Abstract
Background
The lack of reliable human proxies for minor (i.e., non-HLA) histocompatibility loci hampers the ability to leverage these factors toward improving transplant outcomes. Despite conflicting reports of the effect of donor-recipient sex mismatch on renal allografts, the association between acute rejection of renal allografts and the development of human alloantibodies to the male H-Y antigen suggested to us that donor-recipient sex-mismatch deserved re-evaluation.
Objective
To evaluate whether relations between donor sex and allograft failure differed by recipient sex.
Methods
We studied recipients of deceased- (n=125,369) and living-donor (n=63,139) transplants in the United States Renal Data System (USRDS). Using Cox proportional hazards models stratified by donor type we estimated the association between donor-recipient sex-mismatch and death-censored allograft failure with adjustment for known risk factors, with and without use of multiple imputation methods to account for potential bias and/or loss of efficiency due to missing data.
Results
The advantage afforded by male donor kidneys was more pronounced among male than among female recipients (8% vs. 2% relative risk reduction; interaction p<0.01). This difference is of the order of magnitude of several other risk factors affecting donor selection decisions.
Conclusions
Donor-recipient sex mismatch affects renal allograft survival in a direction consistent with immune responses to sexually determined minor histocompatibility antigens. Our study provides a paradigm for clinical detection of markers for minor histocompatibility loci.
Keywords: gender, H-Y, kidney transplant, minor histocompatibility, sex
Introduction
Although HLA alloantigen mismatching is an important and well-defined determinant of kidney allograft rejection and subsequent allograft failure,1 the effect of non-HLA disparity on allograft function is poorly understood. Observations that even in HLA-identical kidney transplants a) acute rejection is commonly observed; and b) maintenance immunosuppression is required for long-term allograft survival suggest that minor-histocompatibility antigens (mHA) may be the target of immune responses leading to allograft dysfunction and premature loss. Some indirect evidence for a clinically significant role for mHA comes from Opelz et al. Using HLA-identical sibling transplants in the European Collaborative Transplant Study (CTS) registry, these authors showed significantly lower 10-year allograft survival estimates with increasing levels of panel reactive antibody (PRA).2 As HLA-identical siblings have no HLA mismatches, this difference in allograft survival would most likely be a result of factors other than immunity to HLA. These findings suggest a clinically significant role for immunity to non-HLA alloantigenic stimuli.
Among the best defined of the mHA candidates are the H-Y antigens encoded by the Y chromosome.3 Although specific immune responses to H-Y have been difficult to measure, we recently demonstrated that de novo anti-H-Y antibody production is associated with acute rejection in female recipients of male donor kidneys.4, This finding suggests how renal allograft survival would be affected by donor-recipient sex mismatch, however previous studies of donor-recipient sex mismatch effects have yielded conflicting results.5,6 These studies have been limited by the short duration of follow-up5 and/or lack of correction for differences in expected allograft size (“nephron supply”) relative to recipient body size (“metabolic demand”). For example, women are known to have smaller kidney weight compared to men,7 and lower transplanted kidney weight or volume is associated with poorer allograft survival.8–10 Body surface area (BSA) is directly correlated with kidney weight7,11; lower donor BSA and higher recipient BSA are independently associated with allograft failure.12 Of greater concern, however, are the methods of analysis. More specifically, none of the previous studies formally compared the effect of donor sex among male recipients to that among female recipients, a crucial comparison for addressing the hypothesis.
To test the hypothesis that immunity to sexually determined alloantigens might influence rates of allograft failure, we evaluated whether the relations between donor sex and allograft failure (i.e., lower risk of allograft failure among recipients of male donor kidneys) differed by recipient sex. Specifically, we hypothesized that male donor kidneys would provide less advantage than expected when transplanted into female recipients.
Patients & Methods
Study Population
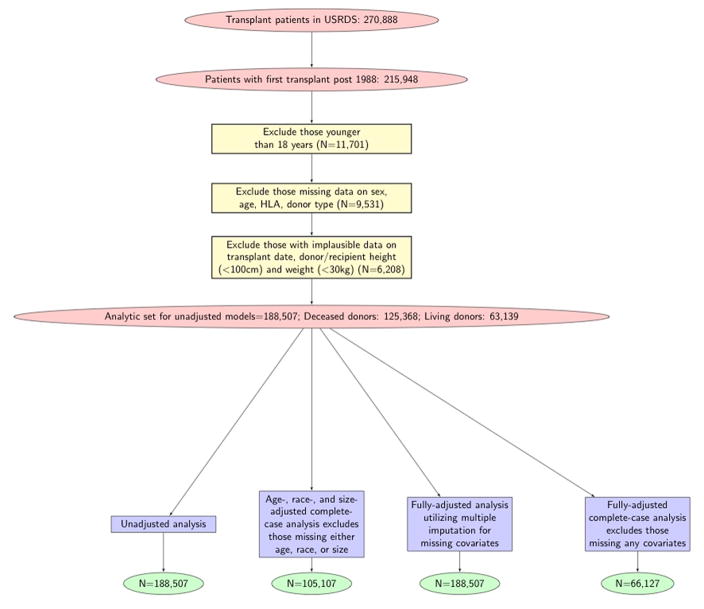
Our analytic sample is a subset of all kidney transplant recipients in the USRDS database who received their first recorded kidney transplant from January 1988 to December 2006 (n=215,948; 143,282 with deceased donors; 71,703 with living donors; and 963 with an unknown donor type). We restricted the sample to include data on the first transplant experience for adults only (excluded n=11,701 subjects younger than 18 years old). We subsequently excluded subjects with missing or unknown data on recipient or donor sex, donor type, age, and HLA mismatch (n=9,531). Those with implausible reported values for donor or recipient weight (<30 kg), donor or recipient height (<100 cm) (n=6,208), or transplant date (n=3) were further excluded. The full analytic cohort contained 188,507 transplant recipients (n=125,368 with deceased donors and n=63,139 with living donors). Figure 1 displays the derivation of our study population including the number of subjects used in analyses.
