Abstract
Previously, we have shown that the intraductal (i.duc) administration of pegylated liposomal doxorubicin (PLD) to Her2/neu transgenic mice is associated with mammary tumor regression and prevention. Exploring the mechanism underlying the protection afforded by PLD, we studied: the effects of i.duc PLD-treatment with a subsequent pregnancy on outgrowth of tumors in Her2/neu mice; whether the i.duc PLD antitumor effect was mediated partially through changes in normal mammary stem cells (MaSCs); and the long-term safety of i.duc PLD into the normal mouse mammary gland. Her2/neu mice were treated with two i.duc injections of PLD given four weeks apart; pregnancy was induced and mice were followed up for changes in physiology, and tumor formation. We found that all pups born to i.duc PLD-treated Her2/neu mice died without weight gain within 7 days after birth. Despite an additional pregnancy, compared to vehicle control PLD-treated Her2/neu mice had a significantly longer latency and lower frequency of tumor development. Mammary epithelial cells isolated from untreated and i.duc PLD-treated 6–8 months-old multiparous FVB/N mice were analyzed for their repopulating ability in mammary fat pads of naïve recipients. Mice were also monitored for abnormalities in mammary gland morphology and function, including tumor formation. PLD-treated FVB/N mice displayed histomorphologic changes and a significant reduction in the outgrowth potential of cells from the mammary glands. Thus, i.duc PLD administration altered the mammary gland structurally and functionally by reducing the MaSC population, which could compromise milk production. Followed long term, i.duc PLD-treated FVB/N mice developed malignant mammary tumors, confirming similar published findings on doxorubicin injected into the mammary gland of rats. Unless there are fundamental species differences in PLD metabolism in rodents and humans, this finding seriously limits the consideration of i.duc PLD use in the clinic for treatment or prevention of breast cancer.
Keywords: Intraductal, Mammary, Carcinogenesis, Doxorubicin, Stem cell
Introduction
Adult tissue-specific stem cells are long-lived, generally quiescent cells that maintain the stem cell pool as well as more committed progeny that repopulate the organ [1, 2]. Normal tissue homeostasis involves a careful balance between cell loss and cell renewal. Stem and progenitor cells perform these biologic processes as functional units of regeneration during both tissue homeostasis and repair. Under normal circumstances, the processes of tissue regeneration or homeostasis are tightly regulated by several morphogen pathways to prevent excessive or inappropriate cell growth. Deregulation of these processes may provide opportunities for carcinogenesis for the long-lived, highly proliferative tissue stem cell population [3].
An important feature of the mammary gland is the regenerative capacity of its epithelium, which is demonstrated in successive reproductive cycles [4]. The activity of mammary stem cells (MaSCs) and their mitotic progeny is fundamental to normal mammary growth, differentiation and regeneration in successive cycles of pregnancy, lactation and involution [5, 6]. Within the mammary arbor, the ductal cells are those that line the ducts of mammary gland. Lobular cells form secretory acinar structures at the end of each branch and, upon pregnancy and lactation, become alveolar cells that produce milk proteins.
We have previously demonstrated that the intraductal (i.duc) administration of 4-hydroxytamoxifen or pegylated liposomal doxorubicin (PLD) to carcinogen-induced mammary tumors in rats and spontaneous mammary tumors in Her2/neu mice is associated with a reduction in mammary tumor volume, eradication of pre-malignant disease, and prevention of new lesions [7, 8]. Recently, we also demonstrated that several chemotherapeutics are equally or more effective than PLD in prevention and treatment of established carcinogen-induced mammary tumors in rats [8]. Moreover, a Phase I clinical trial established the feasibility and safety of intraductal injection of PLD into a single mammary duct in women awaiting mastectomy [8].
Prior to considering i.duc PLD for human use in treatment of ductal carcinoma in situ, several safety features need to be confirmed. Here, we present detailed structural and functional studies on i.duc PLD’s effects on normal mammary gland function, stem cell function, and the effects of pregnancy following i.duc PLD on tumorigenesis. All pups born to i.duc PLD-treated Her2/neu mice died within 7 days without weight gain. I.duc PLD-treated Her2/neu mice developed tumors at significantly lower frequency and longer latency than vehicle treated controls. I.duc PLD treatment significantly reduced mammary stem cell number in wild type FVB/N mice. However, the treated FVB/N mice developed mammary tumors upon long-term follow up. The ability of i.duc PLD to induce mammary malignant tumors in wild type mice raises serious concern for its future use in the clinic for prevention or treatment through this route.
