Abstract
Purpose
Cerebral hyperperfusion following carotid endarterectomy (CEA) occurs in patients with preoperative impairments in cerebral hemodynamics. The aim of the present study was to determine whether late images/early images on preoperative brain 123I-iomazenil (IMZ) single-photon emission computed tomography (SPECT), which correlate with oxygen extraction fraction images on positron emission tomography, could identify patients at risk for cerebral hyperperfusion following endarterectomy for unilateral cervical internal carotid artery (ICA) stenosis.
Methods
In 80 patients, preoperative brain SPECT scans were initiated immediately after (early images) and 180 min after (late images) administration of 123I-IMZ. A region of interest (ROI) was automatically placed in the middle cerebral artery territory in both the cerebral hemispheres using a three-dimensional stereotaxic ROI template. Transcranial regional cerebral oxygen saturation (rSO2) was monitored using near-infrared spectroscope throughout carotid endarterectomy, and a patient was defined as having cerebral hyperperfusion when a ratio of rSO2 at the end of the surgery to rSO2 before ICA clamping was ≥ 1.1.
Results
Cerebral hyperperfusion was observed on intraoperative rSO2 monitoring in eight patients (10%). Preoperative increase in affected side-to-contralateral side asymmetry on late/early 123I-IMZ value was the only significant independent predictor of cerebral hyperperfusion (95% confidence interval [CI], 1.606 to 8.710; P = 0.0010). The preoperative late/early 123I-IMZ asymmetry corresponded to an 88% sensitivity and 89% specificity, with 47% positive- and 98% negative-predictive values for the development of cerebral hyperperfusion.
Conclusions
Preoperative late/early 123I-IMZ images can identify patients at risk for cerebral hyperperfusion following endarterectomy for unilateral cervical ICA stenosis.
Keywords: 123I-iomazenil, brain SPECT, hyperperfusion, carotid endarterectomy
Introduction
Cerebral hyperperfusion following carotid endarterectomy (CEA) is defined as a major increase in ipsilateral cerebral blood flow (CBF) that is well above the metabolic demands of the brain tissue [1,2]. Cerebral hyperperfusion syndrome following CEA is a complication of cerebral hyperperfusion that is characterized by unilateral headache, face and eye pain, seizure, and focal symptoms that occur secondary to cerebral edema or intracerebral hemorrhage [1-4]. Although the incidence of intracerebral hemorrhage is relatively low, the prognosis for patients with this condition is poor [1,5-9]. In addition, recent studies have demonstrated that post-CEA hyperperfusion, even when asymptomatic, causes postoperative cortical neural damage that results in postoperative cognitive impairment [10-12].
Risk factors for cerebral hyperperfusion include long-standing hypertension, high-grade stenosis, poor collateral blood flow, and contralateral carotid occlusion, which often result in impairments in cerebral hemodynamics [13]. Further, a rapid restoration of normal perfusion pressure following CEA may result in hyperperfusion in regions of the brain in which autoregulation is impaired due to chronic ischemia. This hypothesis is similar to the "normal perfusion pressure breakthrough" theory described by Spetzler et al. [14] and is consistent with observations by several investigators that decreased cerebrovascular reactivity to acetazolamide on brain perfusion single-photon emission computed tomography (SPECT) is a significant predictor of post-CEA hyperperfusion [15-17]. However, acetazolamide is associated with frequent and various adverse effects, including headache, nausea, dizziness, tinnitus, numbness of the extremities, motor weakness of the extremities, general malaise, and Stevens-Johnson syndrome [18-20]. In fact, one study demonstrated that 63% of patients who underwent SPECT study with acetazolamide challenge developed such adverse effects between 1 and 3 hours after administration of acetazolamide, and these symptoms lasted for 0.5 to 72 hours and frequently impacted patients’ activities of daily living, including the ability to engage in their jobs [20]. Measurement of cerebrovascular reactivity to acetazolamide using brain perfusion SPECT also requires two instances of administration of a tracer (without and with acetazolamide challenge). Thus, it would be beneficial to develop a simpler SPECT method of predicting post-CEA hyperperfusion that does not require administration of acetazolamide.
123I-iomazenil (IMZ) is a central benzodiazepine receptor ligand (Figure 1) and binds to the same central binding sites as does flumazenil [21-24]. Images obtained at 180 min after administration of 123I-IMZ using brain SPECT (late images) are proportional to the distribution of central benzodiazepine receptor binding potential (CBRBP) [21-24]. Further, CBRBP on late 123I-IMZ SPECT images correlates with neural density in the cerebral cortex, and a reduction in cortical CBRBP indicates cortical neural damage or loss [22-24]. In the cerebral cortex with reduced CBRBP, cerebral metabolism probably decreases in proportion to the degree of neuronal damage. Indeed, late 123I-IMZ SPECT images reportedly correlate with cerebral metabolic rate of oxygen (CMRO2) images on positron emission tomography in the cerebral cortex in patients with carotid artery occlusive diseases [25,26]. On the other hand, several investigators have used assumed values for 123I-IMZ kinetics in the brain in a three-compartment, two-parameter model to calculate the transition rate constant (k1) from the blood to the brain [27]. As a result, the k1 value correlated highly with CBF, suggesting that SPECT images obtained early after administration of 123I-IMZ are influenced by the distribution of CBF [27]. Because oxygen extraction fraction (OEF), which is one of key parameters of cerebral hemodynamics [28,29], is a function of CMRO2/CBF, late images/early images on 123I-IMZ SPECT may reflect OEF. A recent study has demonstrated that early and late/early images on 123I-IMZ SPECT correlate with CBF images and OEF images on PET, respectively, in the cerebral cortex of patients with chronic unilateral major cerebral artery occlusive disease [30].