Figure 1.
The stepwise approach of the excluded population is detailed in this diagram. The analytic cohorts of the four models used in this study are delineated.
Outcomes
The primary outcome of interest was death-censored allograft failure, defined as return to dialysis or subsequent transplant, whichever happened first. Patients who died with a functioning allograft were censored at their time of death. We also considered non-death-censored allograft failure, where allograft failure included death with a functioning allograft.
Factor of Primary Interest: Differential Effect of Donor Sex by Recipient Sex
The primary exposure of interest was donor sex. To determine whether the relation between donor sex and allograft failure differed by recipient sex, however, the interaction between donor and recipient sex, which represents the differential effect of donor sex by recipient sex, was the factor of primary interest.
Effect Modifiers and Confounders
Donor type, transplant era, and donor and recipient BSA were considered potential effect modifiers. To that end, we assessed whether the associations of primary interest varied by levels of these variables. As this was not found to be the case with any of these variables, they were subsequently considered potential confounders. We adjusted for donor and recipient BSA, transplant era, as well as other known confounders of allograft failure, including recipient and donor age, recipient and donor race, peak panel reactive antibody (PRA), primary cause of end stage renal disease (ESRD), number of HLA mismatches, dialysis vintage, and cold ischemia time. Analyses were stratified by deceased- vs. living-donor type. The latter allowed the baseline hazards to vary by donor type, an assumption strongly supported by empirical and biological evidence.
Statistical Analysis
To assess the association of donor-recipient sex mismatch with allograft failure, we compared hazard ratios of death-censored allograft failure for male vs. female donor kidneys among female and male recipients. This comparison is equivalent to testing for an interaction between donor and recipient sex. To address issues posed by missing data, we utilized both a complete-case approach and a multiple imputation-based approach, with our primary results based on the latter. More specifically, the complete-case approach excludes individuals missing at least one variable included in the model, while the multiple imputation approach makes use of the entire cohort by imputing a set of plausible values for each missing covariate multiple times to account for the uncertainty about the imputed value. Multiple imputation methods produce statistically valid results when the data are missing at random (that is, when the probability that an observation is missing depends only on the observed variables and not on the missing values), a reasonable assumption here.13 There are two main approaches to multiple imputation: the joint modeling approach a fully conditional specification approach. Ours is based on the former which has well-established and desirable statistical propoerties. More specifically, a multivariate normal distribution was assumed for the joint distribution of the covariates and an uninformative prior distribution was assumed for the population mean vector of the normal distribution. Bayesian methods (Markov Chain Monte Carlo algorithms) were used to sample from the predictive distribution to impute missing values. We included all variables from the model of interest for the imputation model, which is a widely suggested procedure so as to avoid bias in resulting estimates. This procedure produced 5 imputed data sets and standard rules for combining the results were applied.
We fitted four Cox proportional hazards regression models for each donor type: 1) an unadjusted model that only included the major variables of interest: donor sex, recipient sex and their product (the interaction term) (n=188,507); 2) a complete-case model that adjusts for donor and recipient age, race and BSA and excludes subjects missing data on any of these variables (n=105,107); 3) a complete-case fully adjusted model that adjusts for all known determinants of allograft failure listed above and excludes subjects missing at least one covariate included in the model (n=66,127); and 4) a multiple-imputation based model that adjusts for all risk factors listed above without excluding any of the subjects from the full cohort (n=188,507). See Figure 1 for more details on how the analytic samples were created. The four models above were fitted for recipients of living donors, and then for recipients of deceased kidney donors. Our primary results are based on the latter model described under 4). We displayed Kaplan-Meier product limit estimates to graphically compare allograft failure among the relevant groups. Additionally, we ran analysis on acute rejection events using similar models mentioned above.
We calculated hazard ratios (HR) and 95% confidence intervals (95% CI) from model parameter estimates and standard error estimates, respectively. Graphical techniques such as plots of the log cumulative hazard rates over time were used to establish the validity of the proportionality assumption.13 All tests were two-sided and conducted at the 0.05 level of significance. Statistical analyses were performed in SAS version 9 14 and R version 2.12.1.15
Results
Donor-Recipient Match/Mismatch and Allograft Failure
Study population characteristics across the four recipient-donor sex groups for the deceased and living data sets are shown in Table 1. Note that all features appear comparable across the four groups, with the exception of BSA differences among recipients and donors as expected.
Table 1(a).
