Abstract
Modification-specific antibodies are important tools to examine the dynamics and functions of posttranslational protein modifications in cells. Here, we describe in detail the generation of polyclonal antibodies specific for mono-, di- and trimethylated lysine 51 within the HIV transactivator Tat. Lysine 51 is a highly conserved residue located in the RNA-binding region of Tat and the target of lysine methyltransferases KMT1E (SETDB1) and KMT7 (Set7/9). Using affinity-purified methyl-specific antibodies of Tat, we find that cellular Tat is predominantly monomethylated at lysine 51, a modification enhanced by coexpression of KMT7.
1. INTRODUCTION
The HIV transactivator Tat is an essential viral protein. Without Tat, HIV transcriptional elongation is inefficient and results in the generation of abortive viral transcripts that cannot sustain viral replication (1, 2). In addition, reverse transcription steps in the viral life cycle are dependent on Tat (3). Tat has pleiotropic effects on the host cell’s survival and activation status (4-7), interacts closely with the host cell’s transcription and RNAi machinery (8-10) and can act as a secreted factor on neighboring cells (11, 12). Tat autoregulates its own production within infected cells; once generated from initial viral transcripts, Tat production is sustained as a result of its own activating effects on elongation as well as initiation steps of HIV transcription (13-17). Tat binds cooperatively with the positive transcription elongation factor b (P-TEFb) to TAR RNA, a conserved stem-loop structure that forms spontaneously at the 5′ ends of nascent viral transcripts (18, 19). Tat also strengthens the interaction of P-TEFb with transcription elongation factor ELL2 and the PAF1 complex to support HIV transcription (20, 21).
Tat is a 101–104 amino acids protein encoded by two exons; transactivation and RNA-binding domains lie in the N-terminal 72 amino acids encoded by the first tat exon (22, 23). The N-terminal 72 amino acids of Tat are multiply modified by posttranslational modifications, most of them clustering in the basic RNA-binding domain also called ARM (amino acids 49–59) (Table 1). This region is reversibly acetylated at lysines 50/51 by KAT3B (p300), human KAT2A (GCN5) and the nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase sirtuin 1 (SIRT1) (24-28). Arginines 52 and 53 are methylated by protein arginine methyltransferase 6 (PRMT6) (29-31). While arginine methylation suppresses Tat transcriptional activity by interfering with the formation of the Tat/TAR/cyclin T1 complex, lysine acetylation activates Tat transactivation by recruiting the histone acetyltransferase PCAF and the chromatin remodeling complex SWI/SNF to elongating HIV transcripts (32-36).
Table 1.
Posttranslational modifications in the ARM region of the HIV-1 Tat protein
| Residue Modified | Modification | Enzyme/s | Effect of Modification | Citation/s |
|---|---|---|---|---|
|
|
|
|
|
|
| K50 | Acetylation | KAT3B (p300) |
|
Kiernan et al, 1999 |
| KAT2A (GCN5) | Ott et al, 1999 | |||
| Deng et al, 2000 | ||||
| Col et al, 2001 | ||||
| Mujtaba et al, 2002 | ||||
| Dorr et al, 2002 | ||||
| Bres et al, 2003 | ||||
| Kaehlcke et al, 2003 | ||||
|
|
|
|
|
|
| Deacetylation | SIRT1 |
|
Pagans et al, 2005 | |
|
|
|
|
|
|
| K50 | Methylation | KMT1E (SETDB1) |
|
Van Duyne et al, 2008 |
| K51 | ||||
|
|
|
|
|
|
| K51 | Monomethylation | KMT7 (Set7/9) |
|
Pagans et al, 2010 |
|
|
|
|
|
|
| R52 | Methylation | PRMT6 |
|
Boulanger et al, 2005 |
| R53 | Xie et al, 2007 | |||
| Silvakumaran et al, 2009 | ||||
|
|
|
|
|
|
Recent evidence uncovered that the Tat ARM is also a target of lysine methyltransferases KMT1E (SETDB1) and KMT7 (Set7/9) (37, 38). In general, lysines can be mono-, di- or trimethylated by distinct KMTs, which transfer methyl groups from S-adenosyl-L-methionine to the ε-amino group of lysines generating S-adenosyl-L-homocysteine as a byproduct (39, 40). Lysine methylation of histone proteins is an important posttranslational modification involved in the regulation of transcription, genome integrity and epigenetic inheritance. Methylation of histone proteins can be associated with transcriptional activation or repression depending on the sites of methylation and the number of methyl groups added (41). For example, H3K9me3 is generally associated with pericentric heterochromatin (transcriptionally silenced), while H3K9me1 and H3K9me2 are predominantly enriched in euchromatic regions (transcriptionally active) (42). Recently, lysine methylation of nonhistone proteins has evolved as a novel mechanism that regulates the function of many proteins, especially transcription factors (43, 44). Notably, KMT7 methylates a growing number of nonhistone targets whereas no significant histone methyltransferase activity was detected on nucleosomes (45, 46).