Figure 1.
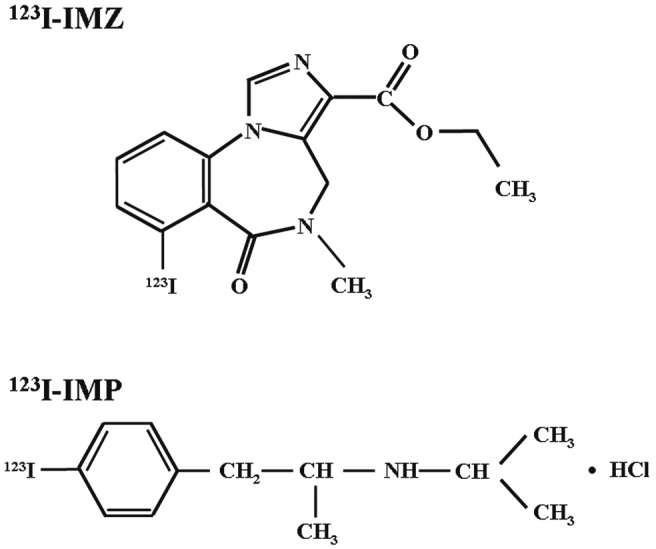
Chemical structures of 123I-iomazenil (IMZ) (upper) and N-isopropyl-p-[123I]-iodoamphetamine (IMP) (lower).
Thus, the purpose of the present study was to determine whether late images/early images on preoperative brain 123I-IMZ SPECT could identify patients at risk for cerebral hyperperfusion following endarterectomy for unilateral cervical internal carotid artery (ICA) stenosis.
Materials and methods
Patients
A total of 80 patients (75 men and 5 women), age 46 to 81 years (mean, 69 years), with unilateral ICA stenosis (³70%) and useful residual function (modified Rankin disability scale 0, 1 or 2), without large cortical infarction on conventional magnetic resonance imaging who underwent CEA between October 2009 and September 2011 were enrolled in the present study. Concomitant disease states and symptoms were recorded, including 72 patients with hypertension, 33 patients with diabetes mellitus and 40 patients with hyperlipidemia. While 59 patients showed ischemic symptoms in the ipsilateral carotid territory, 21 patients exhibited asymptomatic ICA stenosis.
All patients underwent preoperative angiography with arterial catheterization. The overall average degree of ICA stenosis was 87.7 ± 8.4%, with a range of 70-99%. No patient had occlusion or stenosis of greater than 50% in the contralateral ICA or middle cerebral artery.
This protocol was reviewed and approved by the institutional ethics committee, and written informed consent was obtained from all patients or their next of kin.
Brain SPECT study
Brain SPECT studies were performed using a ring-type SPECT scanner (Headtome-SET080, Shimadzu Corp., Kyoto, Japan), which provided 31 tomographic images simultaneously. The spatial resolution of the scanner with a low-energy, all-purpose collimator was 13 mm full width at half maximum (FWHM) at the center of the field of view, and the slice thickness was 25 mm FWHM at the field of view center. Image slices were taken at 5 mm center-to-center spacing, parallel to the orbitomeatal line. The images were reconstructed using the weighted-filtered backprojection technique, in which the attenuation correction was made by detecting the edge of the object. An attenuation coefficient of 0.065 cm-1, a Butterworth filter (cutoff = 0.45 cycle/cm; order = 3), and a ramp filter were used for image reconstruction.
Brain 123I-IMZ SPECT was performed 3 days before surgery. An intravenous injection of approximately 167 MBq 123I-IMZ was administered following a 1-min infusion of physiologic saline at a rate of 20 mL/min. Immediately after administration of the 123I-IMZ, scans were initiated with a scanning duration of 28 min (early images), and 180 min later, scans were also initiated with a scanning duration of 23 min (late images) [30].
Brain perfusion SPECT using N-isopropyl-p-[123I]-iodoamphetamine (IMP) was also performed immediately and three days after surgery only for patients who were defined as having cerebral hyperperfusion on intraoperative transcranial regional cerebral oxygen saturation monitoring as described below. 123I-IMP nonspecifically binds sites for amines according to the distribution of brain perfusion (Figure 1) [32]. The 123I-IMP SPECT study was performed as described previously [32]. Briefly, after a 1-min intravenous infusion of 222 MBq of 123I-IMP (5-mL volume) at a constant rate of 5 mL/min and a 1-min infusion of physiologic saline at the same rate, data acquisition was performed at a mid-scan time of 30 min, for scan duration of 20 min.
All SPECT images were transformed into the standard brain size and shape by linear and nonlinear transformation using SPM99 for anatomic standardization [32]. Thus, brain images from all subjects had the same anatomic format. In 123I-IMZ SPECT images, the ratio of radioactive counts of late images to those of early images was calculated for each pixel and was defined as late/early 123I-IMZ. Three hundred and eighteen constant regions of interest (ROIs) were automatically placed in both cerebral hemispheres using a three-dimensional stereotaxic ROI template (3DSRT) [33]. The ROIs were grouped into nine segments (callosomarginal, pericallosal, precentral, central, parietal, angular, temporal, posterior, and hippocampus) in each hemisphere according to the arterial supply. Five (precentral, central, parietal, angular, and temporal) of these nine segments were combined and defined as a ROI perfused by the middle cerebral artery (MCA) (Figure 2). Mean radioactive count or value of all pixels in the MCA ROI in each hemisphere was calculated on preoperative early 123I-IMZ, preoperative late/early 123I-IMZ images and postoperative 123I-IMP images (immediately and three days after surgery). Then, the ratio of the radioactive count or value in the affected hemisphere to that in the contralateral hemisphere (affected-to-contralateral side asymmetry ratio: ATC-AR) was calculated in each image. Furthermore, the following value, which was defined as CBF increase ratio, was calculated only in patients who were defined as having cerebral hyperperfusion on intraoperative transcranial regional cerebral oxygen saturation monitoring by Equation 1.
