Circulating κ and λ free light chains are associated with elevated risk of AIDS in human immunodeficiency virus (HIV)–infected individuals. Therefore, polyclonal B-cell dysfunction may play an important role in HIV-related immune suppression and predispose to clinical AIDS events.
Abstract
Background. The relevance of B-cell dysfunction for progression to AIDS among human immunodeficiency virus (HIV)–infected individuals has not been clearly defined. We evaluated the association between circulating κ and λ free light chains (FLCs), which are markers of B-cell dysfunction, and risk of developing an AIDS-defining opportunistic infection in HIV-infected men.
Methods. The study included 252 case patients with clinical AIDS and 252 HIV-infected controls from the Multicenter Hemophilia Cohort Study I. Case patients were matched to controls on birth date, specimen type, blood sample collection date, and CD4 cell count. Levels of κ and λ FLCs were measured in serum or plasma collected 0–2.5 years before selection. Elevated FLC levels (κ or λ, above the upper limit of normal) were classified as polyclonal (normal κ-λ ratio) or monoclonal (abnormally skewed κ-λ ratio). We used conditional logistic regression to estimate odds ratios (ORs) for AIDS.
Results. FLC levels were higher in case patients than in controls, for κ (median, 4.03 vs 2.98 mg/dL) and λ (3.77 vs 2.42 mg/dL) FLCs. Compared with normal levels, above-normal FLC levels were associated with AIDS (OR, 3.13 [95% confidence interval (CI), 1.78–5.49] for κ and 3.47 [2.31–5.20] for λ FLCs), and the association with AIDS was strengthened with increasing κ and λ FLC levels (P trends < .0001). Polyclonal FLC elevation was associated with a 4-fold increase in the risk of AIDS (OR, 3.85; 95% CI, 1.97–7.54), but monoclonal FLC elevation was not associated with AIDS.
Conclusions. Circulating FLCs are associated with elevated risk of AIDS in HIV-infected individuals. Polyclonal B-cell dysfunction may contribute to HIV-related immune suppression and predispose to clinical AIDS events.
Abnormally low counts of CD4 T cells have been used for years to identify human immunodeficiency virus (HIV)–infected individuals who are at increased risk of opportunistic infections and malignancies associated with AIDS. Although abnormal B-cell function has also long been recognized in HIV-infected individuals [1, 2], the relevance of these abnormalities for the development of AIDS is poorly defined. Circulating levels of one marker of B-cell activation, soluble CD30, have been associated with progression to AIDS in some studies [3–5] but not in all of them [6].
Immunoglobulin free light chains (FLCs) are produced by B cells along with intact immunoglobulins and have recently been used as markers of B-cell activation [7]. For example, the risk of non-Hodgkin lymphoma (NHL), a specific AIDS-defining condition, is elevated 4-fold with high serum or plasma levels of κ FLCs and 8-fold with high levels of λ FLCs in HIV-infected individuals [8]. However, it is unclear whether this association is specific to NHL or whether FLC levels are also predictive of other AIDS events. In the current case-control study, we evaluated the association between κ and λ FLC levels and nonmalignant AIDS events among HIV-infected men.
METHODS
We conducted a case-control study nested in the Multicenter Hemophilia Cohort Study I (MHCS-I). MHCS-I was initiated in 1982 to prospectively follow up persons with hemophilia or related coagulation disorders for incident HIV infection and HIV-associated outcomes, such as AIDS. Participants were enrolled from 8 US centers during 1982–1985 and 8 additional centers in the United States and Europe during 1987–1990. Semiannually, participants completed physical examinations, medical records were abstracted, and blood samples were collected. AIDS-defining events, specified in the 1993 Centers for Disease Control and Prevention definition of AIDS [9], were abstracted from medical records.