Study population characteristics by recipient-donor sex: Recipients of deceased donors
| Male Recipient | Female Recipient | |||
|---|---|---|---|---|
| Male Donor (%) | Female Donor (%) | Male Donor (%) | Female Donor (%) | |
| N=125,368 | 46,887 (37.4) | 29,600 (23.6) | 29,190 (23.3) | 19,692 (15.7) |
|
| ||||
| Recipient Age (yrs) | ||||
| 18 to 34.9 | 8434 (18) | 4989 (16.9) | 6300 (21.6) | 3873 (19.7) |
| 35 to 49.9 | 17497 (37.3) | 10534 (35.6) | 10623 (36.4) | 6892 (35) |
| 50 to 64.9 | 16483 (35.2) | 10750 (36.3) | 9867 (33.8) | 7043 (35.8) |
| >65 | 4472 (9.5) | 3327 (11.2) | 2400 (8.2) | 1884 (9.6) |
| Recipient Race | ||||
| White | 31312 (66.8) | 19899 (67.2) | 19181 (65.7) | 12793 (65) |
| Black | 12915 (27.5) | 7886 (26.6) | 7972 (27.3) | 5425 (27.5) |
| Other | 2659 (5.7) | 1815 (6.1) | 2037 (7) | 1474 (7.5) |
| Donor Age (yrs) | ||||
| <18 | 7268 (15.5) | 3391 (11.5) | 5127 (17.6) | 2433 (12.4) |
| 18 to 34.9 | 17582 (37.5) | 6535 (22.1) | 11066 (37.9) | 4303 (21.9) |
| 35 to 49.9 | 10941 (23.3) | 8961 (30.3) | 6400 (21.9) | 5821 (29.6) |
| 50 to 64.9 | 6961 (14.8) | 7357 (24.9) | 4075 (14) | 4934 (25.1) |
| >65 | 1136 (2.4) | 1348 (4.6) | 669 (2.3) | 896 (4.6) |
| Missing | 2998 (6.4) | 2008 (6.8) | 1853 (6.3) | 1305 (6.6) |
| Donor Race | ||||
| White | 35942 (76.7) | 23876 (80.7) | 22417 (76.8) | 15803 (80.3) |
| Black | 5302 (11.3) | 2519 (8.5) | 3233 (11.1) | 1747 (8.9) |
| Other/Missing | 5642 (12) | 3205 (10.8) | 3540 (12.1) | 2142 (10.9) |
| Presumed Cause of ESRD | ||||
| Diabetes | 13863 (29.6) | 8691 (29.4) | 8240 (28.2) | 5496 (27.9) |
| Hypertension | 10254 (21.9) | 6402 (21.6) | 4839 (16.6) | 3279 (16.7) |
| Glomerulonephritis | 9702 (20.7) | 6252 (21.1) | 5660 (19.4) | 3746 (19) |
| Cystic Disease | 4123 (8.8) | 2608 (8.8) | 3181 (10.9) | 2201 (11.2) |
| Other | 6244 (13.3) | 3954 (13.4) | 5504 (18.9) | 3773 (19.2) |
| Missing | 2700 (5.8) | 1693 (5.7) | 1766 (6.1) | 1197 (6.1) |
| Peak panel reactive antibody (%) | ||||
| <10 | 27936 (59.6) | 17884 (60.4) | 13702 (46.9) | 9321 (47.3) |
| 10 to 49 | 5292 (11.3) | 3362 (11.4) | 4644 (15.9) | 3174 (16.1) |
| >50 | 1398 (3) | 893 (3) | 3923 (13.4) | 2664 (13.5) |
| Missing | 12260 (26.1) | 7461 (25.2) | 6921 (23.7) | 4533 (23) |
| Time on Dialysis (mos) | ||||
| 0 | 3267 (7) | 1937 (6.5) | 2315 (7.9) | 1489 (7.6) |
| <6 | 3227 (6.9) | 2041 (6.9) | 2012 (6.9) | 1332 (6.8) |
| >6 to 12 | 5292 (11.3) | 3175 (10.7) | 3017 (10.3) | 2028 (10.3) |
| >12 to 24 | 10895 (23.2) | 6771 (22.9) | 6418 (22) | 4248 (21.6) |
| >24 to 36 | 8130 (17.3) | 5345 (18.1) | 4870 (16.7) | 3354 (17) |
| >36 to 48 | 5676 (12.1) | 3764 (12.7) | 3606 (12.4) | 2303 (11.7) |
| >48 | 10399 (22.2) | 6567 (22.2) | 6952 (23.8) | 4938 (25.1) |
| No. of HLA mismatches | ||||
| 0 to 1 | 4429 (9.4) | 2859 (9.7) | 3280 (11.2) | 2183 (11.1) |
| 2 to 4 | 1356 (2.9) | 953 (3.2) | 992 (3.4) | 649 (3.3) |
| 5 to 6 | 25812 (55.1) | 16254 (54.9) | 15940 (54.6) | 10702 (54.3) |
| Unknown/missing | 15289 (32.6) | 9534 (32.2) | 8978 (30.8) | 6158 (31.3) |
| Transplant Era | ||||
| 1988 to 1994 | 16620 (35.4) | 9715 (32.8) | 10722 (36.7) | 6414 (32.6) |
| 1995 to 1999 | 11756 (25.1) | 7809 (26.4) | 7257 (24.9) | 5120 (26) |
| 2000 to 2006 | 18510 (39.5) | 12076 (40.8) | 11211 (38.4) | 8158 (41.4) |
| Cold Ischemia Time (hrs) | ||||
| <12 | 7371 (15.7) | 4451 (15) | 4451 (15.2) | 3063 (15.6) |
| 12 to <24 | 21326 (45.5) | 13352 (45.1) | 13355 (45.8) | 8950 (45.4) |
