Abstract
Temperature and selenium are two environmental parameters that potentially affect reproduction and stock recruitment of sturgeon in the San Francisco Bay / Delta Estuary. To identify proteins whose expression is modified by these environmental stressors, we performed a proteomic analysis on larval green and white sturgeons exposed to 18 or 26°C and micro-injected with Seleno-L-Methionine to reach 8 μg g-1 selenium body burden, with L-Methionine as a control. Selenium and high temperature induced mortalities and abnormal morphologies in both species, with a higher mortality in green sturgeon. Larval proteins were separated by two-dimensional gel electrophoresis and differential abundances were detected following spot quantitation and hierarchical cluster analysis. In green sturgeon, 34 of 551 protein spots detected on gels showed a variation in abundance whereas in white sturgeon only 9 of 580 protein spots were differentially expressed (P<0.01). Gel replicates were first grouped according to heat treatment. Fifteen of these spots were identified using MALDI TOF/TOF mass spectrometry. Proteins involved in protein folding, protein synthesis, protein degradation, ATP supply and structural proteins changed in abundance in response to heat and/or selenium. 40S ribosomal protein SA, FK506-binding protein 10, 65 kDa regulatory subunit A of protein phosphatase 2, protein disulfide isomerase, stress-induced-phosphoprotein 1, suppression of tumorigenicity 13 and collagen type II alpha 1, were differentially expressed in high temperature treatment only. Serine/arginine repetitive matrix protein 1, creatine kinase, serine peptidase inhibitor Kazal type 5 and HSP90 were sensitive to combined temperature and selenium exposure. Valosin-containing protein, a protein involved in aggresome formation and in protein quality control decreased more than 50% in response to selenium treatment. Potential use of such proteins as biomarkers of environmental stressors in larval sturgeons could indicate early warning signals preceding population decline.
Keywords: sturgeon, selenium, temperature, proteomics, valosin-containing protein, biomarker
1. Introduction
Green sturgeons (GS, Acipenser medirostris) and white sturgeons (WS, Acipenser transmontanus) are ancient ray-finned fish endemic to the Pacific Coast of North America. WS has long been valued for its caviar and meat, and has more recently become popular in aquaculture and sport fishery (Chapman et al., 1996). GS is less common but plays an important role in Native American fisheries and culture (Moyle, 2002). Both species are anadromous and can be found in rivers, estuaries and coastal waters. The caviar fishery in the late 19th century, construction of river dams and irrigation projects dramatically reduced the abundance and reproductive habitat of both species. Both distinct population segments (DPS) for GS are currently in decline, with the southern DPS listed as threatened in the Sacramento River under the US Endangered Species Act (NOAA, 2005). The Sacramento River WS is not endangered but is likely to suffer further decline due to climate changes, selenium pollution, and increasing use of river water by the growing population of California. Successful stock recruitment of these species in altered reproductive habitat is of major importance for their conservation and restoration (Dettlaff et al., 1993). To ensure their long-term survival, we must better understand physiological adaptations of early life stages and the mechanisms involved in their responses to a changing and more stressful environment.
River temperature is an important parameter influencing the distribution and health of coldwater fishes. Both GS and WS are seasonal breeders and reproduce at temperatures ranging from 10 to 18°C (Cech and Doroshov, 2004). The dam regulation of river flow combined with annual variation of precipitation results in large fluctuations of river temperatures during the spawning run of sturgeons (van Eenennaam et al., 2005). Construction of dams in the Klamath River (Northern California) has caused fluctuations of daily temperature 3-5°C during the summer and seasonal variation of river temperature from 8°C in the winter to > 25°C during late summer (Bartholow, 2005). A temperature of 17-18°C was found to represent the upper limit of the thermal optimum for developing GS embryos, and higher temperatures result in morphological abnormalities and decreased hatching success (van Eenennaam et al., 2005). Newly hatched GS larvae exhibit a high rate of deformities and significant over-expression of HSP72, HSP78 and HSP89 after acute exposure to 26°C, while the HSP60 level was lowered (Werner et al., 2007). Temperatures between 14 and 16°C were optimal for development and survival of the WS embryos, while temperatures above 20°C were lethal when the eggs were exposed to this temperature during the period from fertilization to hatching (Wang et al., 1985; Wang et al., 1987).
Water contaminants, such as selenium (Se) in the upper San Francisco Bay, may have significant effects on the early life stages of sturgeon. Se is an essential micronutrient in animals but, depending on concentration, chemical form, other dietary factors and interaction with other trace elements, it can be highly toxic (NRC, 2005). The Se cycle in the SF Bay Estuary has undergone dramatic changes in a relatively short time frame, largely due to oil refinery operations (Cutter and Cutter, 2004). In the 1980s, it was demonstrated that dissolved Se was delivered to the North Bay principally by the Sacramento River and by the effluents from oil refineries in the vicinity of Carquinez Strait (Cutter, 1989; Cutter and Diego-McGlone, 1990). At that time, dissolved Se fluxes from refineries accounted for 50–90% of the total input and were predominantly represented by selenite (Cutter and Diego-McGone, 1990). Afterwards, the refinery inputs have significantly decreased during the late 1990's due to treatment and cleanup, with the consequence that selenate and organic selenide are now the predominant forms of dissolved Se (Cutter and Cutter, 2004). However, selenium discharges to the San Francisco Bay-Delta is expected to increase significantly due to the extension of the San Luis Drain to convey agricultural drainage from the western San Joaquin Valley to the North Bay (Presser and Luoma, 2000).
Bioaccumulation of Se in fish mainly happens via the food chain. Tissue Se levels may approach concentrations that adversely affect survival, growth and reproduction of fish, for instance in the San Joaquin River (Saiki et al., 1992). Sturgeons are bottom feeders consuming molluscs, shrimp, amphipods, and fish (Billard and Lecointre, 2001). Since 1986, the Asian clam Potamocorbula amurensis has become the dominant macroinvertebrate in the upper San Francisco Bay and a favorite prey of WS. This bivalve has efficient filtering capacity and bioaccumulates Se at a higher rate than other clam species in the San Francisco Estuary (Linville et al., 2002). WS foraging on Asian clam had higher liver Se concentration (24.4 μg g-1 dw) compared to other carnivorous fish of the San Francisco Bay (Stewart et al., 2004). Several other studies reported elevated Se concentrations in WS muscle and liver, 15 and 30 μg g-1 dw, respectively (Urquhart and Regalado, 1991; Linville et al., 2002). Ovulated eggs collected from six females caught in the Sacramento River had a mean Se concentration of 12.4 ± 3.3 μg g-1 dw (mean ± sem), with a maximum concentration of 29 μg g-1 dw (Kroll and Doroshov, 1991). Presser and Luoma (2000) have predicted that a new selenium disposal plan proposed by the US Bureau of Reclamation to farmers may increase Se concentration in organisms that sturgeons feed on to a level 5 to 40 times the dietary threshold of Se toxicity in fish (Hamilton, 2004).
Systems toxicology has been defined as the application of new “omics” approaches to traditional toxicological studies (Waters and Fostel, 2004). It refers to the interaction between genes and environmental stressors and combines the studies of transcriptomics, proteomics and metabolomics. The use of proteomics in environmental toxicology is still in its infancy due to a number of drawbacks such as the limited number of organisms fully covered in sequence databases. However, several authors have reported that environmental stresses, such as variation of salinity and temperature (Kimmel and Bradley, 2001) or exposure to environmental contaminants, such as heavy metals, xenoestrogen, chlorinated compounds, perfluorinated chemicals (Lopez-Barea and Gomez-Ariza, 2006; Calzolai et al., 2007; Monsinjon and Knigge, 2007; Nesatvy and Suter, 2007; Gillardin et al., 2009) have an impact on protein expression in different tissues of relevant aquatic organisms. Using a proteomic approach, the present study aims to determine the effects of heat stress and selenium exposure on the protein expression profiles in larvae of two sturgeon species.
2. Animals, Materials and Methods
2.1. Exposure experiment
In March 2007, one progeny of GS was obtained by captive breeding of F1 adults reared to maturity at the UC Davis facilities. Three progenies of WS were obtained from nearby commercial sturgeon farms. Hatchery spawning followed procedures detailed by Conte et al. (1988) for WS and by van Eenennaam et al. (2008) for GS. Eggs were incubated at 14-16°C and newly hatched larvae were held in a tank at 17 °C. Approximately 3000 larvae from each progeny were transferred to the UC Davis Center for Aquatic Biology and Aquaculture (CABA) for the exposure experiments. Larvae exhibiting normal morphology were microinjected either with Seleno-L-Methionine (Se) or L-Methionine (Met), and exposed to either 18°C (normal temperature) or 26°C (heat stress). Non-injected larvae (NI) were also exposed to temperature treatments. Consequently, each experiment consisted of 6 treatments (NI18, NI26, Met18, Met26, Se18, Se26), 3 replicate tanks per treatment, and 60 larvae randomly assigned to each tank.
The organic Se was injected as Se-L-Methionine (Sigma-Aldrich, St Louis, MO) to reach a nominal body burden of 8 μg g-1 dw. The injection solutions were prepared based on dry weight of newly hatched larvae, 7.1 mg (WS) and 16.8 mg (GS). Microinjections were conducted using a programmable pressure injector and micromanipulator (Narishige Group, Japan), and aluminosilicate needles calibrated at 400x magnification. Larvae were anesthetized in a 120 ppm tricaine methanesulfonate bath and then placed onto a water-saturated filter paper and injected with 24 nL (WS) or 42 nL (GS) solution into the yolk sac between Cuvier's duct and the vitelline vasculature. Non-injected larvae were exposed to similar procedures, except for injection. Larvae were allowed to recover at 18°C prior to tank stocking. Samples for Se body burden were collected at 24 h after injection (100 pooled larvae from each treatment at 18°C, total dw ~700 mg WS and ~1200 mg for GS) and at stage D45 (3-7 pooled larvae from each treatment tank, dw 31-33 mg). Samples were analyzed for Se using ICP/AES (Fisons Accuris, Fisons Instruments, Inc.). The detection limit for this method was 0.02 μg g-1 for the large samples (24h after injection) and 0.2 μg g-1 for the small samples (stage D45). A certified reference material (TORT-2, NRCC) and a 1 μg g-1 Se overspike of raw bovine liver were used for quality control, with 100% selenium recovery.