Figure 2.
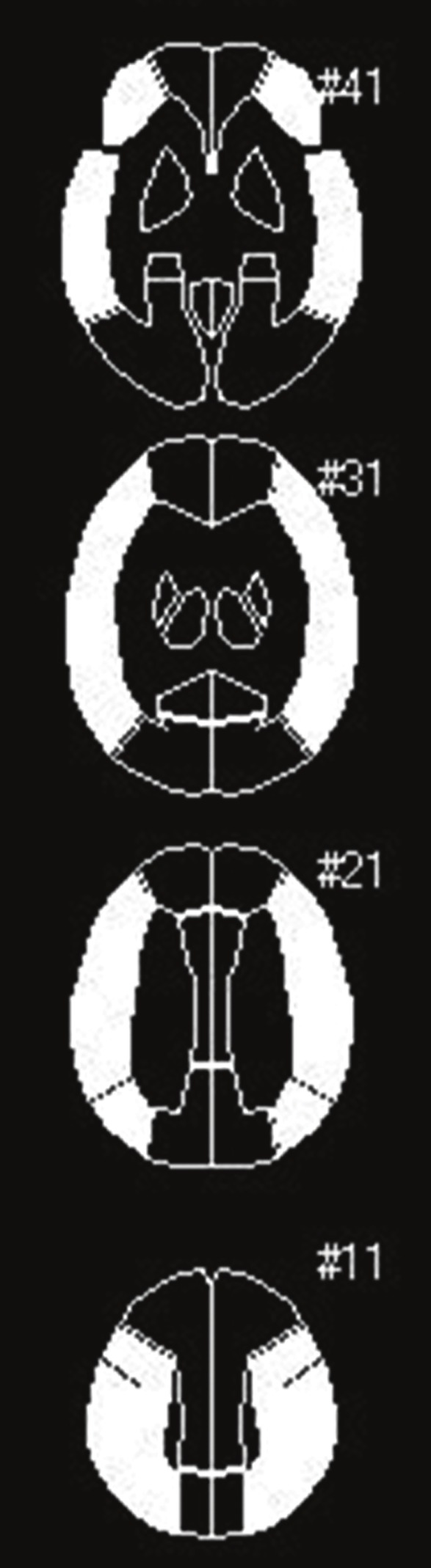
Diagrams showing the regions of interest (ROIs) of a three-dimensional stereotaxic ROI template. The white ROIs (precentral, central, parietal, angular, and temporal segments) indicate territories perfused by the bilateral middle cerebral arteries.
 |
Ten healthy subjects (eight men and two women; age range, 35 to 52 years; mean age, 39 years) underwent 123I-IMZ SPECT study using the same method. The control value of ATC-AR on late/early 123I-IMZ images was 0.999 ± 0.048 when the left cerebral hemisphere was defined as the affected side [30].
Measurement of transcranial regional cerebral oxygen saturation
The near-infrared spectroscope, TOS 96 (Tostec, Tokyo, Japan), with a dual-channel system was used to measure regional cerebral oxygen saturation (rSO2) throughout surgery [34-36]. After induction of general anesthesia, the patient’s skin was thoroughly cleaned, and the sensors were bilaterally and symmetrically placed over the forehead, according to the manufacturer’s instructions. After a stable rSO2 reading was achieved, the margins of the sensors were secured with opaque tape. The rSO2 readings were recorded at 30-second intervals and stored for later analysis. The following data points regarding cerebral oximetry for the side ipsilateral to CEA were gathered and recorded: the mean value in the last 1 minute before ICA clamping (rSO2-0) and the mean value in the last 1 minute at the end of the surgery (rSO2-1). For each patient, the ratio of rSO2-1 to rSO2-0 (rSO2-1/rSO2-0) was calculated, and a patient was defined as having cerebral hyperperfusion when the value was ≥ 1.1, according to previous findings [36].
Intraoperative management
All patients received antiplatelet therapy until the morning of the day on which CEA was performed. Further, all patients underwent surgery under general anesthesia. Blood pressure was kept stable in a range ±20% of the preoperative level throughout the procedure by adjusting the depth of anesthesia or, if needed, by intravenous administration of a vasodilator (nitroglycerin) or a vasoconstrictor (the adrenalin). An intraluminal shunt was not used in these procedures. The mean duration of ICA clamping was 36 minutes, ranging from 28 to 48 minutes.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The relationship between each variable and the development of cerebral hyperperfusion defined by intraoperative rSO2 monitoring was evaluated with univariate analysis using the Mann-Whitney U test or c2 test. A multivariate statistical analysis of factors related to development of cerebral hyperperfusion was also performed using a logistic regression model. Variables with P < 0.2 in the univariate analyses were selected for analysis in the final model. Differences were deemed statistically significant if P < 0.05. The accuracy of ATC-AR in preoperative late/early 123I-IMZ images in predicting the development of cerebral hyperperfusion was assessed using receiver operating characteristic (ROC) curves when the relationship between the two parameters was statically significant. The ROC curve was calculated in increments of 0.5 SD (0.024) from the mean value (0.999) of ATC-AR in late/early 123I-IMZ images obtained in normal subjects.