Materials and methods
Animals, animal surgery and treatment
All the experiments were conducted on parous mice since prior lactation facilitates intraductal injection. Her2/neu transgenic mice in the FVB/N background were kindly provided by Dr. Elizabeth Jaffee (JHSOM) and bred in our animal facility. Six–eight month-old female multiparous wild type FVB/N mice were purchased from NCI-FCRF. Experiments were performed with the approval of Animal Care and Use Committee of Johns Hopkins University School of Medicine.
Intraductal injection
Intraductal injection was performed as previously described [7, 8]. 50 µl of pegylated liposomal doxorubicin (PLD, Doxil; Ortho Biotech Products, LP, Raritan, NJ) was injected into each duct while visualizing the opening under a dissecting microscope. To study effects of pregnancy on the function and tumor incidence of i.duc PLD-treated mammary gland, Her2/neu mice received PLD (20 µg/duct) into all ten ducts on the same day. PLD injection was repeated once, 4 weeks after the 1st injection. In cleared fat pad transplant analysis and studies on long-term effects, PLD (40 µg/duct) was injected into all ten ducts on the same day. PLD injection was repeated once, either 1 week or 4 weeks after the 1st PLD injection. In a replicate experiment, the second i. duc PLD (10, 20 or 40 µg/duct) injection was administered 4 weeks later.
In vivo limiting dilution assay
Single cell suspensions were generated from mammary glands of individual mice by enzymatic digestion as described in [9]. Enriched mammary epithelial (CD45−, Ter119−, CD31−) cells were collected using immunomagnetic bead separation and injected into cleared mammary fat pads (mfps) in limiting dilution as described in [9, 10] and Supplementary Materials. Single cell suspensions were prepared from mammary glands of individual i.duc PLD, or untreated FVB/N mice. 10 µl of cell suspension containing different number of cells (106, 105, 104, 103, 102, and 10) was injected into each of two 4th cleared mfps of pre-pubescent, 3 week-old recipient FVB/N females as described in Supplementary Materials. After 5–8 weeks, fat pads were dissected and ductal outgrowth was estimated by whole mount analysis. Limiting dilution analyses used “statmod” software package for the “R” computing environment (http://www.R-project.org). The frequency of mammary repopulating units (MRUs) in single cell suspensions was calculated as described in [10].
Morphological characterization of mammary glands in i.duc PLD-treated Her2/neu transgenic mice
The number of end buds was counted in 4 fields of each completely mounted mammary gland. Ductal dilatation was evaluated by measuring the diameter of the largest primary ducts [11].
Cell infiltration and fibrosis in the i.duc PLD-treated FVB/N mice
Histomorphologic change was assessed on Hematoxylin- and Eosin-stained specimens of mammary glands from FVB/N mice. These mice were also the donors for cleared fat pad outgrowth analysis. Cell infiltrations in terminal ductal and lobular units were quantified based on individual criteria. Infiltrations of: (1) lymphocyte and plasma cells and (2) neutrophils and macrophages were distinguished. Fibrotic findings were evaluated on ductal branches and end buds excluding main ducts and vessels. Highest score on each specimen was determined as; 0, none; 1, mild; 2, moderate; and 3, severe (criteria detailed in Supplementary Materials).
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Tumor-free survival was estimated using the Kaplan–Meier method and compared using the log-rank test. For whole mount analysis with 4 fields on each slide, Poisson regression was used to model such clustered count data with respect to number of end buds, with the use of the generalized estimating equations method by assuming an exchangeable variance–covariance structure to account for within and between subject variability. Diameter of primary ducts was compared using unpaired t test. The degree of histopathological findings was compared using the exact Wilcoxon rank sum test. Tumor incidence was compared using Fisher’s exact test. Statistical analyses were performed using GraphPad Prism 5 and SAS software 9.2. All tests were two-sided and considered statistically significant at P < 0.05.