| 24 to <36 | 11067 (23.6) | 7221 (24.4) | 7116 (24.4) | 4798 (24.4) |
| >36 | 2984 (6.4) | 1859 (6.3) | 1761 (6) | 1122 (5.7) |
| Unknown/missing | 4138 (8.8) | 2717 (9.2) | 2507 (8.6) | 1759 (8.9) |
| Recipient Body surface area, m2 | ||||
| <1.6 | 7867 (16.8) | 5084 (17.2) | 10521 (36) | 7146 (36.3) |
| 1.6 to 1.8 | 1560 (3.3) | 971 (3.3) | 7783 (26.7) | 5187 (26.3) |
| 1.8 to 2.0 | 15791 (33.7) | 10170 (34.4) | 6557 (22.5) | 4482 (22.8) |
| 2.0 to 2.2 | 12684 (27.1) | 7810 (26.4) | 2172 (7.4) | 1495 (7.6) |
| 2.2 and >2.2 | 6382 (13.6) | 3979 (13.4) | 467 (1.6) | 315 (1.6) |
| Missing | 2602 (5.5) | 1586 (5.4) | 1690 (5.8) | 1067 (5.4) |
| Donor Body surface area, m2 | ||||
| <1.6 | 4570 (9.7) | 8122 (27.4) | 2901 (9.9) | 5434 (27.6) |
| 1.6 to <1.8 | 1631 (3.5) | 4659 (15.7) | 1261 (4.3) | 3320 (16.9) |
| 1.8 to <2.0 | 11630 (24.8) | 5447 (18.4) | 6988 (23.9) | 3464 (17.6) |
| 2.0 to <2.2 | 9555 (20.4) | 2043 (6.9) | 5690 (19.5) | 1269 (6.4) |
| 2.2 and >2.2 | 4722 (10.1) | 725 (2.4) | 2778 (9.5) | 447 (2.3) |
| Missing | 14778 (31.5) | 8604 (29.1) | 9572 (32.8) | 5758 (29.2) |
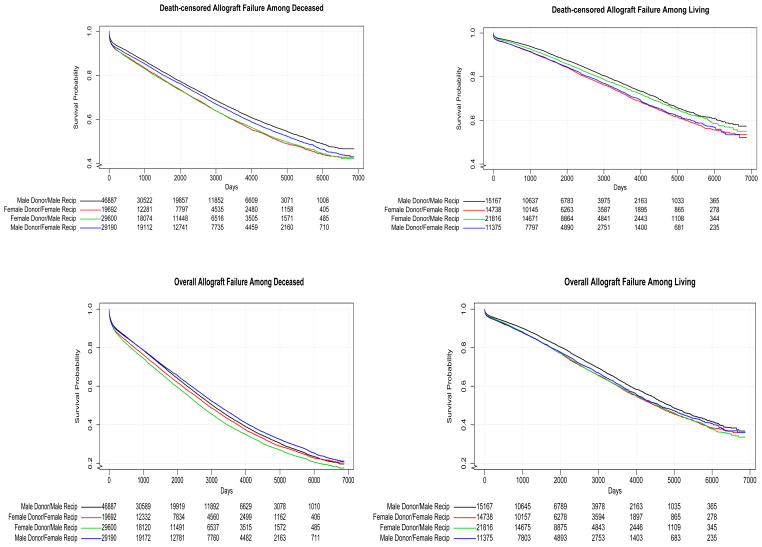
Figure 2 illustrates Kaplan Meier curves of death-censored and overall allograft failure for deceased-and living-donor transplant, respectively. The more pronounced difference between curves for male and female donors among male recipients compared to that among the female recipients is clearly depicted for both donor types. Table 2 displays hazard ratios of allograft failure for male vs. female donor transplants by recipient sex from the four fitted models. The difference in hazard ratios was very similar in every model and those differences were each statistically significant except for the complete-case fully adjusted model, which had a considerably smaller sample size. As expected, receipt of a male donor kidney was advantageous for both women and for men, however this was differentially so. Among female recipients, there was a 2% benefit (HR 0.98, 95% CI 0.95 to 1.01) whereas among male recipients, the benefit was 8% (HR 0.92, 95% CI 0.89 to 0.94) after adjusting for all potential confounders. Results for non-death censored graft failure were directionally similar and of similar magnitude, although not statistically significant.
Figure 2.
Kaplan Meier curves of death-censored (upper panels) and non-death-censored (lower panels) allograft failure for donor-recipient sex among deceased (left) and living (right) donors.
Table 2.
Models for death-censored allograft failure stratified by donor type.
| Hazard Ratios of Allograft Failure for Recipients of Male vs. Female Donors by Recipient Sex
| |||
|---|---|---|---|
| Model | Recipient | HR | Sample Size |
| Unadjusted | Female | 0.91 [0.88, 0.94]† | 188,507 |
| Male | 0.86 [0.84, 0.88] (0.002)†† | ||
| Age-, Race-, and Size-adjusted* | Female | 0.96 [0.92, 1.01] | 105,107 |
| Male | 0.89 [0.86, 0.93] (0.019) | ||
| Fully-adjusted** (MI)*** | Female | 0.98 [0.95, 1.01] | 188,507 |
| Male | 0.92 [0.89, 0.94] (0.001) | ||
| Fully-adjusted (Complete Case) | Female | 0.97 [0.92, 1.03] | 66,127 |
| Male | 0.92 [0.87, 0.96] (0.084) | ||
Adjusts for donor and recipient age, race and BSA.
Includes donor and recipient age, race and BSA, cause of ESRD, peak PRA (%), time on dialysis, no. of HLA mismatches, transplant era, presumed cause of ESRD, cold ischemia time.
Multiple Imputation.
[95% confidence interval];
(p-value of interaction term based on Wald test).