We selected 252 case patients with AIDS-defining opportunistic infections and 252 HIV-infected matched controls from the MHCS-I. We excluded women, because the vast majority of MHCS-I was male. We also excluded participants with Kaposi sarcoma or NHL (because of known associations between FLC levels and NHL [8]). Case patients were required to have had serum or plasma samples collected in the 0–2.5 years before AIDS diagnosis and a CD4 cell count at the time of blood sample collection. For each case patient, we selected a male control who was HIV infected and free of a clinical AIDS diagnosis on the date of the matched case patient's AIDS diagnosis (ie, index date). Controls were matched to case patients for the following characteristics: birth date (±10 years), material type of sample (plasma or serum), CD4 cell count category at blood sample collection (0–49, 50–99, 100–149, 150–199, 200–399, ≥400 cells/mm3), and date of blood sample collection (within 2.5 years before the index date). If >1 sample was available in the 0–2.5-year window with a matching CD4 cell count, we selected the sample that was closest to the middle of the interval.
The κ and λ FLC levels were measured on a SPA PLUS (Special Protein Analyzer) platform using Freelite reagents (Binding Site Ltd). The assay separately measures κ FLC levels (normal range defined by assay manufacturer, 0.33–1.94 mg/dL) and λ FLC levels (normal range, 0.57–2.63 mg/dL) [10]. Using 12 paired quality control samples, we estimated the mean within-batch coefficients of variation to be 1.7% for κ and 2.4% for λ FLCs.
Levels of κ and λ FLCs were assessed based on their relation to the upper limit of the normal range (ULN) (1.94 mg/dL for κ and 2.63 mg/dL for λ FLCs). Measured FLCs were categorized as above or below the ULN, based on multiples of the ULN. Additionally, the ratio of κ to λ FLC levels was classified as normal (0.26–1.65 mg/dL) or abnormal (outside this range). An abnormal ratio indicates an excess of one FLC and suggests the presence of a monoclonal B-cell population. Therefore, we classified FLC elevations (κ or λ FLC levels above ULN) based on this ratio, as monoclonal (abnormal κ-λ ratio) or polyclonal (normal κ-λ ratio) [11].
We used conditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for AIDS, comparing persons with κ and λ FLC levels above or below the ULN and across categories (ie, normal or 1.01–1.49, 1.50–1.99, or ≥2.00 times the ULN). Persons with monoclonal and polyclonal elevations were compared with persons who did not have elevated levels of either FLC. Because of the matched design, all analyses were adjusted for age, type of sample, CD4 cell count category at the blood sample collection date and the date of blood sample collection. A subanalysis additionally adjusted for time since estimated date of HIV infection [12] in 225 matched case-control pairs with available data. Using unconditional logistic regression, additional analyses were stratified by time between blood sample collection and index date (0, 0.1–1.0, or 1.1–2.5 years), with adjustment for matching criteria. For control subjects, we also used logistic regression to measure associations of FLC levels with age, CD4 cell count, and HIV viral load at sample collection.
RESULTS
The study included 252 case patients with AIDS and 252 HIV-infected controls (Table 1). Case patients and controls were predominantly white, and the median age at index date was 31 years for both groups. The median index date was 1992, and with three-quarters of the index dates before 1995 (Table 1), very few men would have been treated with highly active antiretroviral therapy (HAART). CD4 cell counts were similar in case patients and controls at the sample collection date (median, 121 vs 125 cells/mm3), owing to the matched design, but were lower in case patients when measured closest to the index date (51 vs 121 cells/mm3). Among case patients with AIDS, the most common AIDS diagnoses were Pneumocystis pneumonia (31.0%), candidiasis (17.9%) and wasting syndrome (10.3%).
Table 1.