Interestingly, both, KMT1E and KMT7, methylate K51 in the Tat ARM but exert opposing effects on Tat transcriptional activity (37, 38). While methylation by KMT1E reduces formation of the ternary Tat/TAR/P-TEFb complex and decreases HIV transcription (37), KMT7-mediated methylation increases Tat transcriptional activity and enhances the formation of the Tat/TAR/P-TEFb complex (38). Since KMT1E is known as a di- and trimethyltransferase of H3K9 (47) and KMT7 acts mainly as a monomethyltransferase (48), differential methylation of Tat K51 by the two methyltransferases may explain the different outcomes on HIV transcription.
To examine the differential methylation status of K51 in cells, we generated modification-specific antibodies against monomethylated (K51me1), dimethylated (K51me2) and trimethylated (K51me3) Tat. Affinity-purified antibodies were tested in dot blot analysis for specificity on synthetic Tat ARM peptides carrying different numbers of methyl groups at position 51. We detected monomethylated Tat as the predominant methyl-Tat species in cells using immunoprecipitation/western blot analysis. The pool of monomethylated Tat increased when KMT7 was coexpressed.
2. METHOD DESCRIPTION
2.1. Production of polyconal antibodies against K51me1-, K51me2- and K51me3-Tat
Chemically synthesized ARM peptides K51-methylated (K51me1, K51me2, K51me3), were conjugated with keyhole limpet hemocyanin through the sulfhydryl (-SH) group from the C-terminal cysteine residue added to each peptide and injected into rabbits in a 118-day rabbit peptide protocol. The IgG fractions were isolated on affinity columns loaded with the respective antigens (Figure 1).
Figure 1. Scheme of experimental procedure.
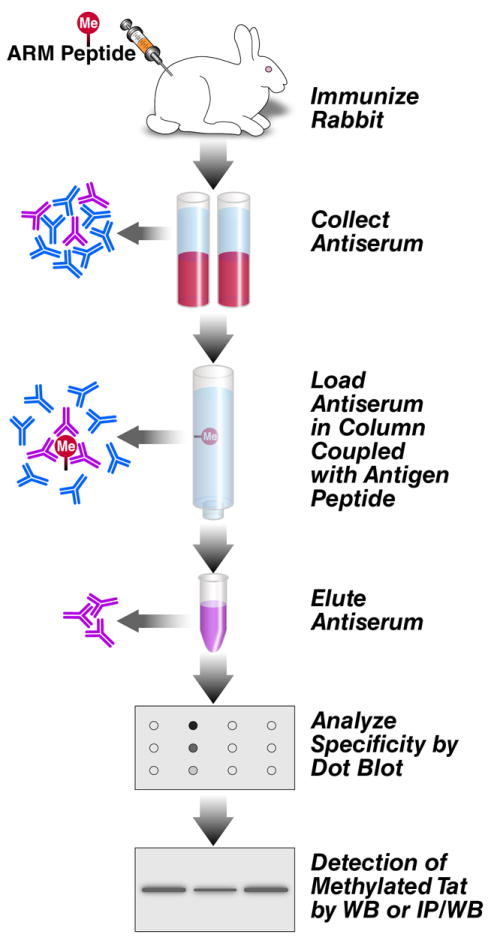
To generate polyclonal antibodies against K51me1, K51me2 and K51me3 in Tat, immunogenic peptides were injected into rabbits, antisera were collected, affinity-purified, validated by dot blot analysis, and examined in cell lysates. See details in the text.
2.1.1. Design and synthesis of the peptides
Designing the peptides for immunization requires attention to the length and the sequence of the peptide. For the generation of anti-methyl Tat antibodies, we used 10-mer peptides corresponding to the ARM region of Tat (aa 45-57) and also included a cysteine residue at the C terminus of the peptides to enable conjugation to the immunogenic carrier protein keyhole limpet hemocyanin (KLH) (Figure 2). Attention should be given to whether other cysteines exist in the in peptide sequence. The synthesis of the peptides was performed on an Applied Biosystems 433A peptide synthesizer by standard Fmoc-Strategy at the Peptide Specialty Laboratories (Heidelberg, Germany). For biotinylated peptides, biotinylation was carried out at the last step of the synthesis. Tat peptides were fully deprotected with deprotecting solution (95% trifluoracetic acid, 4% triethylsilane and 1% H2O2) and purified to homogeneity by reverse-phase high-pressure liquid chromatography (Shimadzu Scientific Instruments Inc., Columbia, MD). The correct molecular masses for the peptides were established by positive-ion ESI mass-spectra recorded on an ion trap Finnigan LCQ mass spectrometer (Bremen, Germany).
Figure 2. ARM peptides used for antibody generation.