Larvae were held in small circular tanks (20 L volume, 1.5 L min-1 flow) of the indoor recirculation systems equipped with biological filter, aeration, YSI thermostat (YSI Environmental, Yellow Springs, OH), chiller and heater. A calibrated thermometer (National Institute of Standards and Technology) was used to adjust system temperatures to either 18°C or 26°C, which was kept within ± 0.1°C throughout the experiment. A natural photoperiod was artificially maintained and tanks were covered with shade-cloth. Dissolved oxygen was maintained within 95 – 100 % air saturation. Since the rates of yolk absorption and development are controlled by temperature (Wang et al., 1987; Dettlaff et al., 1993; Hardy and Litvak, 2004), the exposure duration was different in 18°C (12d) and 26°C (8d) treatments. Diagnostic characters of sturgeon larval stage D45 (absorbed yolk) were used to define the endpoint of exposure (Dettlaff et al., 1993). Dead larvae were counted daily and removed from the tanks. Live abnormal larvae (bent body and edema) were counted daily but not removed. At stage 45 three normal larvae were randomly sampled from each tank for Met and Se groups, snap-frozen in liquid nitrogen and stored at -80°C for proteomic analysis. Larvae weighed (wet weight) between 43.2 and 81.8 mg for GS, and between 29.3 and 53.5 mg for WS. Remaining larvae were euthanized (overdose of tricaine methanesulfonate), counted for percent abnormality, and used for Se analyses, histology and photography. All manipulations with animals were conducted according to animal care protocol approved by the Animal Care and Use Committee of the University of California, Davis.
Larvae collected for histology were fixed in 10% buffered formalin, dehydrated in a series of alcohols, cleared in xylene and infiltrated with paraffin. Paraffin blocks were sectioned at 5 μm and slides were stained by Gomori trichrome and H & E stains. Images of sagittal sections representing normal and bent larvae were obtained using Olympus BH-2 compound scope with digital computer interphase (Nikon ACT-2U).
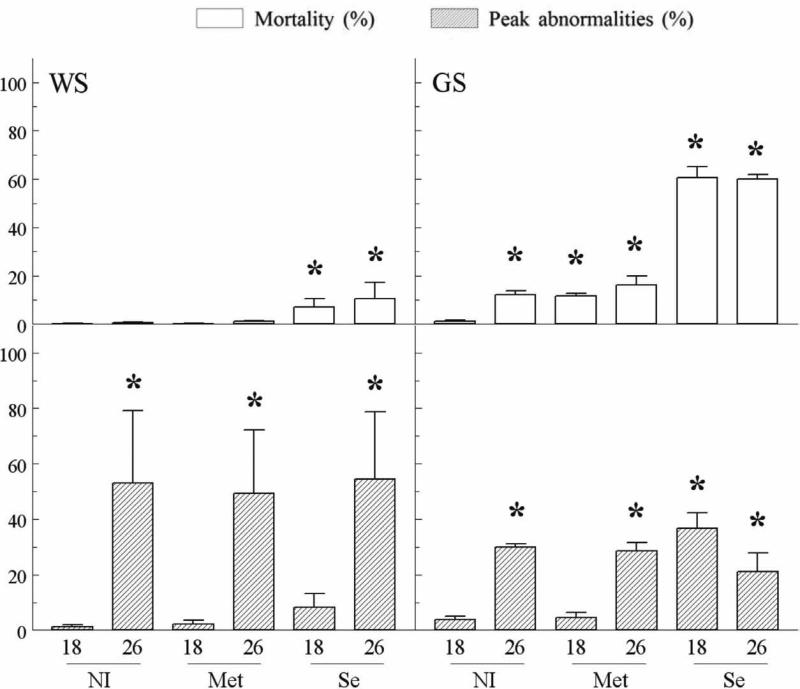
Percent mortality was the endpoint mortality at stage D45. Peak abnormality was a highest percent abnormality in daily observations of live animals in tanks (partial or complete recovery of abnormal larvae was observed at stage D45). Treatment effect in the experiment with WS was tested by two-way ANOVA (Statistica™ version 5.1) for randomized block design (progeny as a blocking factor) and by one-way ANOVA for completely randomized design in the experiment with GS. Percent data were arcsine-transformed to normalize distributions. If the effect was significant (P<0.05), a post-hoc Dunnett test was used to compare all treatment means with non-injected control at 18°C (treatment NI18). Similar ANOVA models were applied to four treatments with injected fish (Met18, Met26, Se18, and Se26, Fig. 1), with a post-hoc Tukey-Kramer HSD test for all treatment pairs.
Fig. 1.
Percent mortality (open bars) and peak abnormality (dashed bars, mean ± sem) of WS and GS larvae in exposure experiments. Asterisks show treatments significantly different from control NI18 (Dunnett test). Superscripts with different letters show significantly different treatments within Met18 – Se26 treatments (Tukey-Kramer test).
2.2. Sample preparation for proteomics
Only larvae belonging to groups Met and Se were used for proteomic analysis. A total of 24 (GS) and 108 (WS) larvae were individually homogenized on ice in 4 volumes of ND-RIPA buffer (50mM Tris-HCl, pH 7.5, 150mM NaCl, 1% v/v Nonidet P-40, 1% v/v Triton X-100, 1% w/v CHAPS, 2mM NaF, 2mM activated Na3VO4, 1x Complete-MiniTM Protease inhibitor cocktail Roche) using a tight-fitting glass homogenizer (Wheaton). Each sample was maintained for 10 min on ice for protein release. The soluble protein fractions were harvested by centrifugation at 19 000 × g for 15 min at 4°C and the pellet discarded. Supernatants were aliquoted into 2 mL siliconized microcentrifuge tubes, and protein concentration was determined using the BCA protein assay (Thermo Fisher Scientific Inc., Rockfeller, USA). For WS, 3 larvae sampled in each tank were pooled to reach a total amount of 300 μg protein, and a total of 3 replicates per treatment were obtained for each progeny (9 replicates per treatment for three progenies). GS supernatants were analysed individually, meaning a total of 6 replicates per treatment for one progeny. A sample volume containing 300 μg proteins was then precipitated for 2 h at -30°C in 4 volumes of precooled 90 % acetone/10 % TCA. Precipitated proteins were centrifuged at 19 000 × g for 5 min at 4°C, and the pellets were rinsed 4 times in pure acetone and 20mM DTT. The pellets were left 30 min on ice in acetone during the last round, and air-dried for 5 min. Proteins were resuspended in 200 μL of IPG rehydration buffer (7 M urea, 2 M thiourea, 2 % CHAPS, 2 % Nonidet P-40, 0.002 % bromophenol blue, 0.5 % IPG buffer 3-10NL and 100 mM DTT).
2.3. Two-dimensional gel electrophoresis
Resuspended proteins (300 μg in 200 μL of rehydration buffer) were added to IPG strips (11 cm, pH 3-10NL, BioRad) by overnight passive rehydration at 4°C. Isoelectric focusing was performed on a Proteome System IsoelectrIQ2 at a maximum of 10 000 V and a total of 55 900 Vh at 20°C and slow ramping mode. Afterwards, IPG strips were stored at -80°C. Prior to SDS-PAGE, focused IPG strips were equilibrated, reduced and alkylated in buffer (375 mM Tris, 6 M urea, 30 % glycerol, 2 % SDS, 0.002% bromophenol blue, pH 8.8) containing 1 % DTT and then 2.5 % iodoacetamide for 2 × 10 min. Strips were then loaded onto a 12 % 1mm thick, acrylamide gel and run in a Criterion Dodeca cell unit (BioRad) at constant 200 V and 10°C until the blue dye front had run off the bottom of the gels. Gels were stained with colloidal Coomassie blue and scanned with an Epson 1680 densitometer as previously described (Valkova and Kültz, 2006).
2.4. Analysis of 2D-gels
Spot detection and matching were performed using Delta 2D gel analysis software (version 3.4, Decodon, Germany). All gels within each treatment were warped to a reference gel. A master gel was then created by fusing all gel images using union fusion type and maximum covered region (Background 50; Spot size 5; Sensitivity 50). Every spot on each gel was quantified and normalized according to the total intensity of all spots in each gel. Using the analysis module of the Delta 2D software and version 1.8 of the freely available software PermutMatrix (http://www.lirmm.fr/~caraux/PermutMatrix/), we performed a multivariate analysis. A one-way ANOVA (Statistica™ version 5.1) among the 4 experimental groups within each sturgeon species first allowed the selection of spots whose abundance was significantly modified at P<0.01. On this basis, a two-way agglomerative hierarchical clustering analysis (HCA) was performed using Pearson's correlation coefficient to calculate the distance matrix, while the agglomerative clustering algorithm complete linkage permitted the construction of the final dendrogram (Meunier et al., 2007). Data were previously standardized by row using the classical zero-mean and unit standard-deviation technique (Satoh et al., 2005). Afterwards, a Fischer LSD post-hoc test allowed comparing groups two by two for spots with significant ANOVA. For this post-hoc analysis, we set as significant a P-level of 0.05 and as highly significant a P-level of 0.01. In order to evaluate the amount of false negatives, we used the freely available software G*Power (http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3/) version 3.0 as described by Faul et al. (2007). We first performed, for GS and WS separately, a sensitivity analysis aimed at computing the critical population effect size as a function of the risk alpha, the power (1-beta), and the sample size. Second, we performed a post-hoc power analysis aimed at computing the power as a function of the risk alpha, the population effect size and the sample size.
2.5. In gel trypsin digestion, MALDI TOF/TOF MS and database searching
Spots of interest were manually excised from 2D gels using a 1mm spot puncher. The same spot was picked from a maximum of 3 gels and pooled for mass spectrometry. Tryptic digestion and peptide extraction were performed in a dust-free environment at room temperature. Each gel plug was washed and destained by shaking the tubes 45 min at room temperature with 50 μL of 5 % acetonitrile/50mM NH4HCO3 and then 3 × 40 min at RT with 50 % acetonitrile/50mM NH4HCO3. Gel plugs were then shrunk and dehydrated using 50 μL of 50 % ACN for 5 min before the solution was discarded and the gel pieces dried. Picked spots were rehydrated and incubated for 16 h at 37°C under gentle shaking in 5 μL of freshly prepared trypsin solution (1 μg of Promega mass spectrometry grade trypsin dissolved in 245 μl of 50 mM NH4HCO3). Peptides were extracted from the gel plug by washing twice for 30 min at RT in 10μL of 60 % ACN/ 1 % trifluoroacetic acid (TFA). Samples were concentrated in a SpeedVac vacuum concentrator (IDT-110, ThermoSavant) at low setting till the final volume was about 1μL, and 9μL of 0.1 % TFA was added. Peptide solutions were cleaned-up with μ-C18 ZipTips (Millipore size P10) and eluted in 1.5 μL of 50 % ACN/ 0.1 % TFA. Protein samples were spotted by 0.5 μL increments on a stainless steel target and they were overlaid with equal amounts of matrix. The matrix, α-cyano-4-hydroxycinnamic acid (HCCA, Sigma, St. Louis, MO), was dissolved at 5 mg mL-1 in 70:30 ACN:H2O, which contained 10 mM ammonium phosphate to reduce the intensity of matrix peaks in MS spectra. The HCCA matrix was recrystallized from 70 % ACN:30 % H2O prior to use. Spotted, trypsin-digested proteins were analyzed using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF/TOF) mass spectrometry on a 4700 Proteomics Analyzer from Applied Biosystems (Foster City, CA) using both MS and tandem MS/MS operating modes. Peptide fragmentation of the 10 most intense peaks in each MS spectrum was achieved in MS/MS mode by both post-source decay (PSD) and collision-induced dissociation (CID) using atmosphere as the collision gas (Lee et al., 2006). Protein identification from MS and MS/MS m/z peaks was carried out with GPS Explorer software version 3.0 (Applied Biosystems) using the Mascot version 1.9 search algorithm (Perkins et al., 1999) to compare observed peaks with in silico digests of proteins in the SwissProt and National Center for Biotechnology Information (NCBI) non-redundant databases. By August 2008, UniProtKB/Swiss-Prot release 56.0 contained 392,667 entries (22 July 2008), while the full NCBInr database release 30 contained 8,572,852 entries (7 July 2008). Mass tolerance settings of 0.3 Da for both parent and fragment ions were applied. Search settings allowed 2 missed cleavages with trypsin, one fixed modification (carbamethylation of cysteine) and one variable modification (oxidation of methionine). Results with confidence interval % (C.I.%) values greater than 95 % were considered to be a positive identification.