Characteristics of Case Patients With AIDS and Controls in Multicenter Hemophilia Cohort Study
| Characteristic | Controls (n = 252) | Case Patients With AIDS (n = 252) |
|---|---|---|
| Age at index date, median (IQR), years | 31 (24–38) | 31 (23–40) |
| Ethnicity, No. (%) | ||
| White | 210 (83.3) | 205 (81.4) |
| Black | 29 (11.5) | 27 (10.7) |
| Other | 13 (5.2) | 20 (7.9) |
| Year of index date, median (IQR) | 1992 (1990–1994) | 1992 (1990–1994) |
| CD4 cell count at sample date, median (IQR), cells/mm3, | 125 (40–285) | 121 (38–230) |
| CD4 cell count closest to index date, median (IQR), cells/mm3 | 121 (40–306) | 51 (16–136) |
| AIDS diagnoses, No. (%) | ||
| Pneumocystis pneumonia | 78 (31.0) | |
| Candidiasis | 45 (17.9) | |
| Wasting syndrome | 26 (10.3) | |
| Mycobacterial infection (excluding tuberculosis) | 18 (7.1) | |
| Dementia | 16 (6.4) | |
| Cytomegalovirus disease | 14 (5.6) | |
| Cryptococcosis | 14 (5.6) | |
| Other | 41 (16.3) | |
| Interval from blood collection to index date, No. (%) | ||
| 0 years | 54 (21.4) | 59 (23.4) |
| 0.1–1 years | 100 (39.7) | 98 (38.9) |
| 1.1–2.5 years | 98 (38.9) | 95 (37.7) |
| FLC level, median (IQR), mg/dL | ||
| κ FLCsa | 2.98 (1.95–4.53) | 4.03 (2.87–6.09) |
| λ FLCsa | 2.42 (1.59–3.82) | 3.77 (2.46–5.52) |
Abbreviations: FLC, free light chain; IQR, interquartile range.
a Median values were significantly different between cases and controls (P < .0001). The median differences in matched case-control sets were 0.99 mg/dL for κ FLCs and 1.12 mg/dL for λ FLCs.
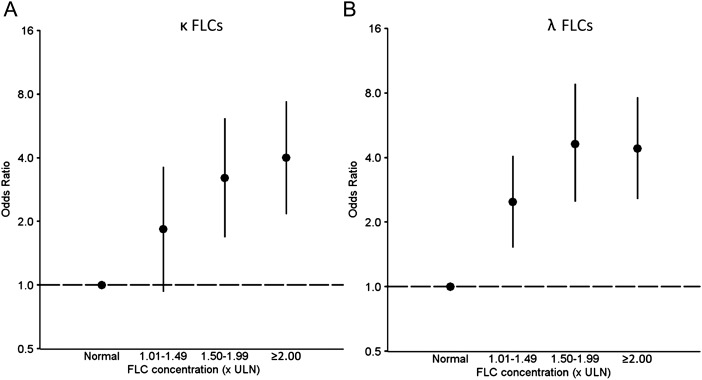
As shown in Table 1, FLC levels were significantly higher in case patients than controls, for both κ (median, 4.03 vs 2.98 mg/dL; P < .0001) and λ (3.77 vs 2.42 mg/dL; P < .0001). The median differences in matched case-control sets were 0.99 mg/dL for κ and 1.12 mg/dL for λ FLCs. Thus, AIDS was more common in HIV-infected individuals with above-normal κ FLC levels (OR, 3.06; 95% CI, 1.74–5.39) or λ FLC levels (OR, 3.47; 95% CI, 2.31–5.20), and this association became stronger with increasing κ and λ FLC levels (P trends < .0001; Figure 1). Polyclonal FLC elevations, but not monoclonal elevations, were strongly associated with AIDS (OR, 3.98; 95% CI, 2.14–7.40) (Table 2).
Figure 1.
Associations of circulating free light chains (FLCs) with risk of AIDS among men with human immunodeficiency virus (HIV) infection. This figure presents the association between the risk of AIDS in HIV-infected men and levels of κ (A) or λ (B) FLCs. FLC results are expressed relative to the upper limit of normal (1.94 mg/dL for κ and 2.63 mg/dL for λ FLCs). Points represent odds ratios; vertical lines, 95% confidence intervals. Abbreviations: FLC, free light chain; ULN, upper limit of normal.
Table 2.