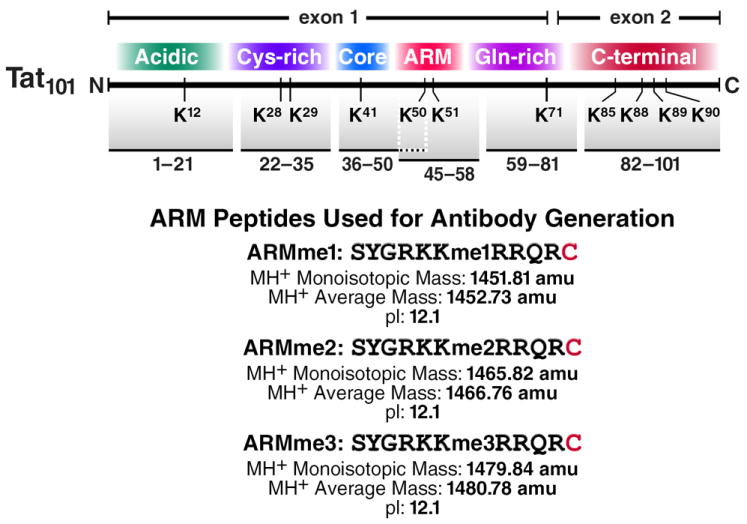
Tat domains and sequences of the ARM peptides used for the generation of antibodies against K51me1, K51me2 and K51me3-Tat.
2.1.2. Conjugation of the peptides
For the effective conjugation of mariculture KLH (mcKLH) to sulfhydryl-containing peptides, KLH is activated by maleimide, which forms covalent crosslinks with sulfhydryl moieties on cysteine residues of peptides.
Reconstitute 2 mg maleimide-activated mcKLH (Pierce) with 200 μl H2O to make a 10 mg/ml solution.
Dissolve 2 mg of peptide in 200 μl conjugation buffer (0.1 M sodium phosphate, 0.15 M NaCl pH 7.2).
Mix maleimide with peptide 1:1 (v/v) and incubate for 2 h at room temperature. Concentration of KLH-peptide is theoretically 4 mg/ml.
Dialyze the KLH-peptide with PBS overnight at 4°C.
Ship the KLH-peptide on dry ice to a company that provides services for the production of antibodies (Covance Research Products, Denver, PA).
2.1.3. Rabbit immunizations
The 118-day rabbit peptide protocol from Covance is as follows:
Day 0: Obtain ~5 ml of pre-immune serum from each rabbit. 500 μg of KLH-peptide are emulsified in Freud’s Complete Adjuvant (FCA) and injected subcutaneously into the rabbits.
Day 21: Rabbits are boosted with 500 μg of KLH-peptide emulsified in Freud’s Incomplete Adjuvant (FIA).
Day 42: Rabbits are boosted with 250 μg of KLH-peptide with FIA.
Day 52: Test bleed (5 ml serum) is withdrawn from the rabbits.
Days 63, 84, 105: Further boosts with 250 μg of KLH-peptide with FIA.
Days 73, 94, and 115: Production bleeds (20 ml serum) are withdrawn.
Day 118: Terminal bleed (55-65 ml serum).
Serum is aliquoted in 15 ml conical tubes and stored at -80°C for long-term. An aliquot of 500 μl is kept at 4°C.
2.1.4. Notes
It is recommended to use at least two rabbits for each immunogen.
2.2. Affinity purification of the methyl-specific antibodies
For affinity purification of the antisera, immunogenic peptides were coupled to the Affi-Gel Activated Affinity Media (Biorad). The Affi-Gel Affinity Media are N-hydroxysuccinimide esters of a derivatized crosslinked agarose gel bead support that couples with high efficiency to ligands that have a primary amino group (i.e., peptides) both in aqueous or nonaqueous conditions. There are two different types of Affi-Gel Affinity Media: Affi-Gel 10, which contains a 10-atom neutral spacer arm; and Affi-Gel 15, which contains a 15-atom positively charged spacer arm. When coupling at neutral pH (6.5–7.5), Affi-Gel 10 is recommended for proteins with a pI over 6.5 (basic proteins), while Affi-Gel 15 is recommended for proteins with a pI below 6.5 (acidic proteins). Because the Tat ARM peptides are rich in arginines and lysines and highly basic (pI 12.1), we used Affi-Gel 10.
2.2.1. Solutions
Solution 1: 1M NaCl, 10 mM Tris HCl pH 7.4
Solution 2: 100 mM Ethanolamine, pH 7.4
Solution 3: 1XPBS
Solution 4: 10 mM Tris HCl pH 7.4
Solution 5: Acidic elution solution: 100 mM glycine (pH 2.5), 10% ethylene glycol
Solution 6: 10 mM Tris HCl pH 8.
Solution 7: Basic elution solution: 100 mM triethylamine (pH 11.5), 10% ethylene glycol
Solution 8. 500 mM NaCl, 10 mM Tris pH 7.4
Solution 9: 1 M Tris pH 8.0
2.2.2. Column preparation
1) Dissolve 4 mg of antigen peptide in 2 ml DMSO.