3. Results
3.1. Larval responses during the exposure experiments
Whole body selenium concentration in non-injected larvae at 24 h after injection was 3.2 μg g-1 in GS and 2.1 μg g-1 in WS, with similar concentrations in Met18 treatments (Table 1). Larvae that received organic Se had a selenium body burden of 7.3 μg g-1 in GS and 8.6 μg g-1 in WS (average for 3 progenies). At stage 45 selenium body burden in surviving GS larvae was only slightly higher in the Se18 treatment compared to non-injected controls, whereas selenium concentrations in WS larvae were 3 times higher in both Se18 and Se26 treatments, compared to NI18 controls. Selenium body burden in Met18 and Met26 treatments did not differ from non-injected controls, but both GS and WS larvae had slightly lower selenium concentrations in all 26°C temperature treatments compared to 18°C treatments (Table 1).
Table 1.
Selenium body burden (mean ± sem, μg g-1 dw) of GS and WS sturgeon at 24 h after injection and at stage D45. Asterisks indicate treatments significantly different from NI18 control (P<0.05).
| Injections | Temp °C | GS | WS | ||
|---|---|---|---|---|---|
| 24h (n=1) | D45 (n=3) | 24 h (n=3) | D45 (n=3) | ||
| Non-injected | 18 | 3.2 | 3.1 ± 0.1 | 2.1 ± 0.1 | 2.4 ± 0.2 |
| 26 | ---- | 2.9 ± 0.1 | ---- | 2.0 ± 1.5 | |
| L-Met | 18 | 3.5 | 3.3 ± 0.1 | 2.1 ± 0.1 | 2.3 ± 0.1 |
| 26 | ---- | 2.8 ± 0.1 | ---- | 2.0 ± 1.8 | |
| Se-L-Met | 18 | 7.3 | 3.8 ± 0.3* | 8.6 ± 1.3* | 7.6 ± 0.3* |
| 26 | ---- | 3.4 ± 0.2 | ---- | 6.8 ± 1.0* | |
End-point mortality of the WS larvae was low in all treatments and was significantly elevated only in two selenium treatments, Se18 and Se26,(P<0.0001, Fig. 1) with no significant difference between these two groups. Morphological abnormalities were significantly elevated in all 26°C treatments compared to 18°C, including non-injected larvae and selenium treatments (P<0,0001, Fig. 1). The peak abnormalities were observed during the 2nd – 5th day of exposure in 26°C treatments and were followed by apparent recovery of affected larvae which straightened their bodies and resumed normal swimming. At the stage 45, only the Se26 treatment had a significantly elevated proportion of abnormal larvae (P< 0.01, not shown). Blocking factor (progeny) had significant effects on mortality (P<0.01) and abnormality (ANOVA P< 0.0001) in WS larvae.
The general patterns of mortality in GS larvae were similar to those in WS larvae, except overall mortality was much higher, about 50 % in two selenium treatments (Se18 and Se26). Mortality in GS larvae was also significantly elevated in treatments NI26, Met18 and Met26, compared to non-injected control at 18°C (P<0.0001, Fig. 1). Mortality was higher in both selenium treatments, compared to Met18 and Met26 treatments but, as in WS larvae, there was no significant difference between mortality in Se18 and Se26 treatments. As in WS, peak abnormalities were higher in all 26°C treatments compared to non-injected controls. However, significantly higher peak abnormality was also observed in Se18 treatment (P< 0.01, Fig. 1). ANOVA for the endpoint abnormality was not significant (P> 0.05) in the GS experiment, indicating that the majority of abnormal larvae died before reaching stage D45.
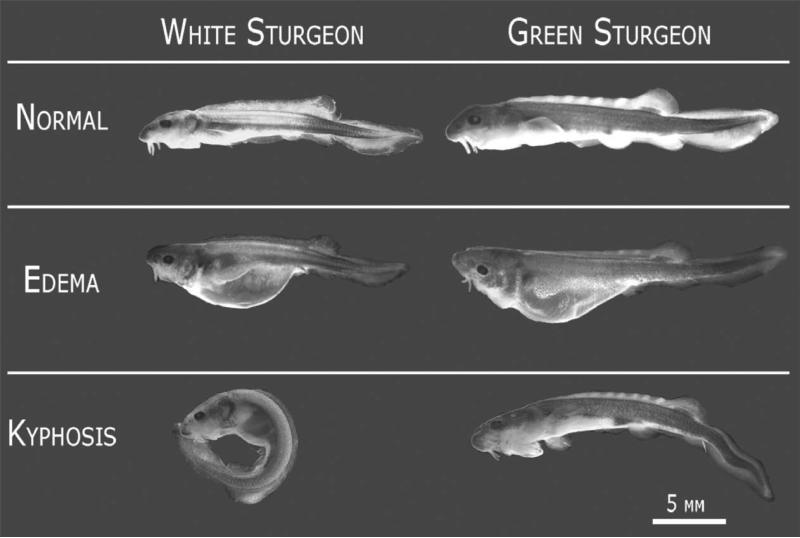
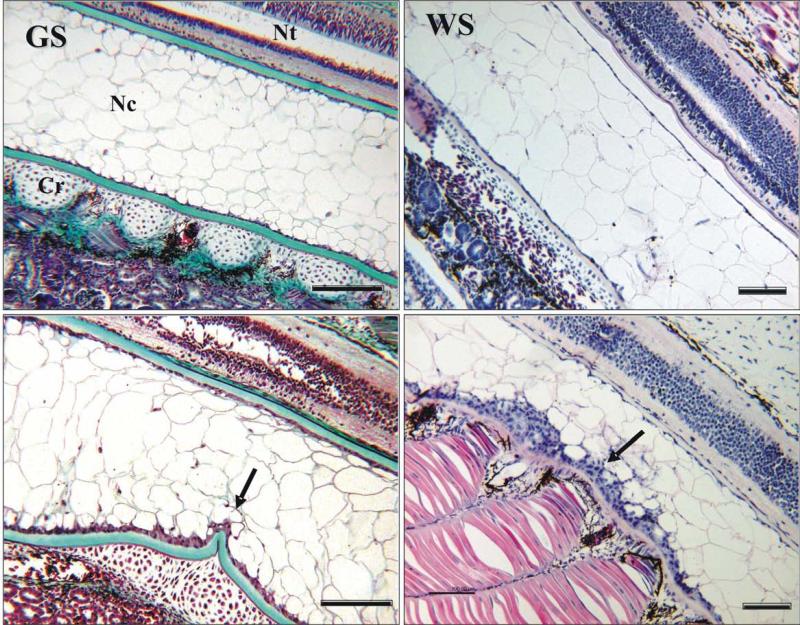
Both GS and WS larvae exhibited stressor-specific abnormalities in response to selenium and heat stress. Peritoneal and pericardial edema, lethargy, bradycardia and hemorrhage were prevalent signs of selenium stress (Se18, Fig. 2), with spinal deformities observed in some larvae. Heat stress mainly elicited kyphotic body curvatures in practically all deformed larvae (NI26 and Met26, Fig. 2), and both stresses combined (Se26) induced both edema and kyphosis. Histological analysis of abnormal larvae revealed an apparent loss of structural integrity of the collagen sheath of the notochord in larvae affected by high heat stress (Fig. 3).
Fig. 2.
Representative abnormalities of WS and GS larvae at the end of exposure experiment, stage D45. Kyphosis was mainly induced by heat treatment and edema by selenium treatment. Distinguishing characteristics of normal stage 45 larvae include differentiated fins, scute rudiments in dorsal finfold, barbels, and differentiated stomach and mid-intestine, previously ‘yolk sac’.
Fig. 3.
Sagittal sections of GS and WS larvae. Normal notochord is shown on the top and abnormal notochord (kyphotic curvature) on the bottom. Arrows indicate constriction of notochord and apparent damage to collagen sheath. GS is stained by Gomori Trichrome, collagen is green (scale bar 250 μm). WS is stained by H & E (scale bar 100 μm). Nc – notochord core cells; Nt - neural tube, Cr = cartilage of sclerotome
3.2. Proteome responses
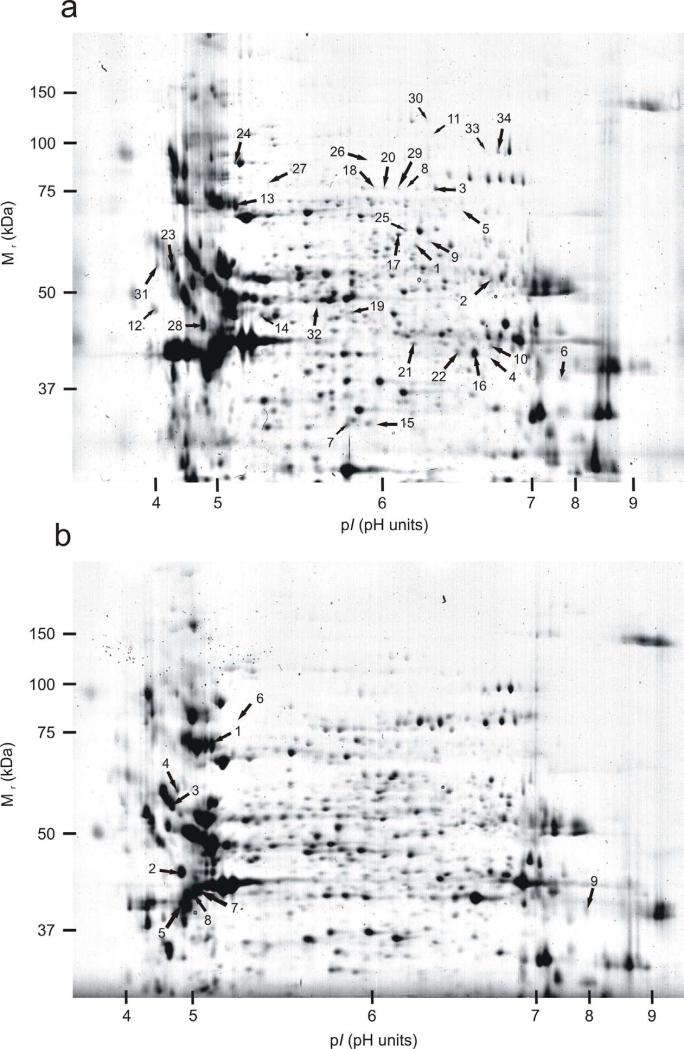
Using Delta 2D software, we consistently detected a total of 551 spots for GS and a total of 580 spots for WS on 2D gels. A coefficient of variability (CV) within four experimental groups (Met18, Se18, Met26, Se26) was calculated on the basis of the % vol for each individual spot with the formula : CV=SD/mean*100. It ranged between 36 and 40 % for GS, and between 34 and 39 % for WS. The ANOVA test among the 4 experimental groups revealed that 34 protein spots (6.2 % of all detected spots) were differentially expressed in GS at P<0.01 (Fig. 4a). In WS, the same statistical analysis allowed the detection of only 9 protein spots (1.6 % of all detected spots) differentially expressed (Fig. 4b). The rigorous level of significance of 0.01 has been chosen to decrease the risk of false positive identifications. However, as a consequence, the analysis can suffer from a high beta risk of false negatives. In order to evaluate the power of the ANOVA analysis, we used the software G*Power to first calculate a sensitivity power analysis for each species separately. With a risk alpha set at 0.01, we calculated that an effect size of 0.93 for GS and 0.72 for WS was detectable in our study with a power of 80 %. This corresponds to a 1.8-fold and 1.6-fold increase, respectively for GS and WS, in protein abundance in one experimental group out of four. The post-hoc power analysis indicated that our study will detect a 2-fold variation in expression in one of four groups with a 95.5 % probability for GS and 99.9 % probability for WS. However, for detecting a 1.5-fold change, the power dropped to 27.9 % for GS and to 59.4 % for WS, and for a 1.2-fold change even to 3.0 % (GS) and to 5.8 % (WS).
