Immediate treatment initiation is optimal for patients aged <30 years regardless of CD4 count under a wide range of assumptions about treatment benefits and harms, whereas the timing of initiation at older ages depends on the assumed magnitude of the cardiovascular risk from treatment and patient preferences toward treatment.
Abstract
We developed a mathematical model to identify the timing of antiretroviral therapy (ART) initiation that optimizes patient outcomes as a function of patient CD4 count, age, cardiac mortality risk, sex, and personal preferences. Our goal was to find the conditions that maximize patient quality-adjusted life expectancy (QALE) in the context of our model. Under the assumption that ART confers disease progression and mortality benefits at any CD4 count, immediate treatment initiation yields the greatest remaining QALE for young patients under most circumstances. The timing of ART initiation depends on the magnitude of benefit from ART at high CD4 counts, the magnitude of increases in cardiac risk, and patients' preferences. If ART reduces HIV progression at high CD4 counts, immediate ART is preferable for most newly infected individuals <35 years even if ART doubles age- and sex-specific cardiac risk.
Modern antiretroviral therapy (ART) has been the mainstay of treatment for human immunodeficiency virus (HIV) in the past decade, yet optimal timing of treatment initiation remains controversial. The pendulum has swung from “hit early, hit hard” in the late 1990s [1], to drug-conserving late initiation strategies [2, 3] at a CD4 cell count threshold of 350 cells/mm3, and now back to earlier initiation thresholds [4–8]. The Strategic Timing of AntiRetroviral Treatment (START) clinical trial [9] should provide guidance on the timing of ART initiation, but the results of this trial are likely years away.
The debate about whether to initiate ART for patients with a CD4 count >500 cells/mm3 hinges on the uncertainty about the balance of toxicities and benefits of ART at high CD4 counts. Several studies suggest that ART may have harmful cardiovascular effects. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) cohort studies found that exposure to protease inhibitors increased the relative rate of myocardial infarction by 16% per year of exposure [10–12]. Other studies found that exposure to abacavir was associated with an increased risk of cardiovascular events and heart failure [13]. On the other hand, the Strategies for Management of AntiRetroviral Therapy (SMART) study suggested that ART is associated with a reduction in cardiac mortality over short periods of time compared with ART discontinuation [14].
The benefits of ART at high CD4 cell counts are also unclear. Whereas Kitahata et al [15] found that the relative risk of death for patients who initiated ART at CD4 counts >500 cells/mm3 was lower than that of patients who deferred therapy until their CD4 count was <500 cells/mm3, the Concerted Action on SeroConversion to AIDS and Death in Europe (CASCADE) study did not observe a mortality benefit at the population level for initiation above 500 cells/mm3 [15, 16]. Other studies find that a low CD4 count is independently associated with an increase in non-HIV mortality, particularly cardiovascular mortality [17–20].
Previous model-based studies on timing of ART initiation have compared only a small number of strategies [21, 22] or have not incorporated patient characteristics such as age and sex [23, 24], characteristics that are central for making informed clinical decisions. No study has attempted to find the optimal strategy for initiating ART while considering age, sex, cardiac toxicities, and therapy initiation at CD4 counts >500 cells/mm3.
In this paper, we estimate the optimal time to initiate ART that maximizes quality-adjusted life expectancy (QALE) as a function of patient age, sex, CD4 count, and personal preferences about taking ART. Because the available clinical trials and cohort studies of ART are of relatively short duration [2, 4–8, 15, 25], we use a modeling approach to balance the long-term risks and benefits of ART exposure. We focus on the cardiovascular implications of long-term ART use because of their prominent role in non-HIV-related morbidity and mortality in infected individuals [10–13]. This problem is especially pertinent to regimens containing protease inhibitors, which are commonly used for both treatment-naive and treatment-experienced patients [26], and have been associated with increased risk of myocardial infarction [10]. Our work provides a general framework for determining optimal treatment initiation decisions for HIV-infected individuals in the face of competing risks from HIV and ART-associated toxicities.