Results
All patients recovered from surgery without new major neurological deficits. The interval between ICA declamping and rSO2-1 determination ranged from 24 to 51 minutes. The interval between rSO2-1 determination and postoperative CBF measurement by brain perfusion SPECT with 123I-IMP ranged from 38 to 72 minutes.
Of the 80 patients studied, eight (10%) met the intraoperative rSO2 criteria for cerebral hyperperfusion in the ipsilateral hemisphere and underwent postoperative CBF measurement by brain perfusion SPECT with 123I-IMP. Figure 3 shows ATC-ARs on preoperative early 123I-IMZ and postoperative 123I-IMP images in these eight patients with cerebral hyperperfusion. While ATC-ARs on preoperative early 123I-IMZ images were 0.772 ± 0.099, the values on 123I-IMP images immediately after surgery were increased (1.490 ± 0.172). As a result, the CBF increase ratio immediately after surgery was more than 1.80 in all eight patients (1.801 to 2.132; mean, 1.934). For these eight patients, intensive control of arterial blood pressure between 100 and 140 mmHg was instituted using intravenous administration of antihypertensive drugs immediately after 123I-IMP SPECT. In six of these eight patients, the ATC-ARs on 123I-IMP images on the third postoperative day were decreased, resulting in CBF increase ratio less than 1.8, and pharmacologic control of blood pressure was discontinued. The postoperative courses were uneventful in these six patients. In the remaining two patients, the ATC-ARs on 123I-IMP images on the third postoperative day were increased or unchanged, resulting in CBF increase ratio more than 1.8; one developed confusion on the fifth postoperative day (Figures 4 and 5), and another patient experienced aphasia and right motor weakness with onset on the seventh postoperative day. Propofol coma was induced in both patients. Following termination of the propofol coma, both patients had no identifiable lesion on magnetic resonance imaging and eventually experienced full recovery.
Figure 3.
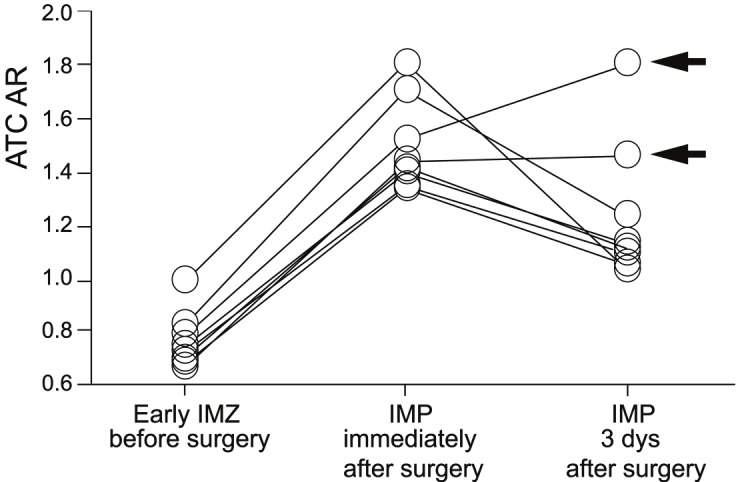
Affected-to-contralateral side asymmetry ratio (ATC-AR) on preoperative early 123I-iomazenil (IMZ) and postoperative N-isopropyl-p-[123I]-iodoamphetamine (IMP) images in patients who were defined as having cerebral hyperperfusion on intraoperative transcranial regional cerebral oxygen saturation monitoring. Arrows indicate patients with cerebral hyperperfusion syndrome after surgery.
Figure 4.
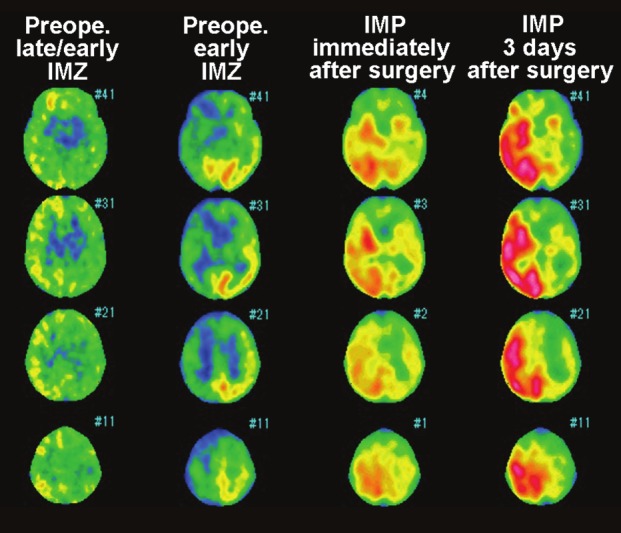
Brain single-photon emission computedtomography images in a 74-year-old man with symptomaticright internal carotid artery (ICA) stenosis(95%) exhibiting hyperperfusion syndrome 5 daysafter carotid endarterectomy (CEA). Preoperativelate/early 123I-IMZ images (first column from the left)show elevation of the values in the right cerebralcortex when compared with those in the left cerebralcortex. Preoperative early 123I-IMZ images (secondcolumn from the left) show reduction of radioactivecounts in the right cerebral cortex when comparedwith those in the left cerebral cortex. 123I-IMP imagesobtained immediately after surgery (second columnfrom the right) show a considerable increase in radioactivecounts in the right cerebral cortex when comparedwith those in the left cerebral cortex. 123I-IMPimages on the third postoperative day (first columnfrom the right) show further increase in radioactivecounts in the right cerebral cortex compared with 123I-IMPimages immediately after surgery.
Figure 5.