2) Add 1 ml Affi-Gel 10 to empty column.
3) Add 5 ml isopropanol and let drain to wash the column. Repeat this step once.
4) Transfer the moist gel to a 15 ml conical tube and add ligand solution from step 1.
5) Incubate on wheel for 3 h at room temperature (coupling peptide).
6) Centrifuge at 2500 rpm, 5 min, and remove supernatant.
7) Add 10 ml of solution 1. Spin at 2500 rpm, 5 min, and remove supernatant.
8) Add 10 ml solution 2, and incubate on wheel overnight at 4°C.
9) Spin at 2500 rpm, 5 min, and remove supernatant.
10) Wash with 10 ml solution 3 and spin at 2500 rpm, 5 min.
10) Add 10 ml solution 3. Mix, transfer to a column and let drain.
11) Add 10 ml solution 4 and let drain.
12) Add 10 ml of the solution 5, and let drain.
13) Add 10 ml of solution 6 and let drain. Check the pH of last drops, and continue until pH is 8.0.
14) Add 10 ml of solution 7 and let drain.
15) Wash with solution 4 (10 mM Tris pH 7.4) until the pH is 7.4.
2.2.3. Antibody purification
Heat inactivate 4 ml of antiserum 30 min at 56°C. Cool, and spin at 2000 rpm, 5 min.
Add 36 ml solution 4 to the supernatant.
Load onto column, let drain. Add flow-through to column again. Repeat 4 times for a total of 5 flow-throughs.
Wash with 20 ml solution 4, then with 20 ml solution 8.
Elute with 10 ml solution 5 (acidic elution), and collect eluate in 1 ml solution 9.
Wash column with solution 6 until pH 8.0.
Elute with 10 ml solution 7 (basic elution) and collect eluate in 1 ml solution 9.
Wash column with solution 4 until pH 7.4.
Inject acidic or basic eluate into Slide-A-Lyzer Dialysis Cassettes 3-12 ml, γ-irradiated (MWCO 10,000, Pierce) and dialyze against PBS (800 ml) in cold room (2 h, change the PBS and then leave overnight).
Remove from cassette and concentrate antibodies in Vivaspin 6 ml concentrator (Vivascience AG) by centrifugation at 3000 rpm in tabletop centrifuge at 4°C until 0.5-1 ml.
Measure protein content, and add sodium azide to 0.1%.
If protein concentration is less than 1 mg/ml, add BSA to a final concentration of 1%. Aliquot the antibodies, and store at −20°C.
2.2.4. Notes
Acidic and basic elution solutions should be made fresh.
If the peptide is already dissolved in H2O, peptide will be mixed with conjugation buffer to a final concentration of 0.1 M MOPS, pH 7.4 before adding the Affi-Gel solution.
For basic peptides (ARM peptides), most of the antibodies are eluted in the acidic elution step. However, both acidic and basic elutions should be tested.
If the analysis in 2.3 reveals unwanted crossreactivity with unmodified or differentially modified peptides, one or multiple depletion steps can be included where the antiserum is first run over a column coated with the ‘unwanted’ peptide followed by affinity purification of the flow-through on a column packed with the ‘wanted’ peptide.
2.3. Analysis of the specificity of the antibodies by dot blot
Using dot blot experiments, we demonstrated that the affinity-purified antibodies displayed specificity for the immunogens; very little signal was detected for the other differentially methylated peptides (Figure 3). We generally use biotinylated peptides for the dot blot analysis and document with streptavidin-horseradish peroxidase (SA-HRP) proper attachment of the peptides to the membrane.
Figure 3. Dot blot analysis of ARM peptides.
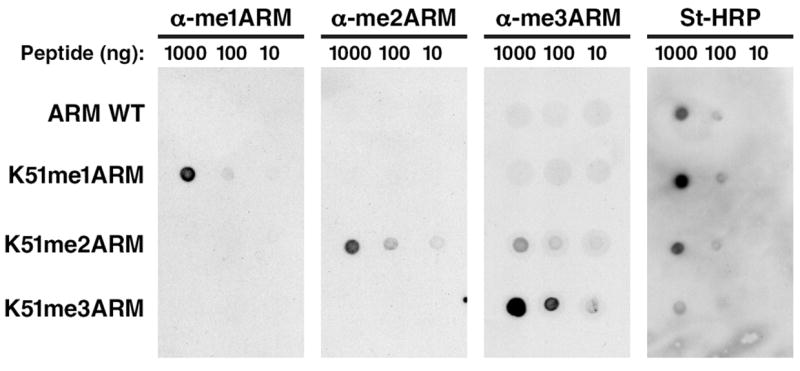
Biotinylated-ARM peptides, either unmodified, mono-, di-, or trimethylated at K51 were spotted and analyzed with α-me1ARM, α-me2ARM, α-me3ARM antibodies or streptavidin-horseradish peroxidase.
2.3.1. Dot blot
Prepare rectangular pieces of 0.2 μM pore size Hybond ECL membranes (RPN 3032D, GE Healthcare).