Fig. 4.
Two-dimensional gel images showing relative protein expression from green (a) and white (b) sturgeon. The numbers in the gels correspond to spots with significant variations in expression following exposure to 18° or 26°C and to Se-L-Met or L-Met (p<0.01).
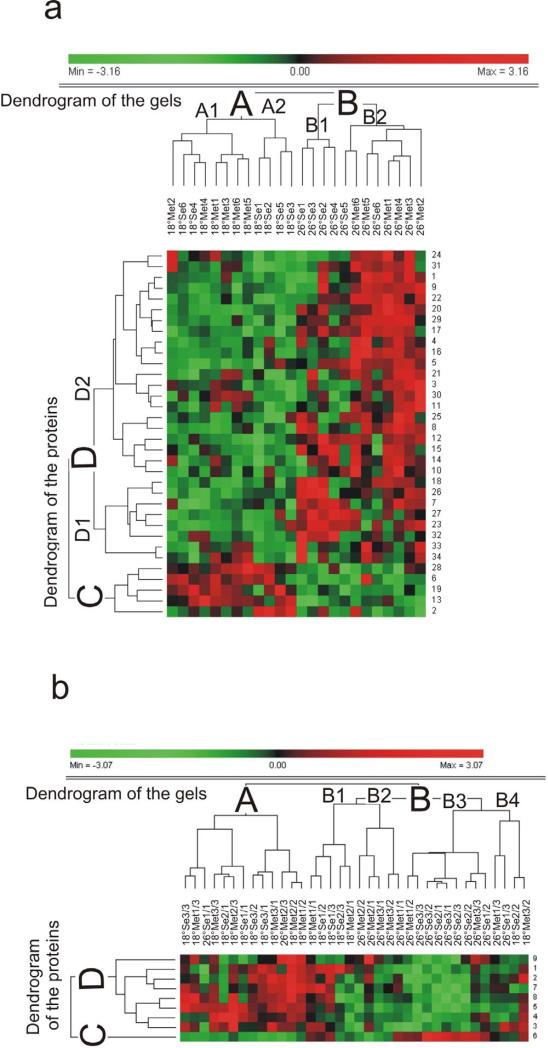
In order to analyse the relationships among the 34 significant spots in GS and among the 9 significant spots in WS, a two-way agglomerative HCA was performed using complete linkage between groups. In the cluster dendrogram for GS (Fig. 5a) we were able to observe that a clear distinction existed between the gels containing proteins of larvae exposed to 18°C (group A) and the proteins of larvae exposed to 26°C (group B). A weaker separation was detected for larvae exposed to Se-L-Met compared to larvae exposed to L-Met. Within group A, the subgroup A2 encompassed 4 of the 6 gel replicates of larvae exposed to Se-L-Met and was separated from subgroup A1 which encompassed the 6 gels of larvae exposed to L-Met. However, 2 replicates of gels from fish exposed to selenium were also included in A1 (Se18-6 and Se18-4). Within group B, a similar analysis could be done since the subgroup B1 encompassed 4 of 6 gel replicates of larvae exposed to Se-L-Met, while the subgroup B2 encompassed all gels of fish unexposed to selenium and 2 gels of fish exposed to selenium (Se26-5 and Se26-6). In WS (Fig. 5b), the dendrogram of the gels revealed that the temperature was also the most important parameter that influenced the protein expression patterns but, compared to GS, the groups were less clearly separated. Most of the gels from fish exposed to 18°C were grouped in A. However, 5 other replicate gels were grouped in B1 while the 2 last gels were found in B4. All replicate gels from fish exposed to 26°C were grouped in B2, B3 and B4, except 2 gels found in A. Regarding Se, the distinction was less clear. We could only discern a group of larvae exposed to Met26 in B2 while 7 replicates of Se26 were found in B3.
Fig. 5.
Two-way hierarchical clustering analysis (processed with PermutMatrix according to the Pearson distance and complete linkage aggregation method) of data set from green (a) and white (b) sturgeon larvae. Heat map representation of the clustered data matrix in which each colored cell represents a protein value according to the color scale at the top of the figure. Each line corresponds to one protein spot (see numbers in Fig. 4 and Table 2). The dendrograms of the gels depict a balanced tree of all analysed 2D gels for sturgeons exposed to 18° or 26°C, and to Se-L-Met (Se) or L-Met (Met). Six gel replicates per condition are included in (a), while the 3 progenies and 3 replicates per progeny (indicated as 1-3/1-3) are included in (b). In (a), larvae exposed to 18°C (cluster A) are clearly separated from larvae exposed to 26°C grouped in a common cluster (B) with a weak distinction between larvae exposed (groups A1 and B1) and unexposed to Se-L-Met (groups A2 and B2). Dendrogram of the proteins clearly distinguishes clusters of coregulated proteins (groups C and D) in terms of up-regulation or down-regulation in larvae exposed to 18°C, respectively. In (b), a slight separation exists between larvae exposed to 18°C (clusters A and B1) and to 26°C (clusters B2, B3 and B4).
Regarding the dendrogram of the proteins in GS, 2 groups were clearly separated. Group C encompassed 5 protein spots whose general expression pattern showed an up-regulation at 18°C compared to 26°C. The other 29 spots were grouped in D and showed a general pattern of over-expression at 26°C. Interestingly, the same distinction could be made for WS. However, most of the proteins (8 spots) were over-expressed at 18°C, while only one protein spot (6) was up-regulated at 26°C.
Of these protein spots identified on 2D gels as differentially expressed, we were able to determine the molecular identity of 12 different protein species in GS and 3 protein species in WS using MALDI-TOF/TOF MS. Proteins identified by MS, together with the PANTHER ID and the Pfam domain are reported in Table 2. Table 3 depicts the ratios of expression among the tested conditions and the corresponding level of significance given by the Fisher LSD post-hoc test. In GS, spots 13 and 28 belong to group C and have been identified as a FK506 binding protein 10 and a 40S ribosomal protein SA, respectively. For both of these proteins, a highly significant decrease in protein abundance was observed in larvae exposed to 26°C compared to 18°C. This decrease was of the same magnitude independent of whether the larvae were exposed to Se or not.
Table 2.
List of protein spots identified by MALDI-TOF/TOF from green (GS) and white (WS) sturgeon larvae following exposure to 18° or 26°C and to Se-L-Met or L-Met (P<0.01).
| No. on gel | Sturgeon species | Mr (kDa) Gel/Theoretical | pI (pH units) Gel/Theoretical | Protein name | Species | Accession No. | PANTHER ID | Pfam domain | Functional pathway | Prot. Score | No. of peptides | Seq. Cov. (%) | Total ion score | Best ion score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | GS | 80/118 | 6.3/8.7 | Serine peptidase inhibitor, Kazal type 5 | Mus musculus | Q148R4 | Kazal 1 Kazal 2 |
Serine protease inhibitor Serine protease inhibitor |
86.0 (99.79%) | 24 | 29 | I | I | |
| 7 | GS | 30/143 | 5.8/5.9 | Collagen, type II, alpha 1 | Xenopus laevis | Q91717 | PTHR10499:SF126 | VWC Collagen COLFI |
Multiprotein complexes Formation of connective tissue Substrate binding |
116 (100%) | 14 | 9 | 88.2 (100%) | 40.0 (98.9%) |
| 12 | GS | 47/40 | 4.0/5.2 | Suppression of tumorigenicity 13 | Danio rerio | Q6NX98 | PTHR22904:SF34 | TPR1 | Assembly of multiprotein complexes | 114 (100%) | 8 | 9 | 77.5 (100%) | 60.2 (100%) |
| 13 | GS | 72/65 | 5.1/5.6 | FK506 binding protein 10 | Bos taurus | Q2HJ89 | FKBP C | Protein folding | 48.7 (0%) | 6 | 8 | 39.1 (97.9%) | 39.1 (97.9%) | |
| 16 | GS | 40/43 | 6.7/6.3 | Creatine kinase | Ictalurus punctatus | Q804Z2 | ATP-gua Ptrans ATP-gua PtransN |
ATP:guanido phosphotransferases ATP:guanido phosphotransferases |
95.6 (100%) | 12 | 13 | 63.4 (100%) | 56.1 (100%) | |
| 17 | GS | 64/62 | 6.1/6.4 | Stress-induced-phosphoprotein 1 (Hsp70/Hsp90-organizing protein) | Danio rerio | Q5RKM3 | PTHR22904:SF14 | TPR 1 | Assembly of multiprotein complexes | 52.7 (47.6%) | 8 | 9 | 28.9 (98.5%) | 28.9 (98.5%) |
| 22 | GS | 40/43 | 6.5/6.3 | Creatine kinase | Ictalurus punctatus | Q804Z2 | ATP-gua Ptrans ATP-gua PtransN |
ATP:guanido phosphotransferases ATP:guanido phosphotransferases |
35.4 (0%) | 4 | 3 | 24.3 (93.6%) | 24.3 (93.6%) | |
| 23 | GS | 57/83 | 4.3/4.9 | Heat shock protein hsp90 beta | Salmo salar | Q9W6K6 | HATPase HSP90 |
ATP binding Protein folding |
93.5 (100%) | 14 | 14 | 54.8 (99.9%) | 24.3 (0%) | |
| 24 | GS | 85/83 | 5.1/5.2 | Valosin containing protein | Oncorhynchus mykiss | Q1M179 | CDC48 N CDC48 2 AAA |
ATP binding ATP binding Unfolding of macromolecules |
102 (100%) | 11 | 12 | 75.1 (100%) | 54.6 (99.9%) | |
| 28 | GS | 43/35 | 4.8/4.8 | 40S ribosomal protein SA | Ictalurus punctatus | Q90YS4 | Ribosomal S2 | Translation | 345 (100%) | 8 | 55 | 297 (100%) | 75.6 (100%) | |
| 33 | GS | 95/101 | 6.8/11.8 | Serine/arginine repetitive matrix protein 1 | Gallus gallus | Q5ZMJ9 | PTHR23148:SF1 | PWI | splicing factor | 64.7 (97.3%) | 16 | 23 | I | I |
| 34 | GS | 95/93 | 6.9/11.9 | Serine/arginine repetitive matrix protein 1 | Xenopus tropicalis | Q28CC2 | PWI | splicing factor | 97.6 (100%) | 24 | 33 | I | I | |
| 2 | WS | 43/35 | 4.8/4.8 | 40S ribosomal protein SA | Ictalurus punctatus | Q90YS4 | Ribosomal S2 | Translation | 355 (100%) | 10 | 35 | 336 (100%) | 83.6 (100%) | |
| 3 | WS | 55/58 | 4.8/4.8 | Protein disulfide isomerase | Mus musculus | P09103 | PTHR18929:SF1 | Thioredoxin | Protein disulfide oxidoreduction | 137 (100%) | 15 | 15 | 99 (100%) | 51.4 (100%) |
| 4 | WS | 60/66 | 4.8/4.9 | Protein phosphatase 2 (formerly 2A), regulatory subunit A, beta isoform | Danio rerio | A5PMV7 | HEAT | Protein-protein interaction | 65.7 (77.2%) | 10 | 21 | 44.8 (98.5%) | 36.7 (90.4%) |
MS data using both post-source decay (PSD) and collision-induced dissociation (CID) MS/MS modes were searched against both SwissProt and NCBInr databases. Isoelectric point (pI) and molecular mass (Mr) are presented for both the top database hit (theoretical) and from 2D gels (gel). Accession numbers are from Uniprot.