Results
I.duc PLD treatment reduced tumor incidence despite subsequent pregnancy
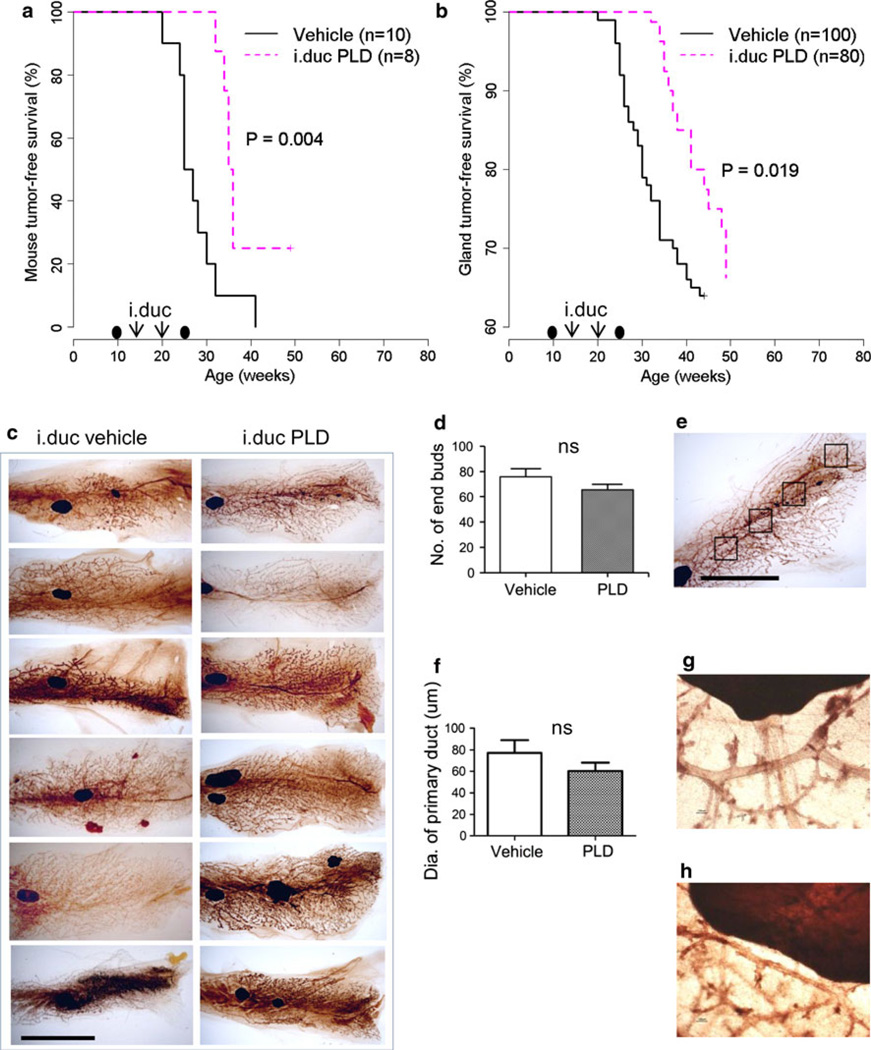
Previously, we have shown that i.duc but not i.v. PLD administered to Her2/neu mice resulted in tumor prevention [7]. Two or more pregnancies are known to stimulate the development of spontaneous mammary tumors [12]. We wanted to study whether the anti-tumor protection afforded by i.duc PLD is still retained notwithstanding the tumor-promoting effects of an additional pregnancy subsequent to treatment. Mice received i.duc PLD or vehicle twice per mammary gland at 16 and 20 weeks of age, respectively. Eight mice in the PLD group and 10 mice in the vehicle group were mated 4 weeks later. All pups born to the PLD-treated mice were normal at birth, but died within 7 days without weight gain, while pups in the vehicle group grew normally. It is possible that prior i.duc PLD exposure resulted in inadequate milk production during lactation. Mothers in both groups were followed up for an additional 5 months (Fig. 1a, b). As seen in the Kaplan–Meier plots of mouse tumor-free survival (Fig. 1a) and gland tumor-free survival (Fig. 1b), the median times to first tumor emergence for i.duc vehicle and i.duc PLD were 26 weeks and 35.4 weeks, respectively (Fig. 1a) and the incidence of tumor in the PLD-treated group was significantly reduced (Fig. 1a, b). Thus, i.duc PLD significantly protected mice by prolonging the latency period and reducing tumor incidence of spontaneous mammary tumors in Her2/neu mice.
Fig. 1.