Associations of Circulating Free Light Chain Elevations With AIDS Among Men With HIV Infection
| Type of FLC Elevation | Odds Ratio (95% CI) |
|||
|---|---|---|---|---|
| Overall | Stratified on Interval Between Sample Collection Date and Index Date |
|||
| 0 Years | 0.1–1 Years | 1.1–2.5 Years | ||
| κ FLCs | ||||
| Normal | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Above ULN | 3.06 (1.74–5.39) | 11.3 (2.95–42.9) | 1.47 (.72–3.00) | 2.28 (.96–5.37) |
| λ FLCs | ||||
| Normal | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Above ULN | 3.47 (2.31–5.20) | 6.00 (2.58–14.0) | 3.84 (2.06–7.15) | 2.46 (1.36–4.44) |
| Clonality | ||||
| Normala | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Monoclonal | 1.24 (.58–2.67) | 4.22 (.52–34.3) | .36 (.10–1.30) | 1.04 (.35–3.14) |
| Polyclonal | 3.98 (2.14–7.40) | 17.1 (3.50–83.7) | 1.87 (.87–4.01) | 3.07 (1.23–7.63) |
Abbreviations: CI, confidence interval; FLC, free light chain; ULN, upper limit of normal.
a Reference group includes subjects without elevated κ or λ FLCs; excluded were 2 case patients and 5 controls without elevated κ or λ FLC levels but with abnormal FLC ratios.
After further adjustment for estimated duration of HIV infection in a subset of case patients and controls (225 case-control pairs), the associations were consistent but attenuated (ORs as follows: elevated κ FLC levels, 2.32 [95% CI, 1.27–4.25]; elevated λ FLC levels, 2.76 [1.82–4.19]; monoclonal elevation, 0.92 [.41–2.04]; and polyclonal elevation, 2.94 [1.54–5.61]). Results did not change when the analysis was restricted to subjects selected in the pre-HAART era (1996 or earlier), and results were similar for patients with Pneumocystis pneumonia examined separately (ORs as follows: elevated κ FLC levels, OR, 3.00 [95% CI, 1.19–7.56]; elevated λ FLC levels, 4.63 [2.15–9.93]; monoclonal elevation, 0.68 [.15–3.10]; and polyclonal elevation, 4.25 [1.48–12.2]).
When stratified by time from blood sample collection date to index date, the strongest associations were observed when FLC levels were measured at the index date (ie, latency of 0 years) (Table 2). Significant associations were also observed between above-normal λ FLC levels and AIDS for samples obtained 0.1–1.0 years (OR, 3.84; 95% CI, 2.06–7.15) and 1.1–2.5 years (OR, 2.46; 95% CI, 1.36–4.44) before the index date and polyclonal FLC elevation at 1.1–2.5 years before the index date (OR, 3.07; 95% CI, 1.23–7.63). Nonetheless, the associations with AIDS for above-normal κ or λ FLC levels or for monoclonal or polyclonal elevation did not vary significantly according to duration of time since FLC measurement (range of P interactions, .09–.94).
Among controls, older age at sample collection date was associated with monoclonal and polyclonal FLC elevations, and a low CD4 cell count was associated with above-normal λ FLC levels, and polyclonal and monoclonal FLC elevation (Table 3). No associations were observed between duration of HIV infection and FLC levels. Furthermore, no associations were observed between HIV viral load and FLC levels, though this analysis was based on only 82 controls with viral load data (not shown).
Table 3.
Associations With Circulating Free Light Chains Among Control Subjects With HIV Infection
| FLC Result | Odds Ratio (95% CI)a |
|
|---|---|---|
| Age (Per 5 Years) | CD4 Cell Count (Per 50 Cells/mm3) | |
| κ FLC levels above ULN | 1.07 (.95–1.21) | .96 (.91–1.02) |
| λ FLC levels above ULN | 1.00 (.89–1.11) | .89 (.83–.96) |
| Monoclonal FLC elevationb | 1.14 (1.00–1.31) | .95 (.89–1.01) |
| Polyclonal FLC elevationb | 1.16 (1.04–1.30) | .94 (.90–.98) |
Abbreviations: CI, confidence interval; FLC, free light chain; ULN, upper limit of normal.
a Odds ratios for age and CD4 cell count are mutually adjusted for each other.
b Reference group includes subjects without elevated κ or λ FLC levels; excluded were 2 case patients and 5 controls without elevated κ or λ FLC levels but with abnormal FLC ratios.