The hazard ratios corresponding to all confounders included in the multiple imputation-based fully adjusted model are shown in Supplementary Table 1. These are well known determinants of allograft failure; they are presented to provide context for the relative effect of donor-recipient sex mismatch. For example, an increase in cold ischemia time from <12 hours to the 12–24 hour range, and then to 24–36 hours each results in a 6% increased risk of allograft failure, Thus, differences in risks experienced by male and female recipients in receipt of male vs. female donors is on par with the additional risk of allograft failure that patients experience when the cold ischemia time is increased from <12 hours to 12–24 hours or from 12–24 hours to 24–36 hours.
Table 3 presents the unadjusted 1-, 5-, and 10-year death-censored allograft survival probabilities for the relevant groups. As expected, the discrepancy due to donor sex at each time point is more pronounced for male recipients than female. In other words, the “male donor advantage” is diminished in female compared with male recipients.
Table 3.
Estimated Survival Probabilities (Without Allograft Failure) at 1, 5, and 10 Years
| Estimated Death-censored Allograft Survival
| ||||
|---|---|---|---|---|
| Recipient Sex | Donor Sex | Ŝ(1) | Ŝ(5) | Ŝ(10) |
| Recipients of Deceased Donors
| ||||
| Male | Male | 0.91 | 0.77 | 0.61 |
| Female | 0.89 | 0.72 | 0.55 | |
| Female | Male | 0.90 | 0.75 | 0.58 |
| Female | 0.89 | 0.72 | 0.54 | |
|
| ||||
| Recipients of Living Donors
| ||||
| Male | Male | 0.96 | 0.87 | 0.73 |
| Female | 0.95 | 0.84 | 0.68 | |
| Female | Male | 0.96 | 0.86 | 0.71 |
| Female | 0.95 | 0.84 | 0.68 | |
Analysis on acute rejection events was conducted on a subset of the analytic cohort due to highly missing data in the outcome, but yielded no significant results as all confidence intervals included the null values. We observed a similar trend where the point estimates are the right direction as the other outcomes.
Discussion
Little is known about the immunological response to sex-mismatched organ transplants. We have recently demonstrated a strong association between acute rejection episodes and development of antibodies against H-Y antigens in female recipients of male kidneys.4 To further test the hypothesis that immunity to H-Y antigens might be associated with allograft failure, we considered donor-recipient sex mismatch as a proxy for H-Y immunity and more generally, for mHA mismatch. In the present report, more than 188,000 subjects were included in our primary analysis, where we tested whether there was an interaction between donor-recipient sex after adjusting for body size and other factors known to affect long-term allograft function. The direction of the estimated hazard ratios for both female and male recipients indicated a benefit from receipt of male donor kidneys but the benefit is less for women (8% benefit for males vs. 2% benefit for females). The magnitude of the effect modification observed was not materially altered with adjustment for body size and other determinants of allograft failure.
While the association of donor sex with transplant allograft failure has been previously investigated, published results have been conflicting. One of the first attempts to address whether donor-recipient sex mismatch affected allograft function was by Ellison et al. (1994).5 These authors performed an analysis of 3-year allograft survival of the four donor-recipient sex combinations in HLA-identical living donor transplants. No differences were observed among the groups, and the authors concluded that there was no evidence for an H-Y mHA effect in zero-mismatched living donor transplants. While the zero-mismatched living donor transplant group was, on some level, the ideal cohort for investigating the clinical significance of non-HLA or mHAs, a major challenge was the low event rate in this group (i.e., low rate of allograft failure). The half-life of zero-mismatched living donor transplant exceeds 20 years16; thus, few events would accrue in three years, reducing the power to detect differences. Three subsequent studies also examined the role of donor and recipient sex. Zeier et al. (2002) made use of the European Collaborative Transplant Study (CTS) registry data to perform a retrospective cohort study in 124,911 transplant recipients of both deceased and living donors and concluded that having a female donor was deleterious for male recipients, but not for female recipients. In 2008, Gratwohl et al. used the same registry to evaluate the allograft survival and death-censored allograft survival in 195,516 female and male recipients from deceased male and female donor kidneys at 1 and 10 years.17 Their analysis showed that when compared with all other combinations of donor and recipient sex, transplantation of male donor kidneys into female recipients was associated with an increased risk of allograft failure during the first year (HR 1.08, 95% CI 1.03 to 1.14) and between 2 and 10 years (HR 1.06, 95% CI 1.01 to 1.10) and concluded that H-Y mHA adversely affects human kidney transplantation. More recently, Kim and Gill performed an analysis of deceased donors using the USRDS database18 They showed that female recipients of male donor kidneys had a 12% increased risk for allograft failure relative to all other groups (female recipients with female donors and all male recipients) at 1 year, but no excess risk at 10 years. In our view, there were several important limitations of the latter report. In particular, they focused their findings on non-death censored allograft failure. As such, male recipients of male (or female) kidneys fared less well than female recipients, owing to the higher mortality rate of men vs. women of this age group. Given the purported mechanism underlying potential adverse effects of a male donor to female recipient mismatch, consideration of death censored allograft failure would have been more advisable. A more important concern with all the previous studies, however, is the way the statistical models were used to address the hypothesis. More specifically, none of the previous studies formally compared the effect of donor gender between male and female recipients. Instead, Gratwohl et al. (2008) and Kim and Gill (2009), compared female recipients of male donors to the three other donor- recipient- sex combinations (i.e., female recipients of female donors and male recipients of both female and male donors.) This comparison, however, does not address whether there is an association between donor-recipient sex mismatch and allograft failure nor would it be suggestive of a possible H-Y effect. The inference relies on an assumption that the other 3 subgroups have comparable allograft survival. If this assumption were to hold, one may be able to correctly conclude that if female recipients of male donors have different allograft survival; this is due to a possible H-Y effect. To illustrate, suppose that female recipients, regardless of donor sex, experience accelerated allograft failure relative to male recipients and further that there is no donor sex effect for either male or female recipients. Then a comparison of female recipients of male donors to the other three groups collectively, could reveal differences in allograft survival and one could falsely conclude that this difference may be due to an H-Y effect. If, however, one were to compare the difference in allograft survival between male and female donors among female recipients to that difference among male recipients, one could assess whether the increased risk, if any, were due to donor sex and if this difference were the same for both male and female recipients, making it less likely to falsely implicate H-Y in this example. Thus, in order to tease out a possible H-Y effect, one must compare the female recipient experience to that of the male recipient experience. That is, the male experience serves as a crucial reference in drawing any conclusions. Ellison et al. (1994) and Zeier et al. (2002) do this empirically. Specifically, Zeier et al. (2002) compare the increase in risk of having a female donor among female recipients to that among male recipients (HRs of 1.15 versus 1.22, respectively). Without a formal test statistic that accounts for the variability of these point estimates, however, it is not possible to conclude, as the authors did, that male recipients had an increased risk. Nor is it possible to conclude as Ellison et al. (1994) did that there is no difference.