Protective effect of i.duc PLD with subsequent pregnancy on tumor incidence and latency in Her2/neu mice. Kaplan–Meier plot of mouse tumor-free survival (a) and gland tumor-free survival (b) with i.duc PLD compared with vehicle by log-rank test. The timing of pregnancies before and after PLD is indicated by black ovals. c Mammary gland whole mount analysis of untreated and PLD-treated mice. Bar 10 mm. d Comparison of numbers of end buds between groups. I.duc vehicle (75.8 ± 6.2, n = 6 specimens × 4 fields = 24) and i.duc PLD (65.6 ± 4.1, n = 6 specimens × 4 fields = 24), P = 0.179. e A representative image shows 4 fields (5 × 5 mm) of a whole mount specimen. Bar 10 mm. f Comparison of the diameters of primary ducts. I.duc vehicle (77.2 ± 11.8, n = 9) and i.duc PLD (60.3 ± 7.7, n = 16), P = 0.225. ns not significant. g PLD #1, 100 µm in diameter of the largest primary duct adjacent to the central lymph node. h Vehicle #5, 50 µm in diameter
Morphological characteristics of mammary glands in the I.duc PLD-treated mice
As mentioned above, pups born to the untreated Her2/neu mothers survived and grew normally, while pups born to the i.duc PLD-treated mice died within 7 days. This raised the possibility that PLD-treated mice did not produce adequate milk to sustain the pups. To investigate whether abnormality in mammary gland architecture occurred in response to PLD, whole mount analysis of mammary glands of the i.duc-vehicle and PLD-treated mice was performed. PLD-treated mice did not show a significant difference in mammary gland architecture (Fig. 1c), the number of end buds (Fig. 1d) and diameter of primary ducts (Fig. 1f) compared with vehicle-treated mice. Of note, this analysis was conducted 6 months following pregnancy. Hence it is likely that multiple estrus cycles normalized the mammary architecture.
MaSC regeneration potential was significantly decreased in i.duc PLD-treated mice compared with untreated FVB/N mice
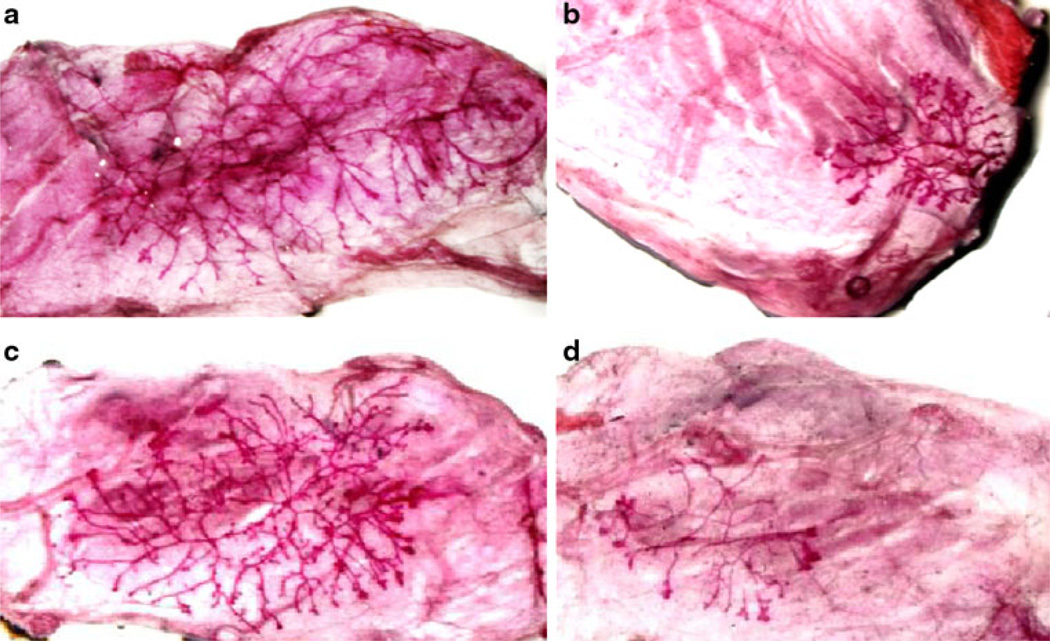
Lack of milk production in i.duc PLD-treated mammary gland, on the other hand, could also be due to a depletion of mammary stem cells in treated mice. To test this hypothesis, we evaluated the effect of i.duc PLD on MaSCs in wild type FVB/N mice. The classical assay for MaSCs is to evaluate ductal tree regeneration potential upon transplantation of a serial dilution of epithelial cells into cleared mfps of recipient mice. PLD injection was repeated one week (n = 13), or 4 weeks (n = 30) after the 1st injection. Ten age- and parity-matched FVB/N mice were used as untreated controls. Mammary glands in i.duc PLD group were harvested 14–31 weeks after 2nd i.duc PLD. Limiting dilution assays were performed by transplanting freshly isolated enriched mammary epithelial cells into cleared mfps. The number of mfps with outgrowths was counted (representative mfp outgrowths shown in Fig. 2). I.duc PLD-treated mammary cells showed MRU frequency of 1 in 2,119 compared to 1 in 118 in untreated mammary cells based on positive outgrowths scored for each cell dilution. This represented an 18-fold decrease in the absolute value of mammary repopulating units in the i.duc PLD-treated compared with untreated mice (Table 1) leading to the conclusion that i.duc PLD significantly reduced the stem cell population in the mammary gland
Fig. 2.