Table 3.
Ratios between normalized volumes in green (GS) and white (WS) sturgeon larvae exposed to 18° or 26°C and to Se-L-Met (Se) or L-Met (Met).
| No. on gel | Sturgeon species | Protein name | Group in HCA | 18Met/26Met | 18Se/26Se | 18Met/18Se | 26Met/26Se |
|---|---|---|---|---|---|---|---|
| 3 | GS | Serine peptidase inhibitor, Kazal type 5 | D3 | 0,68* | 0,84 | 1,31 | 1,62** |
| 7 | GS | Collagen, type II, alpha 1 | D2 | 0,77 | 0,58** | 1,23 | 0,92 |
| 12 | GS | Suppression of tumorigenicity 13 | D3 | 0,57** | 0,60** | 1,00 | 1,07 |
| 13 | GS | FK506 binding protein 10 | C | 1,61** | 1,59** | 1,11 | 1,10 |
| 16 | GS | Creatine kinase | D3 | 0,33** | 0,50* | 1,38 | 2,08** |
| 17 | GS | Stress-induced-phosphoprotein 1 (Hsp70/Hsp90-organizing protein) | D3 | 0,58** | 0,60** | 1,00 | 1,03 |
| 22 | GS | Creatine kinase | D3 | 0,48** | 0,60** | 1,16 | 1,44** |
| 23 | GS | Heat shock protein hsp90 beta | D2 | 0,77* | 0,69** | 0,94 | 0,84* |
| 24 | GS | Valosin containing protein | D3 | 0,78 | 0,78 | 2,13* | 2,15** |
| 28 | GS | 40S ribosomal protein Sa | C | 1,33** | 1,34** | 1,06 | 1,07 |
| 33 | GS | Serine/arginine repetitive matrix protein 1 | D1 | 0,81 | 0,54* | 1,65 | 1,09 |
| 34 | GS | Serine/arginine repetitive matrix protein 1 | D1 | 1,03 | 0,66* | 1,56* | 0,99 |
| 2 | WS | 40S ribosomal protein Sa | D | 1,35* | 1,72** | 1,20 | 1,53* |
| 3 | WS | Protein disulfide isomerase | D | 1,57** | 1,29 | 1,15 | 0,94 |
| 4 | WS | Protein phosphatase 2 (formerly 2A), regulatory subunit A, beta isoform | D | 1,62* | 1,96* | 1,11 | 1,35 |
The P-value corresponds to the Fisher LSD test and indicates a significant level of
0.05
0.01.
Group in HCA refers to the groups defined in the Hierarchical Clustering Analysis as shown in Fig. 3.
Within group D, we distinguished 3 subgroups. D2 encompassed 6 proteins including collagen, type II, alpha 1 (spot 7) and HSP90 beta (spot 23). Both proteins were unambiguously identified on the basis of both MS and MS/MS data with high scores (116 and 93.5 for protein score, respectively). However, the observed molecular weight was lower than the theoretical MW for HSP90 (57 instead of 83 kDa), and for the collagen (30 instead of 143 kDa). This discrepancy between observed sturgeon protein characteristics and database entries could be the result of variation in amino acid sequence between sturgeon proteins and orthologous proteins in species with sequenced genomes (Dowd et al., 2008) or of some proteolytic degradation. For both of these proteins, we observed an increase in abundance at 26°C compared to 18°C. For collagen, this difference was not significant for larvae exposed to L-Met (ratio of 0.77 for Met18/Met26) but was highly significant when fish were exposed to Se-L-Met (ratio of 0.58 for Se18/Se26). For HSP90, ratios represented a significant difference when larvae unexposed to Se were compared for temperature (0.77 for Met18/Met26), and highly significant for larvae exposed to Se and compared for temperature (0.69 for Se18/Se26). Interestingly, while Se exposure seems to have no effect on collagen expression level, fish exposed to Se-L-Met showed an increase in abundance of HSP90 compared to L-Met exposed animals, when they were both exposed to 26°C (ratio of 0.84 for Met26/Se26).
In subgroup D1, we observed 2 protein spots (33 and 34) identified as two forms of a serine/arginine repetitive matrix protein 1 on the basis of MS spectra (97.3 and 100 % for protein score, respectively). Those two spots were localized very close together on 2D gels at 95 kDa and at a pI of 6.8 and 6.9, respectively. This location on 2D gels strengthens the reliability of the identification. For these two proteins, we were able to observe that the treatment Se18 induced a significant decrease in protein abundance compared to treatment Se26. Condition Se18 also caused a decrease in abundance compared to treatment Met18 that was significant for spot 34 (ratio of 1.56 for Met18/Se18) but not significant for spot 33 even if the ratio was high (1.65). This observation suggests that Se decreases the expression of serine/arginine repetitive matrix protein 1 but that this effect is only seen at lower temperature (18°C only). Temperature by itself had no effect on this protein's expression.
In subgroup D3, one protein spot (24) was separated from most of the other spots. This protein has been identified as a valosin containing protein on the basis of both MS and MS/MS data. Clearly, this protein abundance was related to selenium exposure, but not to temperature. Ratios of 2.13 (P<0.05) and 2.15 (P<0.01) illustrate its over-expression in larvae exposed to L-Met compared to those exposed to Se-L-Met, at 18°C and at 26°C, respectively. On the opposite, the other spots belonging to subgroup D3 (spots 3, 12, 16, 17, 22) were all significantly affected by temperature. Spots 16 and 22 have been identified as two creatine kinase forms. For spot 16 we obtained a very high score for both MS and MS/MS data, along with very close matches of the molecular weight and pI to the theoretical values. However, spot 22 had a score of 93.6 % for MS/MS only. Nevertheless, the fact that this spot was very close to spot 16 on the 2D gel and that they both showed the same expression profile strengthens the identification reliability. The expression profile for those two creatine kinase spots revealed a highly significant over-expression at 26°C compared to 18°C for larvae unexposed to Se (ratio 18Met/26Met of 0.33 and 0.48 for spots 16 and 22, respectively). Similarly, their expressions were higher at 26°C than at 18°C for fish exposed to Se-L-Met (ratio 18Se/26Se of 0.50 and 0.60, respectively). Selenium had no effect on either spot at 18°C and the ratio 18Met/18Se was close to 1 (1.38 and 1.16, respectively). However, when we compared larvae exposed to 26°C and L-Met with those exposed to 26°C and to Se-L-Met, a significantly higher protein abundance was seen for both spots in animals not exposed to Se (ratio Met26/Se26 of 2.08 and 1.44, respectively), indicating that the temperature effect was moderated by Se exposure. The same observation was made for spot 3, identified as a serine peptidase inhibitor, Kazal type 5. This spot showed a higher abundance in larvae exposed to 26°C but not to Se (ratios of 0.68 and 1.62 for Met18/Met26 and Met26/Se26, respectively), indicating a moderating effect of selenium. On the other hand, spots 12 and 17 showed a very clear effect of temperature but no effect of Se. For those two spots, protein abundance was highly significantly up-regulated at 26°C compared to 18°C, independent of the Se exposure. Spot 12 has been identified as suppression of tumorigenicity 13 in homology to Danio rerio while spot 17 has been identified as a stress-induced-phosphoprotein 1 (HSP70/HSP90-organizing protein) on the basis of MS/MS data.
In WS, three identified proteins (spots 2, 3, 4) belong to group D in the dendrogram of stress-regulated proteins (Fig. 5b). They were all over-expressed in larvae exposed to 18°C compared to those exposed to 26°C. Those differences were significant or highly significant for ratios Met18/Met26 and Se18/Se26 (between 1.35 and 1.96) except for ratio Se18/Se26 for spot 3 (1.29). This latter result could indicate a reduced effect of high temperature on spot 3 expression when larvae were exposed to Se. We were unable to observe any difference between fish exposed to L-Met and to Se-L-Met at 18°C. However, Se stress decreased the expression of spot 2 at 26°C (ratio of 1.53 for Met26/Se26) indicating that the expression of this protein spot is only affected by co-occuring Se and heat stress. As for spot 28 in GS, spot 2 has been identified in WS as a 40S ribosomal protein SA on the basis of very high protein and peptide scores. Spot 3 has been identified as a protein disulfide isomerase with homology to Mus musculus, while spot 4 is a protein phosphatase 2 regulatory subunit A, beta isoform (total ion scores of 44.8 or 98.5 %).
4. Discussion
4.1. Effects of Se and heat stress on larval development
The patterns of larval mortality and morphological deformations observed during exposure experiments indicate that temperature stress was a strong inducer of notochord abnormality in both species but caused only a moderate mortality in GS. In contrast, microinjections of organic selenium to deliver a body burden of 8μg g-1 induced elevated mortality in WS and an even markedly higher mortality in GS. The mortality in both species did not significantly differ between Se18 and Se26 treatments, suggesting the lack of an additive effect of temperature stress on Se-induced mortality. Higher mortalities of GS in selenium treatments indicate higher sensitivity of this species to selenium stress, compared to WS. However, it should be pointed out here that due to the difficulty of breeding GS in captivity, only one family of offspring was tested in the experiment with GS, compared to three families in the experiment with WS.