On average, recipients of male donor kidneys enjoy better allograft outcome6 While many have postulated as to the reasons for disparate results among male and female donors and among male and female recipients in kidney transplantation, the true mechanisms underlying these findings are unknown. Men tend to have larger kidneys11; kidney weight directly correlated with body size as described by BSA.19 Glomerular filtration rates are higher in men; some have ascribed this to an increased metabolic need when considering supply-demand imbalance in the context of organ transplantation. As such, if the typical immunologic response to organ transplantation were similar in women and men, one would expect men to experience accelerated allograft failure, yet they do not. Thus, the male-female donor-recipient differences we and others have observed reflect effects other than differences in body size. A role for mHA, such as H-Y, would thus appear to be plausible.
Although we suspect H-Y alloimmunity may account for the sex difference results observed, the major obstacle in this type of investigation is in delineating markers that can correctly identify immunity to HY. Thus, further longitudinal studies with anti-H-Y will be needed. We believe that the major findings of this study (i.e., a diminished benefit of male donor kidneys among female recipients), when taken together with our previous report of a correlation between acute rejection episodes and the development of anti-H-Y antibody, continue to build a supportive case for the role of H-Y as a clinically significant mHA in kidney transplantation.
While modest, the magnitude of the donor-recipient sex effects are comparable to other issues about which transplant programs spend substantial time, effort and expense, such as sensitization, HLA mismatch and cold ischemia time. In considering policy implications of these findings, caution is warranted. A naïve approach to the finding of differential benefit afforded to men by receipt of male donor kidneys would be to generally direct female donor kidneys to women and male donor kidneys to men. Such a strategy might enhance overall population-wide allograft survival, but it would place women at a considerable disadvantage; women of larger body size would likely be affected most adversely. More importantly, our approach and findings provide a paradigm for the clinical identification of effects of minor histocompatibility loci.
This study has several strengths. We used a large, comprehensive registry of transplant recipients in the US. We included information from both deceased and living donor types in our analysis. Although we did not find that the associations of interest were modified by donor type, we stratified results by transplant type (deceased and living donor) allowing the baseline hazards for deceased and living donors to vary. Most importantly, we appropriately focused on the interaction of donor and recipient sex in considering allograft failure. We focused on death censored allograft failure, which should be more specific to the immunological response than non-death censored allograft failure. We adjusted for a wide array of observed covariates, and found that results were not materially altered by adjustment for body size and other determinants of allograft failure. Finally, through use of multiple imputation techniques, we were able to utilize the entire analytic cohort of 188,507 when adjusting for all confounders in a statistically valid manner, while a complete-case analysis only used 35% of the cohort when adjusting for confounders, resulting in a substantial loss in efficiency as well as potentially biased estimates.
This study also has several important limitations. While BSA has been widely accepted as the best proxy for metabolic demand and a reasonable proxy for nephron number, it is only a proxy. Other proxies of nephron number that may be less likely to misclassify the exposure include kidney weight,19 kidney length, or cortical volume20 We recently demonstrated that cortical volume measured by magnetic resonance imaging (MRI) relates directly to glomerular number calculated from a method of histomorphometry validated in our laboratory.20 Ideally, a population-wide study of mHA would include measurement of antibody, so that the association between antibody presence as well as titer with allograft failure could be defined and thus provide a much stronger rationale for strategies either to screen for those mHA or institute therapies to reduce mHA.
In summary, we showed a significant interaction between donor and recipient sex and allograft failure, such that women recipients experience measurably less of the “male donor benefit” than do men. These results are above and beyond those explained by differences in donor and recipient body size as well as other donor- and recipient-specific determinants of allograft failure. These findings, combined with our previous finding of an association between de novo H-Y antibodies and acute rejection in females kidney transplant recipients, argues for a clinically significant role for immunity against sexually determined mHA, such in kidney transplantation. In the face of an ever worsening organ shortage, we are confronted with exploring all possible ways of monitoring for clinically relevant immunologic events that may result in decreased long-term allograft survival. Discovering other mHA that may affect allograft function could lead to new strategies to extend the functional life of deceased and living donor organs.
Table 1(b).