Mammary gland reconstitution in transplanted mammary fat pads. Mammary epithelial cells were transplanted into cleared mammary fat pads of 3 week-old recipient female FVB/N mice. Outgrowths in the recipients were analyzed in whole mounts 8 weeks after transplantation. Outgrowths from: a 106 mammary gland epithelial cells isolated 31 weeks after i.duc PLD; b 104 mammary gland epithelial cells isolated 18 weeks after i.duc PLD; c 106 and d 104 epithelial cells isolated from untreated, age and parity matched (to a and b) FVB/N mouse mammary glands
Table 1.
Mammary repopulation efficiency of enriched mammary epithelial cells
| Number of cells injected per mfp |
Number of mammary gland outgrowths/ number of mfp injected |
|
|---|---|---|
| Untreated | i.duc PLD | |
| 106 | 9/9 | 14/14 |
| 105 | 5/6 | 9/10 |
| 104 | 8/8 | 8/10 |
| 103 | 10/10 | 14/21 |
| 102 | 5/8 | 3/12 |
| 10 | 1/8 | 0/12a |
| Repopulating frequency | 1/118 | 1/2,119 |
| (95 % CI) | (1/280–1/50) | (1/3,894–1/1,153) |
| P value | <0.0001 | |
Two-sided 95 % Wald confidence intervals or
one-sided 95 % Clopper-Pearson intervals were used
Histological findings of the mammary glands in i.duc PLD-treated FVB/N mice
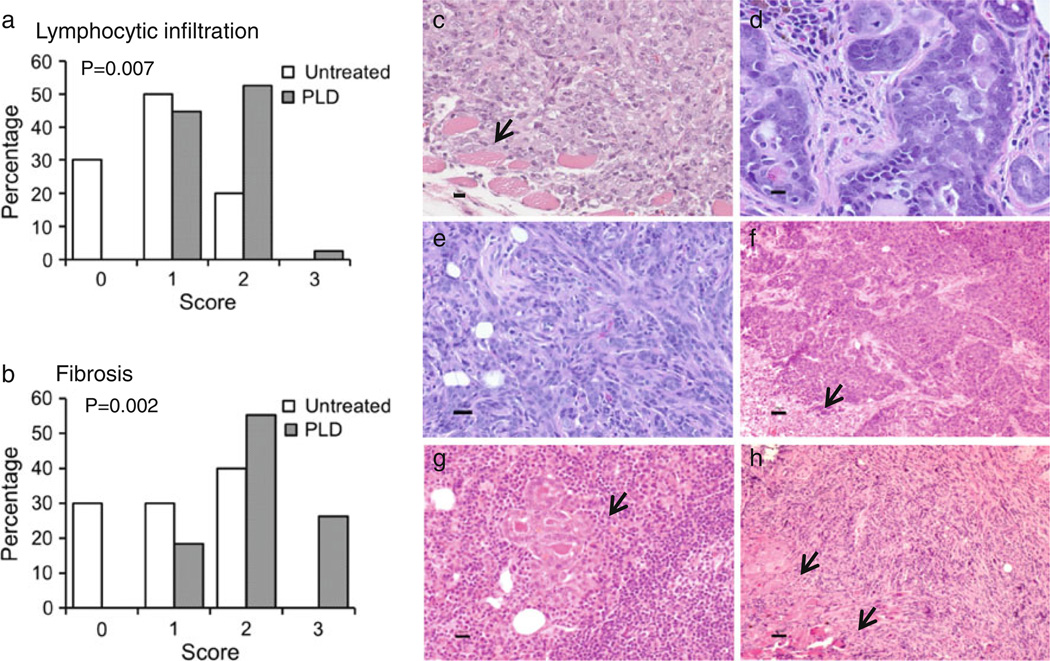
Fourth mammary glands from PLD-treated mice (n = 38) at 14–31 weeks after 2nd i.duc PLD and 4th or 5th mammary glands from untreated mice (n = 10) were subjected to H&E staining. PLD-treated mice had significantly higher degree of lymphocytic infiltrations in the mammary glands (Fig. 3a, P = 0.007), which correlated with fibrosis (Fig. 3b, P = 0.002). Neutrophil infiltration was very infrequent (data not shown).