Make serial dilutions of peptides in H2O (5, 50 and 500 ng/μl).
Accurately spot 2 μl of each dilution peptide on the membrane, allowing a minimum distance of 0.5 cm between dots.
Let the membranes air-dry 30 min-1 h at room temperature.
Block the membrane with blocking solution: 5% non-fat dry milk in TBS-Tween (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% Tween 20) 1 h at room temperature.
Incubate with the appropriate dilution of the antiserum in blocking solution 1 h at room temperature.
Wash the membrane 3-5 times in TBS-Tween.
Incubate with goat anti-rabbit IgG, peroxidase conjugated (#31460, Thermo Scientific) or streptavidin-horseradish peroxidase (#016-030-084, Jackson Immunoresearch Laboratories) in blocking solution 1 h at room temperature.
Wash the membrane 3-5 times in TBS-Tween.
Develop with SuperSignal West Femto Maximum sensitivity substrate (Thermo Scientific) for 10-60 sec and expose to Amersham Hyperfilm (GE Healthcare).
2.3.2.Notes
Different dilutions of the purified antibodies should be tested, usually between 1:500 to 1:5000 (depending on the final concentration of the eluted antibody).
It is recommended to use small volumes of antibody dilutions (1-2 ml) and incubate with the membrane in a sealed plastic bag. Antibodies diluted in blocking solution can also be stored at -20°C and recycled up to 10-15 times.
The SuperSignal West Femto Maximum sensitivity substrate can cause strong background. Background staining can be reduced by washing with H2O for 30 min-1 h before developing.
2.4. Detection of methylated Tat in cells
To examine whether Tat is methylated in cells, we overexpressed a FLAG-tagged Tat protein under the control of the EF-1α promoter (EF-1α-Tat/FLAG) in 293T cells and immunoprecipitate Tat/FLAG from the lysates. Of note, although the antibodies also detect Tat in straight western blotting, we prefer the immunoprecipitation/western blotting technique to enhance sensitivity and to prevent cross reactivity with other methylated proteins such as histones, which run at roughly the same molecular weight as Tat. The immunoprecipitated material was then examined by western blotting with the Tat methylation specific antibodies. A specific signal for K51-me1Tat was detected from the immunoprecipitated Tat protein, but no signal was observed for K51-me2Tat and K51-me3Tat. K51-me1, me2 or me3 Tat signals were not affected after overexpression of KMT1E, but overexpression of KMT7 significantly increased the levels of K51-me1Tat (Figure 4A). We examined the expression of KMT1E/FLAG and KMT7/FLAG by western blotting of cell lysates with FLAG antibody (Figure 4A). Overexpression of KMT1E increased di- and trimethylation of histone H3K9 as expected while overexpression of KMT7 did not affect the methylation status of histone H3K4 in cells consistent with its lack of histone methyltransferase activity on nucleosomes (Figure 4B).
Figure 4. Detection of methylated Tat in cells.
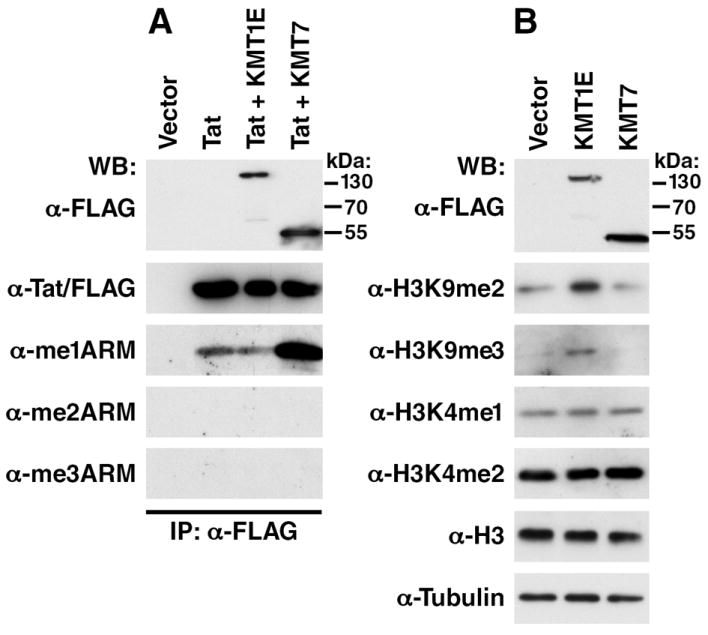
(A) Immunoprecipitation/western blot analysis of Tat/FLAG coexpressed with KMT1E/FLAG or KMT7/FLAG in 293T cells. Cells were transfected with 1 μg of EF-1α-Tat/FLAG expression vector and 6 μg of pCDNA3.1-KMT1E/FLAG, pCDNA3.1-KMT7/FLAG or empty vector. Lysates were immunoprecipitated with α-FLAG M2-agarose, eluted with FLAG peptide and analyzed by western blotting with α-FLAG, α-me1ARM, α-me2ARM or α-me3ARM antibodies.