METHODS
Overview
We developed a stochastic dynamic programming model to find the optimal ART initiation threshold that maximizes remaining QALE for men and women of different ages, CD4 counts, and preferences about taking ART. The model (Supplementary Figure 1) estimates the survival benefits of ART over an extensive range of possible cardiovascular risks. Treatment initiation was considered “optimal” for the patients if their remaining QALE was greater with immediate ART initiation than by delaying initiation and reevaluating the patient after 6 months. Table 1 lists values for all model parameters.
Table 1.
Model Parameters and Sources
| Variable | Base Value |
Source | |
|---|---|---|---|
| General population death rates | Varied by age and sex | [29] | |
| General population cardiac mortality rates | Varied by age and sex | [29] | |
| HIV 6-mo mortality rates with no treatment, by CD4 state | [34] | ||
| <50 | 0.1005 | ||
| 50–99 | 0.02 | ||
| 100–199 | 0.0108 | ||
| 200–349 | 0.006 | ||
| 350–499 | 0.0016 | ||
| 500–649 | 0.001 | ||
| ≥650 | 0.0008 | ||
| Average 6-mo decrease in CD4 count for patients not on treatment (cells/mm3) | 35.25 | [28] | |
| On treatment mortality and progression: “all benefits” case | |||
| HIV 6-month mortality rates on treatment, by CD4 state: | [33] | ||
| <50 | 0.0167 | ||
| 50–99 | 0.0119 | ||
| 100–199 | 0.0085 | ||
| 200–349 | 0.0039 | ||
| 350–499 | 0.0003 | ||
| 500–649 | 0.0002 | ||
| ≥650 | 0.0002 | ||
| Average 6-mo increase in CD4 count (cells/mm3) if treatment was started: | [37] | ||
| 6 mo ago | 100 | ||
| 12 mo ago | 50 | ||
| 18 mo ago | 40 | ||
| 24 mo ago | 40 | ||
| 30 mo ago | 25 | ||
| 36 mo ago | 20 | ||
| 42 mo ago | 20 | ||
| 48 mo ago | 0 | ||
| On treatment mortality and progression: “partial benefits” case | |||
| HIV 6-mo mortality rates on treatment, by CD4 state: | [33] | ||
| <50 | 0.0167 | ||
| 50–99 | 0.0119 | ||
| 100–199 | 0.0085 | ||
| 200–349 | 0.0039 | ||
| 350–499 | 0.0012 | ||
| 500–649 | 0.001 | ||
| ≥650 | 0.0008 | ||
| Average 6-mo increase in CD4 count (cells/mm3) if treatment was started: | [37] | ||
| 6 mo ago | 100 | ||
| 12 mo ago | 50 | ||
| 18 mo ago | 40 | ||
| 24 mo ago | 40 | ||
| 30 mo ago | 25 | ||
| 36 mo ago | 20 | ||
| 42 mo ago | 20 | ||
| 48 mo ago | 0 | ||
| On treatment mortality and progression: “no benefits above 500 cells/mm3” case | |||
| HIV 6-mo mortality rates on treatment, by CD4 state: | [33] | ||
| <50 | 0.0167 | ||
| 50–99 | 0.0119 | ||
| 100–199 | 0.0085 | ||
| 200–349 | 0.0039 | ||
| 350–499 | 0.0012 | ||
| 500–649 | 0.001 | ||
| ≥650 | 0.0008 | ||
| If current CD4 is <500 cells/mm3 | |||
| Average 6-mo increase in CD4 count (cells/mm3) if treatment was started: | [37] | ||
| 6 mo ago | 100 | ||
| 12 mo ago | 50 | ||
| 18 mo ago | 40 | ||
| 24 mo ago | 40 | ||
| 30 mo ago | 25 | ||
| 36 mo ago | 20 | ||
| 42 mo ago | 20 | ||
| 48 mo ago | 0 | ||
| If current CD4 is >500 cells/mm3 | |||
| Average 6-mo increase in CD4 count (cells/mm3) | −35.25 | [28] | |
| Health utilities for each CD4 state | [5, 13, 26] | ||
| Life-years maximizing | With treatment | Without treatment | |
| <50 | 1 | 1 | |
| 50–99 | 1 | 1 | |
| 100–199 | 1 | 1 | |
| 200–349 | 1 | 1 | |
| 350–499 | 1 | 1 | |
| 500–649 | 1 | 1 | |
| ≥650 | 1 | 1 | |
| ART dominating in quality of life at all CD4 counts <650 cells/mm3 | With treatment | Without treatment | |
| <50 | 0.82 | 0.7 | |
| 50–99 | 0.83 | 0.73 | |
| 100–199 | 0.84 | 0.76 | |
| 200–349 | 0.85 | 0.79 | |
| 350–499 | 0.86 | 0.82 | |
| 500–649 | 0.87 | 0.85 | |
| ≥650 | 0.88 | 0.88 | |
| ART-induced decrement in quality of life at highest CD4 state | With treatment | Without treatment | |
| <50 | 0.82 | 0.72 | |
| 50–99 | 0.83 | 0.75 | |
| 100–199 | 0.84 | 0.78 | |
| 200–349 | 0.85 | 0.81 | |
| 350–499 | 0.86 | 0.84 | |
| 500–649 | 0.87 | 0.87 | |
| ≥650 | 0.88 | 0.9 | |