Two types of stressor-specific abnormalities were observed in the exposure experiment. Temperature stress invariably induced kyphotic curvature of the larval body and, in some cases, curling inward reminiscent of embryo posture in chorion. Most larvae were able to swim but their swimming trajectories were greatly affected by body curvature, based on regular observations of larvae in tanks. The majority of larvae partially or completely recovered at stage D45, similar to observations of Werner et al. (2007). The abnormalities induced by selenium (Se18 and Se26) were more severe and suggestive of excessive selenium affecting body fluid homeostasis. In this context it should be noted that newly hatched sturgeon larvae do not have functional mesonephric kidneys until the stage D45 (Wrobel et al., 2002; Lepilina, 2007). Even if changes in the endothelium could contribute to larval edema in response to Se, it is possible that edema cannot be efficiently relieved due to the lack of an appropriate degree of development of osmoregulatory organs such as kidneys and gills.
4.2. Proteome responses
To examine effects of temperature and selenium at the molecular level, we investigated for the first time the protein expression profiles (PEP) of yolk sac larvae of green and white sturgeons exposed to two temperatures (18 and 26°C) and microinjected with Se-L-Met, and with L-Met as a control. We showed that a temperature change of 8°C is the first parameter affecting the PEP. In both species, the HCA analysis first discriminated gel replicates according to the temperature difference. The PEP was quantitatively more affected in GS than in WS. The power of this analysis was estimated to be close in both species, since an effect size of 0.93 and of 0.72 was possibly detected with a power of 80 % in GS and WS, respectively. These data indicate that the risk to miss significant results is about the same in the analysis for both species. It therefore makes sense to compare the total number of differentially expressed protein spots among the two species. However, the current proteomic analysis was carried out on a small fraction of the whole proteome, less than 600 detected protein spots. Less abundant proteins, with pI under 4 or over 7, or less soluble, must be investigated before drawing general conclusions on differential species responsiveness at the whole proteome level. Moreover, the higher mortality rate observed in GS larvae, along with the fact that GS accumulated less Se at D45 stage, indicate that the selenium exposure could exert a higher selection pressure on GS than on WS larvae. It suggests that the survivors of GS are the larvae which accumulated less Se. This fact could at least partly explain why proteomic results were more significant in GS larvae, since PEPs were analysed on surviving animals only.
4.3. Effects of temperature
In this study we have identified proteins that are differentially expressed depending on temperature. In GS, FK506-binding protein 10 and 40S ribosomal protein SA were down-regulated at 26°C compared to 18°C. The same observation has been made in WS for 40S ribosomal protein SA. This latter protein is required for the assembly and/or stability of the 40s ribosomal subunit. Its under-expression indicates that mRNA-directed protein synthesis is impaired at 26°C in both sturgeon species. Temperature is known to interfere with protein synthesis, and the ribosome biogenesis is temperature-dependent in bacteria (Refaii and Alix, 2009). Working on Zebra fish, Connolly and Hall (2008) recently suggested that both transcription and translation are regulated in response to environmental stress such as high temperature. The evaluation of stress-mediated aberrations in translational processes has been proposed to be used as a supplement to the repertoire of techniques for biomonitoring pollution in aquatic organisms (Kalpaxis et al., 2003). The FK506-binding protein 10 is localized in the lumen of the endoplasmic reticulum (ER). It encompasses four peptidylprolyl isomerase domains (PPIase) which accelerate protein folding by catalyzing the cis-trans isomerization of proline imidic peptide bonds in oligopeptides. It is known that PPIase activity can facilitate protein folding of proteins containing proline residues with a cis conformation at low temperature. Furthermore, it has been shown in bacteria that a protein belonging to a similar family (FKBP family) was up-regulated at 4°C compared to 20°C (Suzuki et al., 2004). In GS, the FK506-binding protein 10 was over-expressed at the lower temperature, which is consistent with the assumption that it enhances the efficiency of protein folding at 18°C, while it is no longer efficient at 26°C.
In WS, the 65 kDa regulatory subunit A of protein phosphatase 2 (PR65) was down-regulated in larvae exposed to 26°C. This protein contains the tandem repeat module HEAT which appears to function as a protein-protein interaction surface. The PR65 protein is the scaffolding subunit A of the protein phosphatase 2A (PP2A) enzyme, a highly conserved Ser/Thr phosphatase (Xu et al., 2006). A total absence or a substantial reduction of this scaffolding subunit had been reported to be linked to a variety of primary human tumors (Xu et al., 2006). PP2A is known to dephosphorylate and inactivate the extracellular signal-regulated kinase (ERK) pathway implicated in the regulation of cell proliferation and differentiation. Kim et al. (2005) showed that heat treatment of a mouse melanocyte cell-line inactivated PP2A, inducing sustained ERK activation, therefore reducing melanin synthesis. In WS, further investigations will test the hypothesis that, likewise, heat treatment could decrease PP2A activity, possibly leading to alteration of cellular functions via sustained ERK activation.
Another identified protein, protein disulfide isomerase (PDI), was down-regulated in WS larvae exposed to 26°C. PDI is located in the ER and is known to catalyse the formation, reduction and isomerisation of disulfide bonds, which is essential for correct folding, stability, and/or multimerization of many proteins (Laboissiere et al., 1995). It acts as a molecular chaperone and has the ability to bind to polypeptide chains, increasing the yield of correctly folded proteins (Noiva et al., 1993). As other molecular chaperones, it is synthesized in response to the unfolded protein response pathway (UPR) in the ER lumen (Malhotra and Kaufman, 2007). The data in the present study do not support the hypothesis that high temperature exposure would trigger an UPR due to an accumulation of unfolded or misfolded proteins in the ER lumen. Rather, the down-regulation of PDI at 26°C suggests a diminished capacity for mounting the UPR, which would provide an explanation for the observed occurrence of mortality and developmental abnormalities. Selenium had no significant effect on PDI abundance, consistent with its lack of an effect in the liver of rats subjected to Se deficiencies (Arthur et al., 1991). Unlike mercury, arsenic, lead and cadmium, for which a high induction of PDI has been observed in Chinese crab gills following chronic exposure (Silvestre et al., 2006), selenium is not a sulfhydryl-reactive element potentially disrupting numerous proteins through direct binding to sulfhydryl groups (Quig, 1998), and necessitating PDI as a molecular chaperone.
Other proteins exhibited a higher expression level in GS larvae exposed to 26°C, such as a stress-induced-phosphoprotein 1 (STIP1), suppression of tumorigenicity 13 (ST13), and HSP90 beta. The first two proteins contain a tetratrico peptide repeat motif (TPR) mediating protein-protein interactions and the assembly of multiprotein complexes. STIP1 is a HSP70/HSP90-organizing protein, while ST13 is a HSP70 interacting protein. They belong to the large group of co-chaperones, which regulate and assist major chaperones. The over-expression of HSP90 under the same conditions strengthens the fact that at high temperature, sturgeon larvae endure protein misfolding events, necessitating more chaperone activities to counteract this effect (Lepock, 2005). It is consistent with observations of Werner et al. (2007) who showed the over-expression of two members of the HSP70 family and a HSP89 in GS exposed to heat stress.
Collagen, type II, alpha 1 was over-expressed at 26°C compared to 18°C in GS. Collagens are major structural proteins of extracellular matrix involved in the formation of connective tissue and crucial for morphogenesis during development (Cheah et al., 1991). Type II collagen has been historically recognized as specific for cartilaginous tissue and as essential for the normal embryonic development of the skeleton. Collagen type II mRNA was over-expressed at high temperature in articular cartilage of other vertebrates (Tonomura et al., 2008). In chicken, reducing or elevating the incubation temperature in the early stages of embryo development caused a major increase in collagen type II gene expression in the articular cartilage, which was associated with tibial dyschondroplasia lesions (Yalçin et al., 2007). Collagen type II, alpha 1 purified from sturgeon notochord was characterized by chromatography and found to be chemically identical to that in sturgeon cartilage and similar to type II collagen of higher vertebrates (Miller and Mathews, 1974; Miller and Gay, 1987). Van Eenennaam et al. (2005) showed that GS embryos developed a high rate of axial skeleton deformities when hatched at high temperature. The prevailing abnormalities were lordosis and kyphosis which affected mobility of hatched embryos. Werner et al. (2007) described kyphotic curvature in the GS larvae as a characteristic abnormality induced by high temperature stress, which was also observed in this study and was associated with apparent loss of integrity by the collagen sheath of notochord. We suggest that collagen type II over-expression in GS larvae exposed to high temperature could be used as an indicator of temperature stress and resulting morphological abonormalities in sturgeon.
4.4. Effects of selenium
Effects of Se on the aquatic food web have been reviewed by Hamilton (2004). Bio-concentration of Se in the food chain via benthic organisms has been associated with SF Bay Delta fish population declines (Hamilton, 1999). The expected flow increase from the San Joaquin River to the SF Bay will likely raise Se levels in the estuary. Along with increases of Se in sturgeon food organisms (Presser and Luoma, 2000), this will likely raise selenium concentration in fish tissue and eggs to the levels that would be toxic for larvae, possibly impairing reproduction and stock recruitment (Kroll and Doroshov, 1991). It is known that excess Se in the egg yolk is toxic for the embryos and the yolk-sac larvae of teleosts (Holm et al., 2005). A significant decrease in swimming activity and growth rate (Tashjian et al., 2006) as well as an impairment of the osmoregulatory capacity (Tashjian et al., 2007) were reported in WS juveniles fed with 41.7 μg Se g-1 diet. In WS juveniles, the threshold dietary Se toxicity concentration was estimated to be between 10 and 20 μg g-1 diet (Tashjian et al., 2006). In the present study, both species exhibited abnormalities, bent notochord at high temperature and edema in selenium treatment, but some larvae have recovered at the stage D45. We chose to analyse the protein expression patterns in larvae that presented no obvious abnormalities at stage D45 but could have been affected at the early stages. A proteomic approach is sensitive and has the potential to point out mechanisms of action of xenobiotics before effects at higher levels of organization can be observed, such as morphological or physiological levels. Under these conditions, this study is the first to demonstrate that Se affects the PEP in sturgeon larvae, possibly triggering mechanisms involved in abnormal development.
The decrease in abundance of a valosin containing protein (VCP) in sturgeon larvae exposed to Se-L-Met, in both 18°C and 26°C treatments, indicates a high sensitivity of this protein to Se exposure, suggesting that it could be used as a biomarker of exposure to this element. VCP is a ring-shaped homohexamer member of the ATPase family associated with diverse cellular activities (AAA ATPases). AAA ATPases exert their activity through the energy dependent unfolding of macromolecules, performing chaperone-like functions in the assembly, operation, or disassembly of protein complexes (Neuwald et al., 1999). VCP is known to bind ubiquitinated proteins and participates in transportating ubiquitinated proteins to the aggresome, a cellular organelle in which ubiquitinated and unfolded proteins accumulate (Kopito, 2000). When the capacity of the intracellular protein degradation machinery is exceeded, ubiquitinated protein aggregates are transported to aggresomes via microtubules (Johnston et al., 2002). Song et al. (2008) showed that cadmium is an effective inducer of aggresome formation, and that VCP plays a critical role in this formation. To our knowledge, no data exists concerning a similar role of Se exposure. RNA interference of VCP used in insect and human cells caused significant accumulation of ubiquitinated proteins in multiple small, dispersed cytoplasmic aggregates, but not in the aggresome, suggesting that VCP is required for the formation of this organelle (Wójcik et al., 2004). In GS, we showed a down-regulation of VCP following Se treatment at both 18 and 26°C. This suggests a possible modification of the cellular unfolded protein detoxification machinery and a possible impairment of protein quality control. Though we could not find any indication of modification of the UPS (Ubiquitin-Proteasome System), further studies should investigate this pathway, as well as the aggresome formation, as a possible target of Se toxicity.