(B) Western blot analysis of whole cell lysates after transfection with constructs expressing KMT1E/FLAG or KMT7/FLAG in 293T cells. Cells were transfected with 6 μg of pCDNA3.1-KMT1E/FLAG, pCDNA3.1-KMT7/FLAG or empty vector. Whole lysates were analyzed by western blotting with α-FLAG, α-H3K9me2, α-H3K9me3, α-meH3K4me1, α-H3K4me2, α-H3 or α-tubulin antibodies.
2.4.1.Transfection of Tat/FLAG in 293T cells using lipofectamine
Seed 10 ml of 293T cells (1×105 cells/ml) in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1% Penn/strep, 1% L-glutamine (complete DMEM) in a 10 cm dish.
Culture overnight, obtaining 70-80% confluency.
Prepare DNA mix in Eppendorf tubes maintaining the total amount of DNA constant for all transfections (7 μg /transfection). Add OptiMEM (Reduced Serum Media) to the DNA mix to a final volume of 600 μl.
Dilute Lipofectamine (Invitrogen) in OptiMEM (48 μl Lipofectamine + 552 μl OptiMEM / transfection). Vortex, and incubate at room temperature for 10 min.
Add 600 μl of the diluted Lipofectamine to the diluted DNA, and mix by pippeting up and down. Incubate at room temperature for 30 min.
During this time, rinse the cells once with 10 ml OptiMEM (w/o serum or antibiotics) and add 3.8 ml OptiMEM (w/o serum or antibiotics).
After the 30 min incubation period, add the 1.2 ml of DNA-Lipofectamine mixes to the dish. Incubate 4 to 5 h in tissue culture incubator.
Change the media: aspirate transfection mix by 10 ml complete DMEM.
Culture the cells for 48 h.
2.4.2. Immunoprecipitation of Tat/FLAG from lysates
1) Carefully wash the cells in the dish with ice-cold PBS.
2) Add 1 ml of ice-cold RIPA buffer (50 mM TrisHCl pH 8.0, 150 mM NaCl, 1% NP40, 0.5% Sodium Deoxycholate, 0.1% SDS and protease inhibitors) to the cells, and leave the dish on a rocker at 4°C for 20-30 min for efficient cell lysis.
4) Remove lysed cells with a scraper and transfer to an Eppendorf tube. Centrifuge cell lysates at 13,000 rpm at 4°C for 10 min. Transfer supernatants to a new Eppendorf tube.
5) Wash α-FLAG M2 agarose beads with RIPA buffer: add 800 μl of RIPA buffer, and spin at 1000 rpm at 4°C for 5 min. Repeat this washing step three times.
6) Add 25 μl of M2 agarose slurry to 1 mg of the cellular lysates, and mix in a rotator at 4°C overnight.
7) Spin the agarose beads (1000 rpm at 4°C for 5 min), and discard the supernatant fraction. Wash the beads three times with RIPA buffer.
8) Resuspend agarose beads in 100 μl of elution buffer (RIPA buffer + 200 μg/mL of FLAG peptide). Incubate 30 min, and spin the agarose beads. Keep the supernatant.
2.4.3. Western blotting with the methylation specific antibodies
Load 10 μl of the samples eluted with FLAG peptide or 10 μg/10 μl of whole lysate in a 10% SDS-PAGE gel.
Transfer the gel into a nitrocellulose membrane (0.2 μm, #9179739, GE Healthcare) and block the membrane with blocking solution for 1 h at room temperature.
Incubate with the appropriate dilution of the methyl-antibodies in blocking solution 1 h at room temperature. We also tested commercial α-FLAG M2 (#F-3165 SIGMA), α-histone H3K9me2 (#07-212, Upstate), α-histone H3K9me3 (#07-442, Upstate), α-histone H3K4me1 (#07-436, Upstate), α-histone H3K4me2 (#07-030, Upstate), α-histone H3 (#07-690, Upstate) and α-tubulin (#T6074, SIGMA) antibodies.
Wash the membrane 3-5 times in TBS-Tween.
Incubate with goat anti-rabbit IgG, peroxidase conjugated in blocking solution 1 h at room temperature.
Wash the membrane 3-5 times in TBS-Tween.
Develop with SuperSignal West Femto Maximum sensitivity substrate (for 10-60 sec) and expose to Amersham Hyperfilm.
