have symptoms of pre-syncope or orthostatic intolerance. However, orthostatic change in HR is the most objective and easily measured criterion. Other symptomatic criteria are subjective, harder to quantify, require more clinician experience to properly assess, and can be often overlooked.
The current POTS criteria require only a 10-minute orthostatic study. However, some physicians have gone on to describe a condition termed “delayed POTS” with an orthostatic tachycardia >30 bpm that develops between 10 and 30 minutes of upright posture.[12] While this diagnosis opens the door for the routine use of 30 minute tilt tests for the diagnosis of POTS, it would also “diagnose” many healthy individuals as having POTS. By increasing the heart rate threshold used to diagnose POTS when using a 30 minute tilt test, it would be a much more specific test while losing very little sensitivity.
There are some limitations to this study. The number of POTS patients included is relatively small, but the main focus of our analysis is the effect of different orthostatic maneuvers on ΔHR, and not the disease itself. The numbers from our study cannot be extrapolated to children, as all of our subjects were over 18 years of age. It has recently been shown that the HR criteria for POTS are different for children versus adults.[18] The phase of menstrual cycle during testing, and the changes in HR could be affected by testing in different phases of the menstrual cycle. Each subject’s tilt and stand test were performed within a few days of each other, so it is unlikely that subjects were in different stages of their menstrual cycle for their stand and tilt test. Most test subjects were supine for the entire night before beginning their stand test, which is not the most common clinical test protocol. While it may exaggerate some heart rate changes, this is the most standardized test protocol and ensures that fluid shifts are at steady state.
Conclusions
The variation in HR response leads us to believe that physicians should differentiate between testing patients with a tilt test and a stand test. When using a 30-minute tilt test, a cutoff delta HR of 30 bpm is not specific, and a cutoff of 47 bpm could maximize sensitivity and specificity. If using the 30 bpm criterion, active stand tests are more specific than passive tilt tests, while maintaining comparable sensitivity. Regardless of using tilt or stand, 10 minutes of testing achieved higher specificity while maintaining sensitivity. Thus, 10 minute tests should be used if using the 30 bpm criterion. Finally, POTS is a clinical diagnosis requiring orthostatic symptoms and not just hemodynamic criteria.
Clinical Perspectives.
Postural tachycardia syndrome (POTS) is a disorder of chronic orthostatic intolerance with markedly impaired quality of life. Diagnosis requires an increase in heart rate (HR) ≥30 bpm upon assumption of upright posture from supine. This HR criterion does not differentiate between active stand and passive tilt, which have different physiology. We found that tilt and stand have different HR responses with assumption of upright posture, leading to variation in achievement of the ≥30 bpm HR criterion. The ≥30 bpm HR criterion is reasonable only for short durations (10 min), but not longer tilt durations (30 min). Importantly, physicians should not diagnose POTS solely based on HR criterion, but should also incorporate orthostatic symptoms.
Acknowledgments
RESEARCH FUNDING
Supported in part by NIH grants R01 HL102387, R01 HL071784, R01 NS055670, P01 HL56693, UL1 RR024975 (Clinical and Translational Science Award), and the Paden Dysautonomia Fund.
We would like to thank our research subjects, without whom this research project could not have been performed. We would also like to recognize the highly professional care provided by the Elliot V. Newman Clinical Research Center nursing and nutrition staff.
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- BP
blood pressure
- HR
heart rate
- ΔHR
change in HR (upright HR – supine HR)
- POTS
Postural Tachycardia Syndrome
- ROC
receiver operator curve
- Sn
sensitivity
- Sp
specificity
- STAND
active stand test
- TILT
passive head-up tilt test
Footnotes
CONFLICTS OF INTEREST
None
AUTHORS CONTRIBUTIONS
These protocols were all conducted on the Vanderbilt Clinical Research Center. The following contributions were made by individual authors:
Plash – Analysis & interpretation of data; drafting article
Diedrich – collection, analysis & interpretation of data; critical revisions to draft
Biaggioni – study conception; interpretation of data; critical revisions to draft
Garland - study conception; interpretation of data; critical revisions to draft
Paranjape – collection & analysis of data; critical revisions to draft
Black - study conception; collection of data; critical revisions to draft
Dupont – analysis & interpretation of data; critical revisions to draft
Raj – study conception & design; collection, analysis & interpretation of data; drafting article
All authors have approved the final version of the manuscript.