4.5. Combined effects of temperature and selenium
It appears that two forms of serine/arginine repetitive matrix protein 1 were affected by Se exposure in GS. Moreover, we were able to point out an interaction between temperature and selenium exposure for this protein. The Se treatment and 18°C decreased expression of this protein but at 26°C Se had no effect. Panther classifies this protein as a RNA processing factor. Its PWI domain appears to have an RNA binding capability and has important functions in within splicing complexes. Serine/arginine rich protein (SR proteins) in animals function as essential splicing factors in constitutive pre-mRNA splicing and also regulate alternative splicing by influencing the splice site selection in a concentration-dependent manner (Manley and Tacke, 1996). To our knowledge, our study provides the first evidence for an effect of Se on the expression of this protein.
Creatine kinase plays an important role in vertebrate metabolism, catalyzing the reversible transfer of phosphate from ATP to creatine, generating phosphocreatine and ADP. It functions in buffering the ATP supply during periods of high energy demand by regenerating depleted ATP supplies (Ellington, 2001). Creatine kinase acivity has been shown to increase as high as 328 % as a consequence of high temperature in heart muscle of chicken (Bogin et al., 1996). It was shown in calves that the rises in plasma creatine kinase activity were prevented when they had consumed diets supplemented with Se (Arthur, 1988). In turkeys, muscle damage parameters including creatine kinase activity increased in Se deficient animals (Fischer et al., 2008), suggesting that Se decreases creatine kinase activity. Two spots identified as creatine kinase were significantly over-expressed in GS larvae at 26°C treatments compared to 18°C treatments. When the larvae were exposed to Se, their expression was lowered compared to L-Met at the same temperature (significant at 26°C). Therefore, we can conclude that exposure to Se is antagonistic to the effect of temperature on this enzyme. However, this antagonistic effect is stronger at 26°C than at 18°C, resulting in a modulation of the temperature effect by Se exposure. The same observation was made for a serine peptidase inhibitor, Kazal type 5 in GS. This latter protein belongs to a family inhibiting a number of serine proteases. This result indicates that at high temperature, proteolysis would be counter-acted with the consequence of a potential decrease in protein degradation. Our results also indicate that Se has the capacity to attenuate this effect, thus, possibly promoting an increase in protein degradation.
On the contrary, we observed that some effects of high temperature and Se can be additive. For instance, Se18 (compared to Met18) treatment did not significantly affect HSP90 levels, while Se26 treatment increased HSP90 abundance compared to Met26. It indicates that, when high temperature affects HSP90 expression, Se can trigger additional stress.
Overall, the HCA analysis in the present proteomic approach was well suited to group together gel replicates corresponding to different temperature and Se exposures, and showing similar protein expression profiles. It also permitted to group proteins showing the same pattern of expression in animals exposed to two environmental stresses. Using this approach, we were able to emphasize mechanisms of action of temperature and Se exposure, along with their combined effects. We observed that proteomes of GS and WS larvae were affected by both temperature stress and by Se exposure but in unique, discernible ways. Moreover, the GS, a species classified as threatened, was significantly more sensitive to temperature and Se stress than WS. Proteins involved in correct protein folding, protein synthesis, protein degradation, ATP supply and structural proteins showed significant changes in their abundance, mostly in GS. For the first time, we reported that a trace element Se could decrease the abundance of VCP, a protein involved in aggresome formation and in protein quality control. The fact that its variation in abundance was independent on temperature highlights the possibility to use VCP as a biomarker of Se exposure. The use of such biomarkers in GS would lead to a better health assessment of this species, and would give early warning signals to prevent population decline and monitor early effects of human interventions in the SF Bay Delta water system.
Acknowledgments
This project was supported by CALFED Science Program project number 1035, NSF grant IOS-0542755 and NIH-NIEHS grant 5 P42 ES004699. The authors thank Dr Wes Dowd, UC Davis, for MS/MS analysis. Dr Frederic Silvestre is supported by the Fonds de la Recherche Scientifique FNRS, Belgium, as a post-doc researcher, and by a Fulbright visitor scholarship. Two commercial sturgeon farms, Sterling Caviar (Wilton CA) and Lazy Q Fish Ranch (Dixon CA), provided three progenies of WS for the experiment. Joel Van Eenennaam, Department of Animal Science, UC Davis, conducted hatchery spawning of GS on campus and organized supply of WS larvae from sturgeon farms. Larry Melton, Toxicology Section, California Animal Health and Food Safety Lab System, School of Veterinary Medicine, helped with selenium analysis of sturgeon larvae. All experiments with fish were conducted at CABA facilities, UC Davis.
List of abbreviations
- AAA ATPases
ATPases family Associated with diverse cellular Activities
- BCA
bicinchoninic acid
- CV
coefficient of variability
- D36 and D45
Dettlaff's stages of larval development
- DPS
distinct population segment
- ER
endoplasmic reticulum
- GS
green sturgeon
- HCA
hierarchical clustering analysis
- HCCA
α-cyano-4-hydroxycinnamic acid
- HSP
heat shock protein
- L-Met
L-Methionine
- NCBInr
National Center for Biotechnology Information
- PDI
protein disulfide isomerise
- PEP
protein expression profile
- PP2A
protein phosphatase 2A
- PPIase
peptidylprolyl isomerase domains
- PR65
65 kDa regulatory subunit A of protein phosphatase 2
- Se
selenium
- Se-L-Met
seleno-L-Methionine
- SF Bay Delta
San Francisco Bay and Sacramento-San Joaquin Delta
- SR protein
Serine/arginine rich protein
- ST13
suppression of tumorigenicity 13
- STIP1
stress-induced-phosphoprotein 1
- TPR
tetratico peptide repete motif
- UPR
unfolded protein response
- UPS
ubiquitin-proteasome system
- VCP
valosin-containing protein
- WS
white sturgeon
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arthur JR. Effects of selenium and vitamin E status on plasma creatine kinase activity in calves. J Nutr. 1988;118:747–55. doi: 10.1093/jn/118.6.747. [DOI] [PubMed] [Google Scholar]
- Arthur JR, Nicol F, Grant E, Beckett GJ. The effects of selenium deficiency on hepatic type-I iodothyronine deiodinase and protein disulphide-isomerase assessed by activity measurements and affinity labelling. Biochem J. 1991;274:1297–300. doi: 10.1042/bj2740297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow JM. Recent water temperature trends in the lower Klamath River, California. N Am J Fish Manage. 2005;25:152–62. [Google Scholar]
- Billard R, Lecointre G. Biology and conservation of sturgeon and paddlefish. Rev Fish Biol Fish. 2001;9:355–392. [Google Scholar]
- Bogin E, Peh HC, Avidar Y, Israeli BA, Kahaner A. The effects of long term high environmental temperature on cellular enzyme activities from different organs. Eur J Clin Chem Clin Biochem. 1996;34:625–29. doi: 10.1515/cclm.1996.34.8.625. [DOI] [PubMed] [Google Scholar]
- Calzolai L, Ansorge W, Calabrese E, Denslow N, Part P, Lettieri T. Transcriptomics and proteomics. Applications to ecotoxicology. Comp Biochem Physiol D. 2007;2:245–9. doi: 10.1016/j.cbd.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Cech JJ, Doroshov SI. Environmental requirements, preferences, and tolerance limits of North American sturgeons. In: LeBreton GTO, Beamish FWH, McKinley RS, editors. Sturgeons and paddlefish of North America. Kluwer Academic; Dordrecht: 2004. pp. 73–86. [Google Scholar]
- Chapman FA, VanEenennaam JP, Doroshov SI. The reproductive condition of white sturgeon, Acipenser transmontanus, in San Francisco Bay, California. Fish Bull. 1996;94:628–34. [Google Scholar]
- Cheah KSE, Lau ET, Au PKC, Tam PPL. Expression of the mouth α1(II) collagen gene is not restricted to cartilage during development. Development. 1991;111:945–53. doi: 10.1242/dev.111.4.945. [DOI] [PubMed] [Google Scholar]
- Connolly MH, Hall BK. Embryonic heat shock reveals latent hsp90 translation in zebrafish (Danio rerio). Int J Dev Biol. 2008;52:71–9. doi: 10.1387/ijdb.062241mc. [DOI] [PubMed] [Google Scholar]
- Conte FS, Doroshov SI, Lutes PB, Strange EM. Hatchery manual for the white sturgeon, Acipenser transmontanus, with application to other North American Acipenseridae. Division of Agriculture and Natural Resources, Publication # 3322. University of California Press; Oakland, CA: 1988. [Google Scholar]
- Cutter GA. The estuarine behavior of selenium in San-Francisco Bay. Estuar Coast Shelf Sci. 1989;28:13–34. [Google Scholar]
- Cutter GA, Cutter LS. Selenium biogeochemistry in the San Francisco Bay estuary: changes in water column behavior. Estuar Coast Shelf Sci. 2004;61:463–76. [Google Scholar]
- Cutter GA, Diego-McGlone ML. Temporal variability of selenium fluxes in the San Francisco Bay. Sci Total Environ. 1990:97235–250. [Google Scholar]
- Dettlaff TA, Ginsburg AS, Schmalhausen OI. Sturgeon fishes: developmental biology and aquaculture. Springer-Verlag; New York: 1993. [Google Scholar]
- Dowd W, Wood CM, Kajimura M, Walsh PJ, Kültz D. Natural feeding influences protein expression in the dogfish shark rectal gland: A proteomic analysis. Comp Biochem Physiol D. 2008;3:118–27. doi: 10.1016/j.cbd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Ellington WR. Evolution and physiological roles of phosphagen systems. Annu Rev Physiol. 2001;63:289–325. doi: 10.1146/annurev.physiol.63.1.289. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fischer J, Bosse A, Pallauf J. Effect of selenium deficiency on the antioxidative status and muscle damage in growing turkeys. Arch Anim Nutr. 2008;62:485–97. doi: 10.1080/17450390802453468. [DOI] [PubMed] [Google Scholar]
- Gillardin V, Silvestre F, Dieu M, Delaive E, Raes M, Thomé J, et al. Protein expression profiling in the African clawed frog Xenopus laevis tadpoles exposed to the polychlorinated biphenyl mixture aroclor 1254. Mol Cell Proteomics. 2009;8:596–611. doi: 10.1074/mcp.M800323-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SJ. Hypothesis of historical effects from selenium on endangered fish in the Colorado River Basin. Hum Ecol Risk Assess. 1999;5:1153–80. [Google Scholar]