3. CONCLUDING REMARKS
During the last years, it has become evident that the study of posttranslational modifications of proteins requires the generation of modification-specific antibodies. Selective antibodies are powerful tools of experimental biology, and a carefully studied antibody can provide pivotal information. The methyl-Tat-specific antibodies presented here differentiate robustly between mono-, di- and trimethylation of the Tat K51 residue in vitro. They also recognize differentially modified short peptides with similar sensitivities. We confirmed that monomethylated Tat is detected in cells and that KMT7 is a methyltransferase that can monomethylate K51 in Tat. Our study also underlines important limitations of antibody studies. We cannot detect di- or trimethylated Tat in cells even in the presence of KMT1E, which may indicate that these Tat forms do not exist in cells. However, di- and trimethylated Tat may only be transiently produced or produced at levels below the detection limit of our antibody-based assays. In addition, a co-modification of a neighboring residue (i.e. acetylation of K50 or methylation of R52) may prevent recognition of di- and trimethylated K51 by our antibodies (epitope exclusion). Eventually, a combinatorial approach including antibodies detecting multiple neighboring modifications and mass spectrometry of in vivo isolated Tat will be needed to provide a comprehensive view of Tat methylation in cells.
Acknowledgments
We thank D. Reinberg (NYU School of Medicine), F. Rauscher (The Wistar Institute) and members of the Verdin, Ott and Greene labs (Gladstone Institute of Virology and Immunology) for sharing their reagents and expertise. We thank J. Carroll, A. Wilson and T. Roberts for graphics, G. Howard for editorial, and V. Fonseca for administrative assistance. This work was supported by funds from the Gladstone Institutes, the NIH (RO1AI083139), the University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research and collaborative research with JT Pharma. We gratefully acknowledge postdoctoral fellowship support for S.P. from the American Foundation for AIDS Research (106736-40-RFRL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kao SY, Calman AF, Luciw PA, Peterlin BM. Nature. 1987;330:489–93. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 2.Toohey MG, Jones KA. Genes Dev. 1989;3:265–82. doi: 10.1101/gad.3.3.265. [DOI] [PubMed] [Google Scholar]
- 3.Harrich D, Ulich C, Garcia-Martinez LF, Gaynor RB. EMBO J. 1997;16:1224–35. doi: 10.1093/emboj/16.6.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 5.Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. Science. 1995;268:429–31. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 6.Westendorp MO, Li-Weber M, Frank RW, Krammer PH. J Virol. 1994;68:4177–85. doi: 10.1128/jvi.68.7.4177-4185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E. Science. 1997;275:1481–5. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Huertas MR, Callejas S, Abia D, Mateos E, Dopazo A, Alcami J, Coiras M. Nucleic Acids Res. 2010;38:3287–307. doi: 10.1093/nar/gkq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennasser Y, Le SY, Benkirane M, Jeang KT. Immunity. 2005;22:607–19. doi: 10.1016/j.immuni.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Qian S, Zhong X, Yu L, Ding B, de Haan P, Boris-Lawrie K. Proc Natl Acad Sci U S A. 2009;106:605–10. doi: 10.1073/pnas.0806822106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankel AD, Pabo CO. Cell. 1988;55:1189–93. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 12.Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Nature. 1990;345:84–6. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 13.Laspia MF, Rice AP, Mathews MB. Cell. 1989;59:283–92. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg MB, Baltimore D, Frankel AD. Proc Natl Acad Sci U S A. 1991;88:4045–9. doi: 10.1073/pnas.88.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marciniak RA, Sharp PA. EMBO J. 1991;10:4189–96. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashanchi F, Piras G, Radonovich MF, Duvall JF, Fattaey A, Chiang CM, Roeder RG, Brady JN. Nature. 1994;367:295–9. doi: 10.1038/367295a0. [DOI] [PubMed] [Google Scholar]