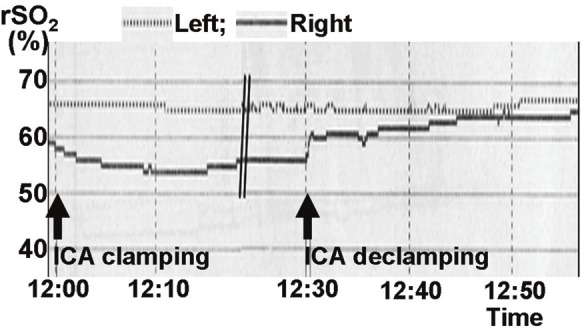
Continuous monitoring of regional cerebral oxygen saturation (rSO2) during CEA in the patient of Figure 3 revealing a drop in the right rSO2 immediately after right ICA clamping. Subsequent to ICA declamping, the rSO2 kept increasing to levels above preclamp rSO2.
Of 80 patients studied, 72 patients who did not meet the intraoperative rSO2 criteria for cerebral hyperperfusion in the ipsilateral hemisphere had uneventful postoperative courses.
Results of univariate analysis of factors related to the development of cerebral hyperperfusion defined by intraoperative rSO2 monitoring are summarized in Table 1. The ATC-AR in preoperative late/early 123I-IMZ images was significantly higher in patients with cerebral hyperperfusion than in those without. Other variables were not significantly associated with the development of cerebral hyperperfusion. After eliminating closely related variables in the univariate analyses, the following confounders (P < 0.2) were adopted in the logistic regression model for the multivariate analysis: degree of ICA stenosis and ATC-AR in preoperative late/early 123I-IMZ images. Multivariate analysis revealed that high ATC-AR in preoperative late/early 123I-IMZ images was significantly associated with the development of cerebral hyperperfusion (95% confidence interval [CI], 1.606 to 8.710; P = 0.0010).
Table 1.
Risk factors for the development of cerebral hyperperfusion defined by intraoperative regional cerebral oxygen saturation monitoring
| Risk factors | Postoperative hyperperfusion | p Value | |
|---|---|---|---|
| Yes (n = 8) | No (n = 72) | ||
| Age (y) (mean ± SD) | 69.6±8.0 | 68.8±6.7 | 0.6297 |
| Male gender | 8 (100%) | 67 (93%) | >0.9999 |
| Hypertension | 8 (100%) | 64 (89%) | >0.9999 |
| Diabetes mellitus | 3 (38%) | 30 (42%) | >0.9999 |
| Hyperlipidemia | 6 (75%) | 34 (47%) | 0.2633 |
| Symptomatic lesions | 7 (88%) | 52 (72%) | 0.6736 |
| Degree of ICA stenosis (%)(mean ± SD) | 90.6±8.6 | 87.4±8.4 | 0.1158 |
| Duration of ICA clamping (min) (mean ± SD) | 36.3±5.2 | 36.0±5.3 | 0.8850 |
| ATC-AR in preoperative late/early IMZ images | 1.112±0.061 | 1.007±0.054 | 0.0001 |
SD, standard deviation; ICA, internal carotid artery; ATC-AR, affected-to-contralateral side asymmetry ratio; IMZ, iomazenil.
Sensitivity and specificity for the ATC-AR in preoperative late/early 123I-IMZ images in the cut-off point lying closest to the left upper corner of the ROC curve in predicting development of cerebral hyperperfusion were 88% (7/8) and 89% (64/72) (cut-off point = 1.071: the mean +1.5SD of the control value obtained from normal subjects), respectively (Figure 6). In the cut-off point, positive- and negative-predictive values were 47% (7/15) and 98% (64/65), respectively.
Figure 6.
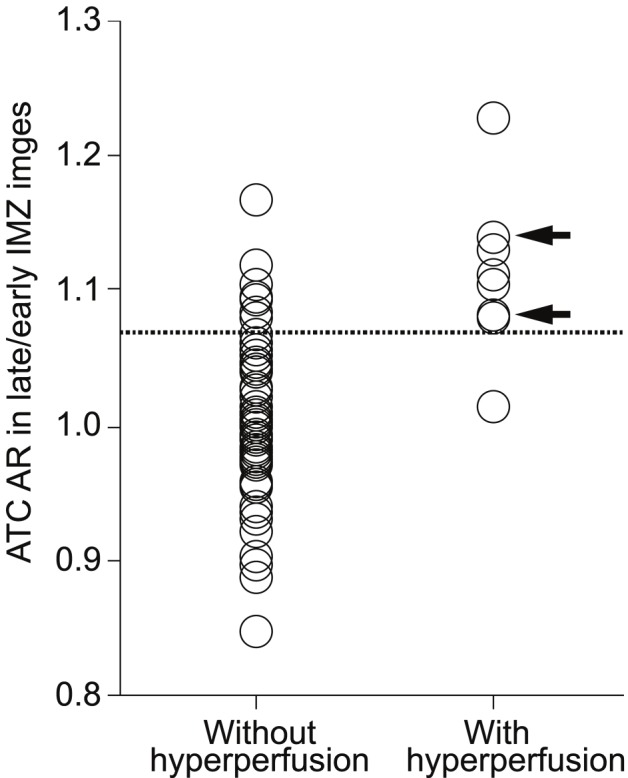
Relationship between ATC-AR in preoperative late/early IMZ images and postoperative hyperperfusion. Arrows indicate patients with cerebral hyperperfusion syndrome after surgery. Dashed horizontal line denotes mean + 1.5 standard deviation (1.071) of ATC-AR obtained in healthy volunteers.