Fig. 3.
Histopathology of mammary glands and tumors in FVB/N mice. a Lymphocytic infiltration and b fibrosis were determined in each specimen. Representative histology is shown of: c invasive undifferentiated mammary carcinoma arising in mfp transplanted with epithelial cells from PLD-treated mouse, d mammary intraepithelial neoplasia, and e–h mammary tumors developing in i.duc PLD treated mice, e poorly differentiated epithelial carcinoma, f carcinoma, solid pattern with necrosis (arrow), g carcinoma invading lymph nodes (arrow) and h sarcoma-like neoplasm with EMT and local invasion of skeletal muscle (arrow). Bars 20 µm
Long-term observation of i.duc PLD-treated FVB/N mice
A group of eight mice was observed for 33 weeks after 2nd i.duc PLD injection. Mammary glands from five of the eight mice were subjected to in vivo limiting dilution assay. Enriched mammary epithelial cells (103–106) were transplanted into the 4th cleared mfps of recipient females. Recipient mice developed solid tumors in their fat pads by 8 weeks after transplantation. Tumors developed in 19 mfps, pure ductal outgrowths developed in 2 mfps, while 20 mfps showed no outgrowth (Supplementary Table 1). The tumors were solid, aggressive neoplasms with undifferentiated histopathology (Fig. 3c), with foci of metastasis in the lung in one recipient. This finding suggested that transformed cells were present in the donor mammary glands prior to transfer to naïve recipient mice.
To test whether microscopic tumors pre-existing in the mammary glands of PLD-treated mice were the cause of tumors in recipient mice, the mammary glands of the remaining three mice were subjected to histopathological examination. The mammary glands of two mice showed foci consistent with hyperplasia and mammary intraepithelial neoplasia in mammary glands (Fig. 3d) while those of the third showed foci of hyperplasia. Retrospective histological examination showed that 8 of 38 mammary glands harvested at 14–31 weeks after 2nd i.duc PLD displayed rare nodules with ductal proliferation and dense desmoplastic stroma. Thus, it is possible that preneoplastic cells existed in the donor mammary glands that progressed following transplantation to the mfp.
To standardize observation times, to compare the effects of two doses of PLD injected at weekly intervals versus at 4 weekly intervals, and to confirm the findings discussed above, a replicate experiment was performed. Here, three groups received i.duc PLD at doses of 10, 20, and 40 µg/duct, respectively, one group received vehicle and the last group was left untreated. Palpable tumors appeared starting at 16 weeks after the 2nd injection in PLD-treated groups during the 42 weeks of observation, but not in the two control groups (Table 2 and Supplementary Table 2). Figure 3e–h illustrates the different types of tumor histology observed in these mice. Thus, long-term observation confirmed that PLD is carcinogenic to the mouse mammary gland epithelium, generating tumors that are invasive, sometimes metastatic and with histopathological features of epithelial-mesenchymal transition (EMT).
Table 2.
Mammary tumor incidence in parous FVB/N mice receiving i.duc PLD
| i.duc/duct | Tumor incidencea |
P value | Tumor type |
|---|---|---|---|
| PLD 40 µg | 6/9 | 0.009 | carcinoma, carcinosarcoma |
| PLD 20 µg | 3/7 | 0.077 | carcinoma |
| PLD 10 µg | 3/9 | 0.206 | carcinoma, sarcoma, b LN tumor |
| Vehicle (50 µl) | 0/7 | >0.999 | |
| No treatment | 0/8 | Reference |
The mice were observed for 42 weeks
One case of microscopic epithelial tumor in the lymph node (LN) without visible or additional microscopic lesions in the mammary gland sections examined
An exact logistic regression analysis showed there was no change in tumor incidence with a change in PLD dosage (P = 0.449)
Discussion
Previous studies have shown that the i.duc administration of 4-hydroxytamoxifen and PLD to rodents with carcinogen-induced mammary tumors, and of PLD to Her2/neu mice was associated with regression of established mammary tumors and prevention of new lesions [7, 8]. In this study, we investigated the safety of i.duc PLD and mechanism underlying PLD’s protective effects. We found that i.duc PLD, despite an additional pregnancy, remained protective against tumorigenesis in the Her2/neu mice by reducing spontaneous tumor incidence and prolonging latency compared with vehicle treated mice. In the wild type FVB/N mice, i.duc PLD resulted in reduction in the number of MaSC compared to untreated controls. However, development of mammary tumors in the treated mice after long-term follow up showed that PLD is carcinogenic in the normal mouse mammary gland.