DISCUSSION
Although B-cell dysfunction is well-documented in HIV infection [1], prior studies have not established whether it is associated with future risk of developing AIDS. Circulating levels of soluble CD30, a marker of B-cell dysfunction, were not associated with AIDS risk in 1 study [6], but 2 other studies found an increased risk of AIDS with increasing soluble CD30 levels [3, 4]. In the present investigation, we observed a significant association of AIDS with elevated levels of κ and λ FLCs and with polyclonal FLC elevation, indicating that elevated FLCs are associated with a range of opportunistic infections as AIDS events. Significant associations were observed for λ FLCs and polyclonal FLC elevation in samples collected 1.1–2.5 years before AIDS, suggesting that B-cell dysfunction precedes and predicts development of AIDS.
During immunoglobulin production, more light chains are produced than heavy chains, and excess FLCs enter circulation where they can be measured in the blood [7]. FLCs are therefore markers of nonspecific polyclonal B-cell activation and hypergammaglobulinemia [1, 7]. The spectrum of B-cell hyperactivity in HIV-infected individuals includes increased polyclonal B-cell activation, cell turnover, expression of activation markers, differentiation of B cells to plasmablasts, production of autoantibodies, and hypergammaglobulinemia [2]. B-cell dysfunction and activation are driven by continuous HIV replication and the constant effort of the immune system to clear HIV infection [2, 13]. The secretion of proinflammatory cytokines, including interleukin 6, 10, and 15, also contribute [2, 13].
We observed an inverse association between CD4 cell count and FLC levels. This observation is consistent with a role of prolonged HIV infection and T-cell depletion in causing B-cell dysfunction in HIV-infected individuals. Furthermore, the prevalence of polyclonal FLC elevations among our subjects increased with age. This finding is of interest, because B-cell function declines with age [14], and the prevalence of monoclonal gammopathy of undetermined significance, including the newly recognized light chain subtype, increases [15, 16]. In this context, the association between older age and B-cell dysfunction in HIV-infected individuals may become more important as the HIV-infected population ages, potentially resulting in an increased occurrence of diseases associated with B-cell dysfunction, such as bacterial infections, NHL [8], and, as we demonstrate here, AIDS.
Soluble CD30 has been shown to be associated with future risk of NHL, a specific AIDS-defining event [5]. In addition, in our prior study that used FLC levels to measure B-cell dysfunction, elevated FLC levels were also associated with increased NHL risk [8]. However, the results of the current study show that the association between FLC levels and AIDS-defining events is not specific to NHL but that FLC levels are also associated with a number of AIDS-defining opportunistic infections. Furthermore, the associations between FLC levels and NHL assessed in the prior study are similar in magnitude to those observed between FLC levels and AIDS-defining opportunistic infections [8].
Our findings may support a model whereby chronic B-cell dysfunction contributes to HIV-related immunosuppression and the development of clinical AIDS. An association between B-cell dysfunction and infections has been demonstrated in other diseases. For example, patients with chronic lymphocytic leukemia, a malignancy that results in chronic dysfunction of B-cells, are at an increased risk of a number of infections [17, 18], although these infections are not entirely consistent with those that define AIDS in HIV-infected individuals. Alternatively, it is possible that high levels of FLCs are indirect markers of T-cell dysfunction or HIV viremia.
The main strength of our study was its nested case-control design with serum or plasma specimens available before diagnosis for a substantial fraction of case patients with AIDS, which allowed us to assess the longitudinal relationship between B-cell dysfunction and subsequent AIDS risk. Although we observed significant associations between B-cell dysfunction and AIDS 1.1–2.5 years before AIDS diagnosis, it is still possible that developing or undiagnosed AIDS events may have caused the elevations in FLC levels, rather than the elevation of FLC levels preceding the AIDS events. Further limitations were the lack of HIV viral load data (missing in 65% of subjects), and the lack of data from the HAART era. Because HIV viral load data were missing for the majority of case patients and controls, we were unable to assess whether FLC levels are associated with AIDS independent of HIV replication.