Study population characteristics by recipient-donor sex: Recipients of living donors
| Male Recipient | Female Recipient | |||
|---|---|---|---|---|
| Male Donor (%) | Female Donor (%) | Male Donor (%) | Female Donor (%) | |
| N=63,139 | 15,182 (24.0) | 21,827 (34.6) | 11,385 (18.0) | 14,745 (23.4) |
|
| ||||
| Recipient Age (yrs) | ||||
| 18 to 34.9 | 4752 (31.3) | 5697 (26.1) | 3444 (30.3) | 4594 (31.2) |
| 35 to 49.9 | 5199 (34.2) | 7754 (35.5) | 4096 (36) | 4978 (33.8) |
| 50 to 64.9 | 4173 (27.5) | 6743 (30.9) | 3247 (28.5) | 4298 (29.1) |
| >65 | 1058 (7) | 1633 (7.5) | 598 (5.3) | 875 (5.9) |
| Recipient Race | ||||
| White | 11988 (79) | 17618 (80.7) | 8673 (76.2) | 11213 (76) |
| Black | 2219 (14.6) | 2972 (13.6) | 1932 (17) | 2598 (17.6) |
| Other | 975 (6.4) | 1237 (5.7) | 780 (6.9) | 934 (6.3) |
| Donor Age (yrs) | ||||
| <18 | 13 (0.1) | 9 (0) | 8 (0.1) | 6 (0) |
| 18 to 34.9 | 6458 (42.5) | 6232 (28.6) | 4478 (39.3) | 4934 (33.5) |
| 35 to 49.9 | 6211 (40.9) | 10567 (48.4) | 4704 (41.3) | 6959 (47.2) |
| 50 to 64.9 | 2370 (15.6) | 4767 (21.8) | 2038 (17.9) | 2708 (18.4) |
| >65 | 128 (0.8) | 250 (1.1) | 155 (1.4) | 137 (0.9) |
| Missing | 2 (0) | 2 (0) | 2 (0) | 1 (0) |
| Donor Race | ||||
| White | 10931 (72) | 16265 (74.5) | 7975 (70) | 10409 (70.6) |
| Black | 1861 (12.3) | 2408 (11) | 1723 (15.1) | 2232 (15.1) |
| Other/Missing | 2390 (15.7) | 3154 (14.4) | 1687 (14.8) | 2104 (14.3) |
| Presumed Cause of ESRD | ||||
| Diabetes | 3363 (22.2) | 5486 (25.1) | 2588 (22.7) | 3392 (23) |
| Hypertension | 2438 (16.1) | 3290 (15.1) | 1458 (12.8) | 1946 (13.2) |
| Glomerulonephritis | 3831 (25.2) | 5055 (23.2) | 2277 (20) | 3036 (20.6) |
| Cystic Disease | 1399 (9.2) | 2394 (11) | 1207 (10.6) | 1570 (10.6) |
| Other | 3133 (20.6) | 4242 (19.4) | 3044 (26.7) | 3833 (26) |
| Missing | 1018 (6.7) | 1360 (6.2) | 811 (7.1) | 968 (6.6) |
| Peak panel reactive antibody (%) | ||||
| <10 | 9992 (65.8) | 14479 (66.3) | 6777 (59.5) | 8595 (58.3) |
| 10 to 49 | 1149 (7.6) | 1559 (7.1) | 1372 (12.1) | 1798 (12.2) |
| >50 | 249 (1.6) | 342 (1.6) | 783 (6.9) | 1148 (7.8) |
| Missing | 3792 (25) | 5447 (25) | 2453 (21.5) | 3204 (21.7) |
| Time on Dialysis (mos) | ||||
| 0 | 3223 (21.2) | 4804 (22) | 2831 (24.9) | 3442 (23.3) |
| <6 | 3213 (21.2) | 4406 (20.2) | 2117 (18.6) | 2760 (18.7) |
| >6 to 12 | 3049 (20.1) | 4264 (19.5) | 2079 (18.3) | 2773 (18.8) |
| >12 to 24 | 3057 (20.1) | 4582 (21) | 2240 (19.7) | 2949 (20) |
| >24 to 36 | 1306 (8.6) | 1781 (8.2) | 969 (8.5) | 1202 (8.2) |
| >36 to 48 | 572 (3.8) | 868 (4) | 440 (3.9) | 626 (4.2) |
| >48 | 762 (5) | 1122 (5.1) | 709 (6.2) | 993 (6.7) |
| No. of HLA mismatches | ||||
| 0 to 1 | 2417 (15.9) | 2613 (12) | 1553 (13.6) | 2316 (15.7) |
| 2 to 4 | 1124 (7.4) | 1367 (6.3) | 851 (7.5) | 1181 (8) |
| 5 to 6 | 9286 (61.2) | 12939 (59.3) | 6893 (60.5) | 9188 (62.3) |
| Unknown/missing | 2355 (15.5) | 4908 (22.5) | 2088 (18.3) | 2060 (14) |
| Transplant Era | ||||
| 1988 to 1994 | 3434 (22.6) | 3965 (18.2) | 2281 (20) | 3113 (21.1) |
| 1995 to 1999 | 3749 (24.7) | 5576 (25.5) | 2976 (26.1) | 3791 (25.7) |
| 2000 to 2006 | 7999 (52.7) | 12286 (56.3) | 6128 (53.8) | 7841 (53.2) |
| Cold Ischemia Time (hrs) | ||||
| <12 | 10438 (68.8) | 14817 (67.9) | 7837 (68.8) | 10090 (68.4) |
| 12 to <24 | 61 (0.4) | 103 (0.5) | 50 (0.4) | 70 (0.5) |
| 24 to <36 | 82 (0.5) | 108 (0.5) | 46 (0.4) | 68 (0.5) |
| >36 | 71 (0.5) | 122 (0.6) | 51 (0.4) | 54 (0.4) |
| Unknown/missing | 4530 (29.8) | 6677 (30.6) | 3401 (29.9) | 4463 (30.3) |
| Recipient Body surface area, m2 | ||||
| <1.6 | 2468 (16.3) | 3174 (14.5) | 4061 (35.7) | 5266 (35.7) |
| 1.6 to <1.8 | 471 (3.1) | 554 (2.5) | 3322 (29.2) | 4244 (28.8) |
| 1.8 to <2.0 | 4954 (32.6) | 7154 (32.8) | 2346 (20.6) | 3086 (20.9) |
| 2.0 to <2.2 | 4085 (26.9) | 6217 (28.5) | 801 (7) | 1076 (7.3) |
| 2.2 and >2.2 | 2261 (14.9) | 3476 (15.9) | 190 (1.7) | 239 (1.6) |
| Missing | 943 (6.2) | 1252 (5.7) | 665 (5.8) | 834 (5.7) |
| Donor Body surface area, m2 | ||||
| <1.6 | 656 (4.3) | 4914 (22.5) | 537 (4.7) | 3108 (21.1) |