Reference List
- 1.Bagai K, Song Y, Ling JF, Malow B, Black BK, Biaggioni I, Robertson D, Raj SR. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7:204–210. [PMC free article] [PubMed] [Google Scholar]
- 2.Borst C, Wieling W, van Brederode JF, Hond A, de Rijk LG, Dunning AJ. Mechanisms of initial heart rate response to postural change. Am J Physiol. 1982;243:H676–H681. doi: 10.1152/ajpheart.1982.243.5.H676. [DOI] [PubMed] [Google Scholar]
- 3.Dupont WD. A Simple Introduction to the Analysis of Complex Data. Cambridge University Press; Cambridge, U.K: 2009. Statistical Modeling for Biomedical Researchers. [Google Scholar]
- 4.Dzurik MV, Diedrich A, Black B, Paranjape SY, Raj SR, Byrne DW, Robertson D. Endogenous substance P modulates human cardiovascular regulation at rest and during orthostatic load. J Appl Physiol. 2007;102:2092–2097. doi: 10.1152/japplphysiol.00969.2006. [DOI] [PubMed] [Google Scholar]
- 5.Faraji F, Kinsella LJ, Rutledge JC, Mikulec AA. The comparative usefulness of orthostatic testing and tilt table testing in the evaluation of autonomic-associated dizziness. Otol Neurotol. 2011;32:654–659. doi: 10.1097/MAO.0b013e3182117769. [DOI] [PubMed] [Google Scholar]
- 6.Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13:304–310. doi: 10.1136/jcp.13.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 8.Gauer ON, Thron HL. Postural changes in the circulation. In: Hamilton WF, Dow P, editors. Handbook of Physiology. American Physiological Society; Washington, DC: 1965. pp. 2409–2439. [Google Scholar]
- 9.Hellebrandt FA, Franseen EB. Physiological study of vertical stance in man. Physiological Reviews. 1943;23:220–255. [Google Scholar]
- 10.Lambrecht R, McNeeley K, Tusing L, Chelimsky T. Evaluation of a brief cardiovascular autonomic screen. Auton Neurosci. 2007;131:102–106. doi: 10.1016/j.autneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 12.Mayuga KA, Butters KB, Fouad-Tarazi F. Early versus late postural tachycardia: a re-evaluation of a syndrome. Clin Auton Res. 2008;18:155–157. doi: 10.1007/s10286-008-0472-1. [DOI] [PubMed] [Google Scholar]
- 13.Petersen ME, Williams TR, Gordon C, Chamberlain-Webber R, Sutton R. The normal response to prolonged passive head up tilt testing. Heart. 2000;84:509–514. doi: 10.1136/heart.84.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 15.Rossberg F, Penaz J. Initial cardiovascular response on change of posture from squatting to standing. Eur J Appl Physiol Occup Physiol. 1988;57:93–97. doi: 10.1007/BF00691245. [DOI] [PubMed] [Google Scholar]
- 16.Rowell LB. Regulation During Physical Stress. Oxford University Press; USA, New York: 1986. Human Circulation. [Google Scholar]
- 17.Sander-Jensen K, Secher NH, Astrup A, Christensen NJ, Giese J, Schwartz TW, Warberg J, Bie P. Hypotension induced by passive head-up tilt: endocrine and circulatory mechanisms. Am J Physiol. 1986;251:R742–R748. doi: 10.1152/ajpregu.1986.251.4.R742. [DOI] [PubMed] [Google Scholar]
- 18.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr. 2012;160:222–226. doi: 10.1016/j.jpeds.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JJ, Ebert TS. General response to orthostatic stress. In: Smith JJ, editor. Circulatory Response to the Upright Posture. CRC Press; Boca Raton, FL: 1990. [Google Scholar]
- 20.Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34:375–386. doi: 10.1002/j.1552-4604.1994.tb04977.x. [DOI] [PubMed] [Google Scholar]
- 21.van Dijk N, de Bruin IG, Gisolf J, de Bruin-Bon HA, Linzer M, van Lieshout JJ, Wieling W. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J Appl Physiol. 2005;98:584–590. doi: 10.1152/japplphysiol.00738.2004. [DOI] [PubMed] [Google Scholar]