Because our analysis was primarily limited to the pre-HAART era, it is unknown whether the associations between serum FLC levels and AIDS risk would remain in HIV-infected individuals treated with HAART. Treatment with HAART dramatically reduces the risk of AIDS [19], and suppression of HIV replication with effective treatment reverses many, but not all, B-cell abnormalities [2]. Furthermore, recent HIV treatment guidelines suggest that most HIV-infected patients would benefit from receiving HAART, particularly if the CD4 cell count is <500 cells/mm3 [20]. Because more than half of HIV-infected individuals in the United States present for care with CD4 cell counts <350 cells/mm3 [21], HAART would probably be initiated immediately for most patients, without regard for markers of B-cell dysfunction. Thus, it is unclear whether measurement of FLC levels would have clinical utility in identifying HIV-infected individuals at highest risk of developing AIDS and therefore at greatest need of HAART initiation.
In conclusion, circulating FLCs are associated with the risk of developing AIDS in HIV-infected individuals. Our findings highlight the importance of B-cell dysfunction in contributing to immune suppression. FLC levels may be a useful predictor of AIDS risk in treatment-naive patients, but additional work is needed to confirm their utility in the HAART era.
Notes
Acknowledgments. The authors thank the staff at the National Heart, Lung, and Blood Institute for their assistance with dataset preparation and specimen selection.
Financial support. This work was supported by the Intramural Research Program of the National Cancer Institute.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 2.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzolo G, Vinante F, Morosato L, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–5. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Rizzardi GP, Tambussi G, Barcellini W, et al. Soluble CD30, tumour necrosis factor (TNF)-alpha, and TNF receptors in primary HIV-1 infection: relationship with HIV-1, RNA, clinical outcome and early antiviral therapy. J Biol Regul Homeost Agents. 1997;11:43–9. [PubMed] [Google Scholar]
- 5.Breen EC, Fatahi S, Epeldegui M, Boscardin WJ, Detels R, Martinez-Maza O. Elevated serum soluble CD30 precedes the development of AIDS-associated non-Hodgkin's B cell lymphoma. Tumour Biol. 2006;27:187–94. doi: 10.1159/000093022. [DOI] [PubMed] [Google Scholar]
- 6.Sabin CA, Bofill M, Phillips AN, Elford J, Janossy G, Lee CA. Relation between soluble CD30 levels measured soon after HIV seroconversion and disease progression in men with hemophilia. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:279–83. doi: 10.1097/00042560-199712010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Hutchison CA, Landgren O. Polyclonal immunoglobulin free light chains as a potential biomarker of immune stimulation and inflammation. Clin Chem. 2011;57:1387–9. doi: 10.1373/clinchem.2011.169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landgren O, Goedert JJ, Rabkin CS, et al. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol. 2010;28:773–9. doi: 10.1200/JCO.2009.25.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 10.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–44. [PubMed] [Google Scholar]
- 11.Maurer MJ, Cerhan JR, Katzmann JA, et al. Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia. Blood. 2011;118:2821–6. doi: 10.1182/blood-2011-04-349134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroner BL, Rosenberg PS, Aledort LM, Alvord WG, Goedert JJ. HIV-1 infection incidence among persons with hemophilia in the United States and western Europe, 1978–1990. Multicenter Hemophilia Cohort Study. J Acquir Immune Defic Syndr. 1994;7:279–86. [PubMed] [Google Scholar]
- 13.De Milito A. B lymphocyte dysfunctions in HIV infection. Curr HIV Res. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 14.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10:330–5. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 17.Molica S, Levato D, Levato L. Infections in chronic lymphocytic leukemia. Analysis of incidence as a function of length of follow-up. Haematologica. 1993;78:374–7. [PubMed] [Google Scholar]
- 18.Ravandi F, O'Brien S. Immune defects in patients with chronic lymphocytic leukemia. Cancer Immunol Immunother. 2006;55:197–209. doi: 10.1007/s00262-005-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 20.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012 Rockville, MD: US Department of Health and Human Systems Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf . Accessed 20 August 2012. [Google Scholar]
- 21.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–20. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]