Discussion
Measurement of rSO2 via near-infrared spectroscopy used in the present study has the advantage of being continuous, noninvasive, portable, and technically simple to perform. Theoretically, the rSO2 reflects a balance between cerebral oxygen supply and oxygen consumption. Thus, if cerebral oxygen consumption and arterial oxygen saturation remain unchanged, an increase in rSO2 must reflect an increase in CBF. One study demonstrated that intraoperative monitoring of the rSO2 is a reliable modality to identify patients with hyperperfusion after CEA; in that study, the sensitivity and specificity of the rSO2-1/rSO2-0 for detecting post-CEA hyperperfusion were 100% when the cut-off point of the value was 1.1 [36]. Thus, the same definition was applied in the present study.
A recent study has demonstrated a strong correlation between affected-to-contralateral side asymmetry in the MCA ROI on early 123I-IMZ SPECT images and that on CBF images of positron emission tomography, indicating that early 123I-IMZ SPECT images are proportional to the distribution of CBF in the MCA cortical territory [30]. 123I-IMP SPECT imaging also can display post-CEA hyperperfusion as an increase in radioactive counts in the hemisphere ipsilateral to surgery when compared with those in the contralateral hemisphere [15-17]. Thus, in patients who were defined as having cerebral hyperperfusion on intraoperative rSO2 monitoring, the present study compared preoperative early 123I-IMZ and postoperative 123I-IMP SPECT images to confirm development of cerebral hyperperfusion after CEA; radioactive counts in the cerebral hemisphere ipsilateral to surgery were considerably lower than those in the contralateral hemisphere on preoperative early 123I-IMZ SPECT images, and the affected-to-contralateral side asymmetry of radioactive counts was reversed on 123I-IMP SPECT images immediately after surgery. As a result, the CBF increase ratios immediately after surgery were more than 1.80. The CBF increase ratio implies a ratio of postoperative CBF to preoperative CBF in the cerebral hemisphere ipsilateral to surgery if CBF in the contralateral cerebral hemisphere is unchanged before and after surgery. Considering that early 123I-IMZ SPECT images show a propensity to underestimate brain perfusion at regions with higher CBF, resulting in underestimation of the affected-to-contralateral side asymmetry [30], the cerebral hemisphere ipsilateral to surgery in patients with the CBF increase ratios > 1.80 may have a postoperative CBF increase of ³ 100% compared with preoperative values. This is consistent with previous findings that only patients with this degree of CBF increase can develop cerebral hyperperfusion syndrome [15-17].
Investigators have proposed various mechanisms for the development of post-CEA hyperperfusion [3]. In cases of severe ICA stenosis and deficient collateral circulation, hemispheric perfusion pressure is severely reduced distal to the ICA stenosis. This may result in reduction of perfusion pressure below the compensatory capacity of autoregulatory mechanisms, thus leading to maximal dilation of resistance vessels and chronic hypoperfusion or “misery perfusion”. After restoration of normal perfusion pressure following CEA, chronically impaired autoregulatory mechanisms may require several days to adjust to the new steady state, resulting in hyperperfusion in the interim. The present study demonstrated that high ATC-AR in preoperative late/early 123I-IMZ images was a significant independent predictor of post-CEA hyperperfusion. Because high ATC-AR in preoperative late/early 123I-IMZ images suggests the presence of “misery perfusion” in the affected hemisphere [30] and resistance vessels maximally dilate due to autoregulatory mechanisms in the misery perfusion, the present findings support the theory that cerebral hyperperfusion results from loss of normal vasoconstriction secondary to chronic cerebral ischemia and maladaptive autoregulatory mechanisms.
The ROC analysis in the present study showed that ATC-AR in preoperative late/early 123I-IMZ images provided low positive- and high negative-predictive values for the prediction of post-CEA hyperperfusion. Both values were approximately equal to previous data obtained using cerebrovascular reactivity to acetazolamide measured by SPECT [15,16]. Thus, when compared with measurement of cerebrovascular reactivity to acetazolamide using brain perfusion SPECT, preoperative late/early 123I-IMZ images may be a useful and alternative SPECT method of predicting post-CEA hyperperfusion as they only require one instance of intravenous injection of a radioactive tracer and because they do not require administration of acetazolamide with its associated frequent and various adverse effects.
As described previously, the use of the present SPECT method has certain limitations. For example, 123I-IMZ is relatively unavailable in Western countries and is not suitable for application in patients with large cortical infarction [30]. In addition, the present study included only patients with unilateral ICA stenosis and used affected side-to-contralateral side asymmetry on SPECT images to detect hemodynamic impairment in the affected cerebral hemisphere. Impairments in cerebral hemodynamics are more severe in patients with bilateral ICA steno-occlusive disease than in those with unilateral ICA stenosis [37], and impairments in bilateral cerebral hemodynamics in patients with bilateral major cerebral arterial occlusive disease may not be detected by the present SPECT method using affected side-to-contralateral side asymmetry.