Whole mount analysis in Her2/neu transgenic mice showed no effects on the structure of the mammary glands, such as the number of end buds (Fig. 1d) or ductal dilatation of primary ducts (Fig. 1f). Structural changes observed in whole mount analysis and histopathological findings, depend on aging, duration after injection, and estrus phase [5]. Therefore, a retrospective analysis based on the long-term follow-up data did not provide an adequate explanation for death of pups born to i.duc PLD-treated Her2/neu mice. Prospective studies on the effect of i.duc PLD on milk production are necessary to resolve this question. In fact, MNU-treated rats treated by i.duc PLD examined within 4 weeks showed a loss of density of ductal outgrowth and periductal inflammation compared to untreated controls [8]. These results are consistent with a severe depletion of stem cells in the mammary gland and decrease of the number of MRUs in i.duc PLD-treated mice seen in this study. Taken together, the major reason for death of all pups born to PLD-treated mice appears not to be due to structural abnormalities, but possibly a compromised functioning of mammary gland epithelial cells.
I.duc PLD significantly protected mice by prolonging the latency and decreasing incidence of spontaneous mammary tumor development in Her2 mice (Fig. 1a, b). A likely explanation for the protection could be a reduction in MaSC function. However, formation of tumors in wild type FVB/N mice that received i.duc PLD over the long term is a serious pitfall. PLD is a formulation of doxorubicin in poly (ethylene glycol)-coated (stealth) liposomes with a prolonged circulation time and unique toxicity profile [13]. Numerous anti-neoplastic agents including doxorubicin are known carcinogens, and rare occurrence of second malignancies in cancer patients treated with single or multiple drugs has been documented [14, 15]. Rodents are particularly susceptible to anthracycline-induced mammary tumors [16–18]. Howell et al. [19] reported that four of 12 rats given an intramammary injection of daunorubicin (4 or 8 µg) developed mammary tumors in the injected area within 6.5 months of injection. The inability of rodent mammary tissue to metabolize the drug was cited as a possible cause of drug-induced tumors in rats [19]. Similarly, it is possible that i.duc PLD-treated mouse mammary glands were unable to metabolize PLD which led to its accumulation in the mammary glands. However, unlike rodents, metabolism of PLD does occur in human breast tissue. Our recent clinical trial showed the presence of metabolites such as doxorubicinol in the breast tissue and blood of women who received PLD into one duct prior to mastectomy [8]. Obviously, metabolism of anthracyclines in breast tissue between rodents and humans is different; this needs to be addressed with comparative studies on pharmacokinetics of PLD in the mammary gland and serum in the future. Transfer of milk contaminated with poorly metabolized PLD in the mammary gland from the mother to the pups as a cause of their death also needs to be ruled out. However, at the present time, the risk of developing cancer in the breast after a long latency due to PLD exposure remains a valid concern.
One can speculate on how PLD causes malignant mammary tumors in i.duc exposed mice. As stem cells self-renew throughout life and are long-lived, one possibility is that accumulating genetic anomalies over time could compromise their genomic integrity and potentially give rise to cancer [1–3].
I.duc treatment has a significant potential for both breast cancer prevention and treatment. Treating patients with a family history or with other high risk factors for developing breast cancer holds the promise of reducing the cancer burden in this vulnerable population [20]. Therefore before proceeding with clinical translation of this novel technique, the first priority is the selection of appropriate treatment agents which are proven safe and fully metabolized in mammary glands when administered intraductally.
In conclusion, we have presented data to demonstrate that i.duc PLD protects Her2/neu mice despite the tumor-promotional effects of an additional pregnancy. Some of the protective effects could be attributed to a reduction in normal MaSC population in the mouse mammary gland. Upon long-term follow up, i.duc PLD-treated mice developed malignant mammary tumors. This finding under-scores the need for further research into metabolism of PLD in the human breast, and a continuing search for new agents for intraductal use.
Supplementary Material
Acknowledgments
We thank Windy Hill II (SS), Susan Love Foundation (SS), and Mary Kay Ash (VS) for funding.
Abbreviations
- i.duc
Intraductal
- MaSC
Mammary stem cell
- PLD
Pegylated liposomal doxorubicin
- MFP
Mammary fat pad
- EMT
Epithelial mesenchymal transition
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-012-2138-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Yong Soon Chun, Email: chunysmd@gachon.ac.kr.