- Hamilton SJ. Review of selenium toxicity in the aquatic food chain. Sci Total Environ. 2004;326:1–31. doi: 10.1016/j.scitotenv.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Hardy RS, Litvak MK. Effects of temperature on the early development, growth, and survival of shortnose sturgeon, Acipenser brevirostrum, and Atlantic sturgeon, Acipenser oxyrhynchus, yolk-sac larvae. Environ Biol Fish. 2004;70:145–54. [Google Scholar]
- Holm J, Palace V, Siwik P, Sterling G, Evans R, Baron C, et al. Developmental effects of bioaccumulated selenium in eggs and larvae of two salmonid species. Environ Toxicol Chem. 2005;24:2373–81. doi: 10.1897/04-402r1.1. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Illing ME, Kopito RR. Cytoplasmic dynein/dynactin mediates the assembly of aggresomes. Cell Motil Cytoskeleton. 2002;53:26–38. doi: 10.1002/cm.10057. [DOI] [PubMed] [Google Scholar]
- Kalpaxis DL, Amarantos I, Tsibouxi A, Papapetropoulou M. Regulation of translation initiation in mussels (Mytilus galloprovincialis) following contamination stress. J Toxicol Environ Health A. 2003;66:481–94. doi: 10.1080/15287390306452. [DOI] [PubMed] [Google Scholar]
- Kim D, Park S, Kwon S, Youn S, Park E, Park K. Heat treatment decreases melanin synthesis via protein phosphatase 2A inactivation. Cell Signal. 2005;17:1023–31. doi: 10.1016/j.cellsig.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Kimmel DG, Bradley BP. Specific protein responses in the calanoid copepod Eurytemora affinis (Poppe, 1880) to salinity and temperature variation. J Exp Mar Biol Ecol. 2001;266:135–49. [Google Scholar]
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–30. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Kroll KJ, Doroshov SI. Vitellogen: potential vehicle for selenium bioaccumulation in oocytes of the white sturgeon (Acipenser transmontanus). In: Williot P, editor. Acipenser. CEMAGREF Publications; Bordeaux: 1991. pp. 99–106. [Google Scholar]
- Laboissiere MC, Sturley SL, Raines RT. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J Biol Chem. 1995;270:28006–9. doi: 10.1074/jbc.270.47.28006. [DOI] [PubMed] [Google Scholar]
- Lee J, Valkova N, White MP, Kultz D. Proteomic identification of process and pathways characteristic of osmoregulatory tissues in spiny dogfish shark (Squalus acanthias). Comp Biochem Physiol D. 2006:328–43. doi: 10.1016/j.cbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lepilina IN. Development of mesonephros in prolarvae of Acipenserids (Acipenseridae). J Ichthyol. 2007;47:81–6. [Google Scholar]
- Lepock JR. How do cells respond to their thermal environment? Int J Hyperthermia. 2005;21:681–7. doi: 10.1080/02656730500307298. [DOI] [PubMed] [Google Scholar]
- Linville RG, Luoma SN, Cutter L, Cutter GA. Increased selenium threat as a result of invasion of the exotic bivalve Potamocorbula amurensis into the San Francisco Bay-Delta. Aquat Toxicol. 2002;57:51–64. doi: 10.1016/s0166-445x(01)00265-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Barea J, Gomez-Ariza JL. Environmental proteomics and metallomics. Proteomics. 2006;6:51–62. doi: 10.1002/pmic.200500374. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–31. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley JL, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–79. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- Meunier B, Dumas E, Piec I, Béchet D, Hébraud M, Hocquette J. Assessment of hierarchical clustering methodologies for proteomic data mining. J Proteome Res. 2007;6:358–66. doi: 10.1021/pr060343h. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:1–39. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Mathews MB. Characterization of notochord collagen as a cartilage-type collagen. Biochem Biophys res Commun. 1974;60:424–30. doi: 10.1016/0006-291x(74)90221-6. [DOI] [PubMed] [Google Scholar]
- Monsinjon T, Knigge T. Proteomic applications in ecotoxicology. Proteomics. 2007;7:2997–3009. doi: 10.1002/pmic.200700101. [DOI] [PubMed] [Google Scholar]
- Moyle PB. Inland Fishes of California. University of California Press; Berkeley, CA: 2002. [Google Scholar]
- Nesatyy VJ, Suter MJ. Proteomics for the analysis of environmental stress responses in organisms. Environ Sci Technol. 2007;41:6891–900. doi: 10.1021/es070561r. [DOI] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- NOAA (National Oceanic & Atmospheric Administration) Endangered and threatened wildlife and plants: proposed threatened status for southern distinct population segment of North American green sturgeon. Federal Register. 2005;70:17386–401. [Google Scholar]
- Noiva R, Freedman RB, Lennarz WJ. Peptide binding to protein disulfide isomerase occurs at a site distinct from the active sites. J Biol Chem. 1993;268:19210–7. [PubMed] [Google Scholar]
- NRC (National Research Council) Selenium mineral tolerance of animals. National Academy Press; Washington DC: 2005. [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–67. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Presser TS, Luoma SN. Forecasting selenium discharges to the San Francisco Bay-delta estuary: ecological effects of a proposed San Luis Drain extension. US Geological Survey; 2000. Open file report 00-416. [Google Scholar]
- Quig D. Cysteine metabolism and metal toxicity. Altern Med Rev. 1998;3:262–70. [PubMed] [Google Scholar]
- Refaii AA, Alix J. Ribosome biogenesis is temperature-dependent and delayed in Escherichia coli lacking the chaperones DnaK or DnaJ. Mol Microbiol. 2009;71:748–62. doi: 10.1111/j.1365-2958.2008.06561.x. [DOI] [PubMed] [Google Scholar]
- Saiki MK, Jennings MR, May TW. Selenium and other elements in freshwater fishes from the irrigated San Joaquin valley, California. Sci Total Environ. 1992;126:109–37. [Google Scholar]
- Satoh M, Haruta-Satoh E, Omori A, Oh-Ishi M, Kodera Y, Furudate SI, et al. Effect of thyroxine on abnormal pancreatic proteomes of the hypothyroid rdw rat. Proteomics. 2005;5:1113–24. doi: 10.1002/pmic.200401117. [DOI] [PubMed] [Google Scholar]
- Silvestre F, Dierick J, Dumont V, Dieu M, Raes M, Devos P. Differential protein expression profiles in anterior gills of Eriocheir sinensis during acclimation to cadmium. Aquat toxicol. 2006;76:46–58. doi: 10.1016/j.aquatox.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Song C, Xiao Z, Nagashima K, Li CH, Lockett SJ, Dai R, et al. The heavy metal cadmium induces valosin-containing protein (VCP)-mediated aggresome formation. Toxicol Appl Pharmacol. 2008;228:351–63. doi: 10.1016/j.taap.2007.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AR, Luoma SN, Schlekat CE, Doblin MA, Hieb KA. Food web pathway determines how selenium affects aquatic ecosystems: a San Francisco Bay case study. Environ Sci Technol. 2004;38:4519–26. doi: 10.1021/es0499647. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Haruki M, Takano K, Morikawa M, Kanaya S. Possible involvement of an FKBP family member protein from a psychrotrophic bacterium Shewanella sp. SIB1 in cold-adaptation. Eur J Biochem. 2004;271:1372–81. doi: 10.1111/j.1432-1033.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- Tashjian D, Cech J, Hung S. Influence of dietary l -selenomethionine exposure on the survival and osmoregulatory capacity of white sturgeon in fresh and brackish water. Fish Physiol Biochem. 2007;33:109–19. [Google Scholar]
- Tashjian DH, Teh SJ, Sogomonyan A, Hung SSO. Bioaccumulation and chronic toxicity of dietary L-selenomethionine in juvenile white sturgeon (Acipenser transmontanus). Aquat Toxicol. 2006;79:401–9. doi: 10.1016/j.aquatox.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Tonomura H, Takahashi KA, Mazda O, Arai Y, Shin-Ya M, Inoue A, et al. Effects of heat stimulation via microwave applicator on cartilage matrix gene and HSP70 expression in the rabbit knee joint. J Orthop Res. 2008;26:34–41. doi: 10.1002/jor.20421. [DOI] [PubMed] [Google Scholar]
- Urquhart KA, Regalado K. Selenium verification study, 1988- 1990. California State Water Resources Control Board Report; Sacramento, CA: 1991. [Google Scholar]
- Valkova N, Kültz D. Constitutive and inducible stress proteins dominate the proteome of the murine inner medullary collecting duct-3 (mIMCD3) cell line. Biochim Biophys Acta. 2006;1764:1007–20. doi: 10.1016/j.bbapap.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Van Eenennaam JP, Linares-Casenave J, Deng X, Doroshov SI. Effect of incubation temperature on green sturgeon embryos, Acipenser medirostris. Environ Biol Fish. 2005;72:145–54. [Google Scholar]
- Van Eenennaam JP, Linares-Casenave J, Muguet J-B, Doroshov SI. Induced spawning, artificial fertilization, and egg incubation techniques for green sturgeon. N Am J Aquacult. 2008;70:434–45. [Google Scholar]
- Wang YL, Buodington RK, Doroshov SI. Influence of temperature on yolk utilization by the white sturgeon, Acipenser transmontanus. J Fish Biol. 1987;30:263–71. [Google Scholar]
- Wang YL, Binkowski F, Doroshov SI. Effect of temperature on early development of white and lake sturgeon, Acipenser transmontanus and A. fulvescens. Environ Biol Fish. 1985;14:43–50. [Google Scholar]
- Waters MD, Fostel JM. Toxicogenomics and systems toxicology: aims and prospects. Nat Rev Genet. 2004;5:936–48. doi: 10.1038/nrg1493. [DOI] [PubMed] [Google Scholar]
- Werner I, Linares-Casenave J, Van Eenennaam J, Doroshov SI. The effect of temperature stress on development and heat-shock protein expression in larval green sturgeon (Acipenser mirostris). Environ Biol Fish. 2007;79:191–200. [Google Scholar]
- Wójcik C, Yano M, DeMartino GN. Interference of valosin-containing protein (VCP/p97) reveals multiple cellular roles linked. J Cell Sci. 2004;117:281–92. doi: 10.1242/jcs.00841. [DOI] [PubMed] [Google Scholar]
- Wrobel K-H, Hees I, Schimmel M, Stauber E. The genus Acipenser as a model system for vertebrate urogenital development : nephrostomial tubules and their significance for the origin of the gonads. Anat Embryol. 2002;205:67–80. doi: 10.1007/s00429-002-0228-y. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, et al. Structure of the protein phosphatase 2A holoenzyme. Cell. 2006;127:1239–51. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Yalçin S, Molayoglu HB, Baka M, Genin O, Pines M. Effect of temperature during the incubation period on tibial growth plate chondrocyte differentiation and the incidence of tibial dyschondroplasia. Poult Sci. 2007;86:1772–83. doi: 10.1093/ps/86.8.1772. [DOI] [PubMed] [Google Scholar]