| 1.6 to <1.8 | 63 (0.4) | 1762 (8.1) | 52 (0.5) | 1173 (8) |
| 1.8 to <2.0 | 2395 (15.8) | 3417 (15.7) | 1864 (16.4) | 2135 (14.5) |
| 2.0 to <2.2 | 2777 (18.3) | 999 (4.6) | 2080 (18.3) | 697 (4.7) |
| 2.2 and >2.2 | 1355 (8.9) | 168 (0.8) | 1048 (9.2) | 116 (0.8) |
| Missing | 7936 (52.3) | 10567 (48.4) | 5804 (51) | 7516 (51) |
Acknowledgments
Funding was provided to Dr. Tan by the Katherine McCormick Faculty Development Award at Stanford University. Drs. Chertow and Desai were supported by K24 DK085446. We thank Dr. David Miklos for his intellectual support in this project and for reviewing our manuscript. In addition, we thank Dr. Mark Cullen for insightful discussions that contributed to this work.
Footnotes
Disclosure
The Authors do not have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003 Jun;3(6):665–673. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 2.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005 Apr-May;365(9470):1570–1576. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 3.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005 Apr 1;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan JC, Wadia PP, Coram M, et al. H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation. 2008 Jul 15;86(1):75–81. doi: 10.1097/TP.0b013e31817352b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison MD, Norman DJ, Breen TJ, Edwards EB, Davies DB, Daily OP. No effect of HY minor histocompatibility antigen in zero-mismatched living-donor renal transplants. Transplantation. 1994 Aug 27;58(4):518–520. doi: 10.1097/00007890-199408270-00020. [DOI] [PubMed] [Google Scholar]
- 6.Zeier M, Dohler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002 Oct;13(10):2570–2576. doi: 10.1097/01.asn.0000030078.74889.69. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, Umen AJ. The influence of age, sex, race, and body habitus on kidney weight in humans. Arch Pathol Lab Med. 1986 Jan;110(1):55–60. [PubMed] [Google Scholar]
- 8.Giral M, Nguyen JM, Karam G, et al. Impact of graft mass on the clinical outcome of kidney transplants. J Am Soc Nephrol. 2005 Jan;16(1):261–268. doi: 10.1681/ASN.2004030209. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Moon JI, Kim DK, Kim SI, Park K. Ratio of donor kidney weight to recipient bodyweight as an index of graft function. Lancet. 2001 Apr 14;357(9263):1180–1181. doi: 10.1016/S0140-6736(00)04377-4. [DOI] [PubMed] [Google Scholar]
- 10.Saxena AB, Busque S, Arjane P, Myers BD, Tan JC. Preoperative renal volumes as a predictor of graft function in living donor transplantation. Am J Kidney Dis. 2004 Nov;44(5):877–885. [PubMed] [Google Scholar]
- 11.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003 Feb;83:S31–37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 12.Chertow GM, Milford EL, Mackenzie HS, Brenner BM. Antigen-independent determinants of cadaveric kidney transplant failure. JAMA. 1996 Dec 4;276(21):1732–1736. [PubMed] [Google Scholar]
- 13.Klein J, Moeschberger M. Survival analysis: techniques for censored and truncated data. 2. New York: Elsevier; 1997. [Google Scholar]
- 14.The MI Procedure. SAS Institute Inc; 1999. [Google Scholar]
- 15.Verhave JC, Gansevoort RT, Hillege HL, De Zeeuw D, Curhan GC, De Jong PE. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004 May;15(5):1316–1322. [PubMed] [Google Scholar]
- 16.UNOS. UNOS 2009 Annual Data Report. UNOS; 2009. [Google Scholar]
- 17.Gratwohl A, Dohler B, Stern M, Opelz G. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet. 2008 Jul 5;372(9632):49–53. doi: 10.1016/S0140-6736(08)60992-7. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Gill JS. H-Y incompatibility predicts short-term outcomes for kidney transplant recipients. J Am Soc Nephrol. 2009 Sep;20(9):2025–2033. doi: 10.1681/ASN.2008101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992 Feb;232(2):194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 20.Tan JC, Busque S, Workeneh B, et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010 Oct;78(7):686–692. doi: 10.1038/ki.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]