Takahiro Yoshida, Email: tyoshid9@jhmi.edu.
Tsuyoshi Mori, Email: t252m@belle.shiga-med.ac.jp.
Zhe Zhang, Email: zzhang16@jhmi.edu.
Vered Stearns, Email: vstearn1@jhmi.edu.
Brandy Perkins, Email: bperkin6@jhmi.edu.
Richard J. Jones, Email: rjjones@jhmi.edu.
References
- 1.Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 3.Perryman SV, Sylvester KG. Repair and regeneration opportunities for carcinogenesis from tissue stem cells. J Cell Mol Med. 2006;10:292–308. doi: 10.1111/j.1582-4934.2006.tb00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GH. Stem cells and mammary cancer in mice. Stem Cell Rev. 2005;1:215–223. doi: 10.1385/SCR:1:3:215. [DOI] [PubMed] [Google Scholar]
- 5.Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 6.Smith GH, Chepko G. Mammary epithelial stem cells. Microsc Res Tech. 2001;15:190–203. doi: 10.1002/1097-0029(20010115)52:2<190::AID-JEMT1005>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Murata S, Kominsky SL, Vali M, Zhang Z, Garrett-Mayer E, Korz D, Huso D, Baker SD, Barber J, Jaffee E, Reilly RT, Sukumar S. Ductal access for prevention and therapy of mammary tumors. Cancer Res. 2006;66:638–645. doi: 10.1158/0008-5472.CAN-05-4329. [DOI] [PubMed] [Google Scholar]
- 8.Stearns V, Mori T, Jacobs LK, Khouri NF, Gabrielson E, Yoshida T, Kominsky SL, Huso DL, Jeter S, Powers P, Tarpinian K, Brown RJ, Lange JR, Rudek MA, Zhang Z, Tsangaris TN, Sukumar S. Preclinical and clinical evaluation of intraductally administered agents in early breast cancer. Sci Transl Med. 2011;3(106) doi: 10.1126/scitranslmed.3002368. 106ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stingl J, Eirew P, Ricketson I, Shackleton M, Vailant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 10.Shackleton M, Vailant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 11.Atabai K, Fernandez R, Huang X, Ueki I, Kline A, Li Y, Sadatmansoori S, Smith-Steinhart C, Zhu W, Pytela R, Werb Z, Sheppard D. Mfge8 is critical for mammary gland remodeling during involution. Mol Biol Cell. 2005;16(12):5528–5537. doi: 10.1091/mbc.E05-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Liu W, Jakubczak JL, Erexson GL, Tindall KR, Chan R, Muller WJ, Adhya S, Garges S, Merlino G. Genetic instability favoring transversions associated with ErbB2-induced mammary tumorigenesis. Proc Natl Acad Sci USA. 2002;99(6):3770–3775. doi: 10.1073/pnas.052710299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabizon AA. Pegylated liposomal doxorubicin: meta-morphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001;19:424–436. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 14.Fornari FA, Randolph JK, Yalowich JC, Ritke MK, Gewirtz DA. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 1994;45:649–656. [PubMed] [Google Scholar]
- 15.Rieche K. Carcinogenicity of antineoplastic agents in man. Cancer Treat Rev. 1984;11:39–67. doi: 10.1016/0305-7372(84)90016-1. [DOI] [PubMed] [Google Scholar]
- 16.Bertazzoli C, Chieli T, Solcia E. Different incidence of breast carcinomas or fibroadenomas in daunomycin or adriamycin treated rats. Experientia. 1971;27(10):1029–1036. doi: 10.1007/BF02286933. [DOI] [PubMed] [Google Scholar]
- 17.Bucciarelli E. Mammary tumor induction in male and female Sprague-Dawley rats by adriamycin and daunomycin. J Natl Cancer Inst. 1981;66:81–84. [PubMed] [Google Scholar]
- 18.Solcia E, Ballerini L, Ballini O, Sala L, Bertazzoli C. Mammary tumors induced in rats by Adriamycin and Daunomycin. Cancer Res. 1978;38:1444–1446. [PubMed] [Google Scholar]
- 19.Howell SK, Stephens LC, Wang YM. Daunorubicin-induced mammary tumors in the rat. Eur J Cancer Clin Oncol. 1989;25:1549–1554. doi: 10.1016/0277-5379(89)90296-4. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs L, Sukumar S, Stearns S. Intraductal therapy for the prevention of breast cancer. Curr Opin Investig Drugs. 2010;11(6):646–652. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.