- 17.Raha T, Cheng SW, Green MR. PLoS Biol. 2005;3:e44. doi: 10.1371/journal.pbio.0030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. Cell. 1998;92:451–62. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 19.Tahirov TH, Babayeva ND, Varzavand K, Cooper JJ, Sedore SC, Price DH. Nature. 2010;465:747–51. doi: 10.1038/nature09131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He N, Liu M, Hsu J, Xue Y, Chou S, Burlingame A, Krogan NJ, Alber T, Zhou Q. Mol Cell. 2010;38:428–38. doi: 10.1016/j.molcel.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobhian B, Laguette N, Yatim A, Nakamura M, Levy Y, Kiernan R, Benkirane M. Mol Cell. 2010;38:439–51. doi: 10.1016/j.molcel.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia JA, Harrich D, Pearson L, Mitsuyasu R, Gaynor RB. EMBO J. 1988;7:3143–7. doi: 10.1002/j.1460-2075.1988.tb03181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuppuswamy M, Subramanian T, Srinivasan A, Chinnadurai G. Nucleic Acids Res. 1989;17:3551–61. doi: 10.1093/nar/17.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng L, de la Fuente C, Fu P, Wang L, Donnelly R, Wade JD, Lambert P, Li H, Lee CG, Kashanchi F. Virology. 2000;277:278–95. doi: 10.1006/viro.2000.0593. [DOI] [PubMed] [Google Scholar]
- 25.Col E, Caron C, Seigneurin-Berny D, Gracia J, Favier A, Khochbin S. J Biol Chem. 2001;276:28179–84. doi: 10.1074/jbc.M101385200. [DOI] [PubMed] [Google Scholar]
- 26.Kiernan RE, Vanhulle C, Schiltz L, Adam E, Xiao H, Maudoux F, Calomme C, Burny A, Nakatani Y, Jeang KT, Benkirane M, Van Lint C. EMBO J. 1999;18:6106–18. doi: 10.1093/emboj/18.21.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ott M, Schnolzer M, Garnica J, Fischle W, Emiliani S, Rackwitz HR, Verdin E. Curr Biol. 1999;9:1489–92. doi: 10.1016/s0960-9822(00)80120-7. [DOI] [PubMed] [Google Scholar]
- 28.Pagans S, Pedal A, North BJ, Kaehlcke K, Marshall BL, Dorr A, Hetzer-Egger C, Henklein P, Frye R, McBurney MW, Hruby H, Jung M, Verdin E, Ott M. PLoS Biol. 2005;3:e41. doi: 10.1371/journal.pbio.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulanger MC, Liang C, Russell RS, Lin R, Bedford MT, Wainberg MA, Richard S. J Virol. 2005;79:124–31. doi: 10.1128/JVI.79.1.124-131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie B, Invernizzi CF, Richard S, Wainberg MA. J Virol. 2007;81:4226–34. doi: 10.1128/JVI.01888-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivakumaran H, van der Horst A, Fulcher AJ, Apolloni A, Lin MH, Jans DA, Harrich D. J Virol. 2009;83:11694–703. doi: 10.1128/JVI.00499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorr A, Kiermer V, Pedal A, Rackwitz HR, Henklein P, Schubert U, Zhou MM, Verdin E, Ott M. EMBO J. 2002;21:2715–23. doi: 10.1093/emboj/21.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mujtaba S, He Y, Zeng L, Farooq A, Carlson JE, Ott M, Verdin E, Zhou MM. Mol Cell. 2002;9:575–86. doi: 10.1016/s1097-2765(02)00483-5. [DOI] [PubMed] [Google Scholar]
- 34.Bres V, Tagami H, Peloponese JM, Loret E, Jeang KT, Nakatani Y, Emiliani S, Benkirane M, Kiernan RE. EMBO J. 2002;21:6811–9. doi: 10.1093/emboj/cdf669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmoudi T, Parra M, Vries RG, Kauder SE, Verrijzer CP, Ott M, Verdin E. J Biol Chem. 2006;281:19960–8. doi: 10.1074/jbc.M603336200. [DOI] [PubMed] [Google Scholar]
- 36.Treand C, du Chene I, Bres V, Kiernan R, Benarous R, Benkirane M, Emiliani S. EMBO J. 2006;25:1690–9. doi: 10.1038/sj.emboj.7601074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Duyne R, Easley R, Wu W, Berro R, Pedati C, Klase Z, Kehn-Hall K, Flynn EK, Symer DE, Kashanchi F. Retrovirology. 2008;5:40. doi: 10.1186/1742-4690-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagans S, Kauder SE, Kaehlcke K, Sakane N, Schroeder S, Dormeyer W, Trievel RC, Verdin E, Schnolzer M, Ott M. Cell Host Microbe. 2010;7:234–44. doi: 10.1016/j.chom.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray K. Biochemistry. 1964;3:10–5. doi: 10.1021/bi00889a003. [DOI] [PubMed] [Google Scholar]
- 40.Bannister AJ, Kouzarides T. Methods Enzymol. 2004;376:269–88. doi: 10.1016/S0076-6879(03)76018-2. [DOI] [PubMed] [Google Scholar]
- 41.Martin C, Zhang Y. Nat Rev Mol Cell Biol. 2005;6:838–49. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 42.Peters AH, Kubicek S, Mechtler K, O’Sullivan RJ, Derijck AA, Perez-Burgos L, Kohlmaier A, Opravil S, Tachibana M, Shinkai Y, Martens JH, Jenuwein T. Mol Cell. 2003;12:1577–89. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 43.Morgunkova A, Barlev NA. Cell Cycle. 2006;5:1308–12. doi: 10.4161/cc.5.12.2820. [DOI] [PubMed] [Google Scholar]
- 44.Huang J, Berger SL. Curr Opin Genet Dev. 2008;18:152–8. doi: 10.1016/j.gde.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Nishioka K, Chuikov S, Sarma K, Erdjument-Bromage H, Allis CD, Tempst P, Reinberg D. Genes Dev. 2002;16:479–89. doi: 10.1101/gad.967202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P, Zhang Y. Mol Cell. 2001;8:1207–17. doi: 10.1016/s1097-2765(01)00405-1. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, An W, Cao R, Xia L, Erdjument-Bromage H, Chatton B, Tempst P, Roeder RG, Zhang Y. Mol Cell. 2003;12:475–87. doi: 10.1016/j.molcel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Nature. 2003;421:652–6. doi: 10.1038/nature01378. [DOI] [PubMed] [Google Scholar]