Conclusion
The present study demonstrated that preoperative late/early 123I-IMZ images can identify patients at risk for cerebral hyperperfusion after endarterectomy for unilateral cervical ICA stenosis. When compared with measurement of cerebrovascular reactivity to acetazolamide using brain perfusion SPECT, these images may be a useful and alternative SPECT method of predicting post-CEA hyperperfusion as they only require one instance of intravenous injection of a radioactive tracer and because they do not require administration of acetazolamide with its associated frequent and various adverse effects.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Piepgras DG, Morgan MK, Sundt TM Jr, Yanagihara T, Mussman LM. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg. 1988;68:532–536. doi: 10.3171/jns.1988.68.4.0532. [DOI] [PubMed] [Google Scholar]
- 2.Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy, with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981;56:533–543. [PubMed] [Google Scholar]
- 3.Bernstein M, Fleming JF, Deck JH. Cerebral hyperperfusion after carotid endarterectomy: a cause of cerebral hemorrhage. Neurosurgery. 1984;15:50–56. doi: 10.1227/00006123-198407000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Solomon RA, Loftus CM, Quest DO, Correll JW. Incidence and etiology of intracerebral hemorrhage following carotid endarterectomy. J Neurosurg. 1986;64:29–34. doi: 10.3171/jns.1986.64.1.0029. [DOI] [PubMed] [Google Scholar]
- 5.Dalman JE, Beenakkers IC, Moll FL, Leusink JA, Ackerstaff RG. Transcranial Doppler monitoring during carotid endarterectomy helps to identify patients at risk of postoperative hyperperfusion. Eur J Vasc Endovasc Surg. 1999;18:222–227. doi: 10.1053/ejvs.1999.0846. [DOI] [PubMed] [Google Scholar]
- 6.Jansen C, Sprengers AM, Moll FL, Vermeulen FE, Hamerlijnck RP, van Gijn J, Ackerstaff RG. Prediction of intracerebral haemorrhage after carotid endarterectomy by clinical criteria and intraoperative transcranial Doppler monitoring: results of 233 operations. Eur J Vasc Surg. 1994;8:220–225. doi: 10.1016/s0950-821x(05)80464-5. [DOI] [PubMed] [Google Scholar]
- 7.Ouriel K, Shortell CK, Illig KA, Greenberg RK, Green RM. Intracerebral hemorrhage after carotid endarterectomy: incidence, contribution to neurologic morbidity, and predictive factors. J Vasc Surg. 1999;29:82–89. doi: 10.1016/s0741-5214(99)70362-9. [DOI] [PubMed] [Google Scholar]
- 8.Pomposelli FB, Lamparello PJ, Riles TS, Craighead CC, Giangola G, Imparato AM. Intracranial hemorrhage after carotid endarterectomy. J Vasc Surg. 1988;7:248–255. [PubMed] [Google Scholar]
- 9.Riles TS, Imparato AM, Jacobowitz GR, Lamparello PJ, Giangola G, Adelman MA, Landis R. The cause of perioperative stroke after carotid endarterectomy. J Vasc Surg. 1994;19:206–216. doi: 10.1016/s0741-5214(94)70096-6. [DOI] [PubMed] [Google Scholar]
- 10.Chida K, Ogasawara K, Suga Y, Saito H, Kobayashi M, Yoshida K, Otawara Y, Ogawa A. Postoperative cortical neural loss associated with cerebral hyperperfusion and cognitive impairment following carotid endarterectomy: 123I-iomazenil SPECT study. Stroke. 2009;40:448–453. doi: 10.1161/STROKEAHA.108.515775. [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara K, Kobayashi M, Suga Y, Chida K, Saito H, Komoribayashi N, Otawara Y, Ogawa A. Significance of postoperative crossed cerebellar hypoperfusion in patients with cerebral hyperperfusion following carotid endarterectomy: SPECT study. Eur J Nucl Med Mol Imaging. 2008;35:146–152. doi: 10.1007/s00259-007-0588-x. [DOI] [PubMed] [Google Scholar]
- 12.Ogasawara K, Yamadate K, Kobayashi M, Endo H, Fukuda T, Yoshida K, Terasaki K, Inoue T, Ogawa A. Postoperative cerebral hyperperfusion associated with impaired cognitive function in patients undergoing carotid endarterectomy. J Neurosurg. 2005;102:38–44. doi: 10.3171/jns.2005.102.1.0038. [DOI] [PubMed] [Google Scholar]
- 13.Reigel MM, Hollier LH, Sundt TM Jr, Piepgras DG, Sharbrough FW, Cherry KJ. Cerebral hyperperfusion syndrome: a cause of neurologic dysfunction after carotid endarterectomy. J Vasc Surg. 1987;5:628–634. [PubMed] [Google Scholar]
- 14.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 15.Hosoda K, Kawaguchi T, Shibata Y, Kamei M, Kidoguchi K, Koyama J, Fujita S, Tamaki N. Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke. 2001;32:1567–1573. doi: 10.1161/01.str.32.7.1567. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, Inoue T, Ogawa A. Prediction and Monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg. 2003;99:504–510. doi: 10.3171/jns.2003.99.3.0504. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimoto T, Houkin K, Kuroda S, Abe H, Kashiwaba T. Low cerebral blood flow and perfusion reserve induce hyperperfusion after surgical revascularization: case reports and analysis of cerebral hemodynamics. Surg Neurol. 1997;48:132–139. doi: 10.1016/s0090-3019(97)90106-3. [DOI] [PubMed] [Google Scholar]
- 18.Derick RJ. Carbonic anhydrase inhibitors. In: Mauger TF, Craig EL, editors. Hevener's Ocular Pharmacology. St Louis: CV Mosby; 1994. pp. 567–571. [Google Scholar]
- 19.Ogasawara K, Tomitsuka N, Kobayashi M, Komoribayashi N, Fukuda T, Saitoh H, Inoue T, Ogawa A. Stevens-Johnson syndrome associated with intravenous acetazolamide administration for evaluation of cerebrovascular reactivity. Case report. Neurol Med Chir (Tokyo) 2006;46:161–163. doi: 10.2176/nmc.46.161. [DOI] [PubMed] [Google Scholar]
- 20.Saito H, Ogasawara K, Suzuki T, Kuroda H, Kobayashi M, Yoshida K, Kubo Y, Ogawa A. Adverse effects of intravenous acetazolamide administration for evaluation of cerebrovascular reactivity using brain perfusion single-photon emission computed tomography in patients with major cerebral artery steno-occlusive diseases. Neurol Med Chir (Tokyo) 2011;51:479–483. doi: 10.2176/nmc.51.479. [DOI] [PubMed] [Google Scholar]
- 21.Millet P, Graf C, Moulin M, Ibanez V. SPECT quantification of benzodiazepine receptor concentration using a dual-ligand approach. J Nucl Med. 2006;47:783–792. [PubMed] [Google Scholar]
- 22.Nakagawara J, Sperling B, Lassen NA. Incomplete brain infarction of reperfused cortex may be quantitated with iomazenil. Stroke. 1997;28:124–132. doi: 10.1161/01.str.28.1.124. [DOI] [PubMed] [Google Scholar]
- 23.Hatazawa J, Satoh T, Shimosegawa E, Okudera T, Inugami A, Ogawa T, Fujita H, Noguchi K, Kanno I, Miura S. Evaluation of cerebral infarction with iodine 123-iomazenil SPECT. J Nucl Med. 1995;36:2154–2161. [PubMed] [Google Scholar]
- 24.Hatazawa J, Shimosegawa E, Satoh T, Kanno I, Uemura K. Central benzodiazepine receptor distribution after subcortical hemorrhage evaluated by means of [123I] iomazenil and SPECT. Stroke. 1995;26:2267–2271. doi: 10.1161/01.str.26.12.2267. [DOI] [PubMed] [Google Scholar]
- 25.Dong Y, Fukuyama H, Nabatame H, Yamauchi H, Shibasaki H, Yonekura Y. Assessment of benzodiazepine receptors using iodine-123-labeled iomazenil single-photon emission computed tomography in patients with ischemic cerebrovascular disease. A comparison with PET study. Stroke. 1997;28:1776–1782. doi: 10.1161/01.str.28.9.1776. [DOI] [PubMed] [Google Scholar]
- 26.Chida K, Ogasawara K, Kuroda H, Aso K, Kobayashi M, Fujiwara S, Yoshida K, Terasaki K, Ogawa A. Central benzodiazepine receptor binding potential and cerebral blood flow images on SPECT correlate with oxygen extraction fraction images on PET in cerebral cortex with unilateral major cerebral artery occlusive disease. J Nucl Med. 2011;52:511–518. doi: 10.2967/jnumed.110.084186. [DOI] [PubMed] [Google Scholar]
- 27.Yanagimoto S, Ono S, Sone T, Morita K, Nagai K, Otsuka N, Mimura H, Tomomitsu T, Fukunaga M, Muranaka A, Itaya M, Kitayama A. Compartment analysis of 123I-iomazenil brain on early and delayed SPECT. Kaku Igaku. 1997;34:371–377. [PubMed] [Google Scholar]
- 28.Gibbs JM, Wise RJ, Leenders KL, Jones T. Evaluation of cerebral perfusion reserve in patients with carotid-artery occlusion. Lancet. 1984;1:310–314. doi: 10.1016/s0140-6736(84)90361-1. [DOI] [PubMed] [Google Scholar]
- 29.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki T, Ogasawara K, Kuroda H, Chida K, Aso K, Kobayashi M, Fujiwara S, Yoshida K, Terasaki K, Ogawa A. Comparison of early and late images on 123I-iomazenil SPECT with cerebral blood flow and oxygen extraction fraction images on PET in the cerebral cortex of patients with chronic unilateral major cerebral artery occlusive disease. Nucl Med Comm. doi: 10.1097/MNM.0b013e32834de94e. In press. [DOI] [PubMed] [Google Scholar]
- 31.Ogasawara K, Ito H, Sasoh M, Okuguchi T, Kobayashi M, Yukawa H, Terasaki K, Ogawa A. Quantitative measurement of regional cerebrovascular reactivity to acetazolamide us ing 123I-N-isopropyl -p-iodoamphetamine autoradiography with SPECT: validation study using H2 15O with PET. J Nucl Med. 2003;44:520–525. [PubMed] [Google Scholar]
- 32.Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi R, Matsuda H, Yoshioka K, Yonekura Y. Cerebral blood flow SPET in transient global amnesia with automated ROI analysis by 3DSRT. Eur J Nucl Med Mol Imaging. 2004;31:578–589. doi: 10.1007/s00259-003-1406-8. [DOI] [PubMed] [Google Scholar]
- 34.Litscher G, Hadolt I, Eger E. Transcranial optical spectroscopy: A comparison of the TOS 96 and INVOS 3100 cerebral oximeters. Biomed Tech (Berl) 1998;43:133–136. doi: 10.1515/bmte.1998.43.5.133. [DOI] [PubMed] [Google Scholar]
- 35.Nara I, Shiogai T, Hara M, Saito I. Comparative effects of hypothermia, barbiturate, and osmotherapy for cerebral oxygen metabolism, intracranial pressure, and cerebral perfusion pressure in patients with severe head injury. Acta Neurochir. 1998;71:22–26. doi: 10.1007/978-3-7091-6475-4_7. [DOI] [PubMed] [Google Scholar]
- 36.Ogasawara K, Konno H, Yukawa H, Endo H, Inoue T, Ogawa A. Transcranial regional cerebral oxygen saturation monitoring during carotid endarterectomy as a predictor of postoperative hyperperfusion. Neurosurgery. 2003;53:309–314. doi: 10.1227/01.neu.0000073547.86747.f3. [DOI] [PubMed] [Google Scholar]
- 37.Reinhard M, Muller T, Roth M, Guschlbauer B, Timmer J, Hetzel A. Bilateral severe carotid artery stenosis or occlusion - cerebral autoregulation dynamics and collateral flow patterns. Acta Neurochir (Wien) 2003;145:1053–1060. doi: 10.1007/s00701-003-0137-8. [DOI] [PubMed] [Google Scholar]