All CD4 values are reported as cells/mm3.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
The approach allows solving complex decisions in which every combination of health states—including in this analysis age, CD4 count, and quality of life—is accounted for. For example, a dynamic programming model that tracked age in 6-month intervals between ages 20 and 100 for 7 CD4 cell count categories would be equivalent to a decision tree with 1134 health states [= 81 years × 2 (twice-yearly evaluations) × 7 CD4 categories]. Dynamic programming provides a tractable means of finding optimal solutions of such complex decision problems [27]. Life expectancy is calculated recursively. By that we mean that to compute the remaining life expectancy at the current age given a treatment decision, we first compute the life expectancy from the next assessment onward given this decision, discount it, multiply it by the probability of surviving until the next assessment, and then add it to the current expectation of surviving until the next evaluation. The Supplementary Data provides a specific representation of a dynamic programming model (Supplementary Figure 2) and its solution.
HIV Progression
A patient's HIV disease state was determined by CD4 count. Changes in CD4 count over time depended on duration of disease and treatment status. We discretized CD4 counts into 7 categories: <50, 50–99, 100–199, 200–349, 350–499, 500–649, and ≥650 cells/mm3 [15]. To operationalize the changes in CD4 count, we assumed that within each category, CD4 counts are uniformly distributed. Before treatment initiation, patients' CD4 cell counts decreased by 70.5 cells/mm3 annually on average [28]. We assumed that, once initiated, ART is continued for the duration of the patient's life. This assumption of continuous effective treatment is consistent with the variety of currently available ART drugs and drug classes [26].
Cardiovascular Risk
We decomposed cardiac mortality into ART-related mortality and age- and sex-specific mortality. We estimated the background cardiac mortality by multiplying age- and sex-specific mortality rates [29] by the age- and sex-specific proportion of deaths occurring due to cardiac disease [30]. Our data on the cardiovascular risk associated with ART were drawn from data on myocardial infarctions. We assumed that the rate of myocardial infarctions could be used as an upper limit on cardiac mortality, and used the rate of myocardial infarctions as a proxy for the cardiovascular death rate.
The evidence on the magnitude and longitudinal pattern of the association between ART and cardiovascular events is inconclusive. One D:A:D study suggests a fixed multiplicative risk increase [31]; another suggests a linearly increasing risk for each year on treatment [10]. We based the increased cardiovascular risk associated with ART on published estimates suggesting a constant percentage increase above the age- and sex-matched mortality in the absence of ART [31, 32]. Thus, if the background cardiac mortality rate at age t is r(t) and if c is the fixed percentage increase, the resulting cardiac mortality rate at age t is (1 + c)r(t). We varied the relative rate, c, between 0% and 400% in sensitivity analysis (Supplementary Data). To inspect the cases when ART offers some cardioprotective effects, we also examined other forms of risk association, such as a relative increase in the cardiovascular mortality rate for CD4 counts <500 cells/mm3 independent of treatment [17–20], and an initial decrease in cardiovascular death rate from ART (Supplementary Data) [14].
Mortality Estimation
We calculated total mortality as the sum of background mortality, HIV-related mortality, and ART-related cardiac mortality. ART-related cardiac mortality was assumed to be 0 for patients not on treatment. We computed background mortality by removing cardiovascular and HIV mortality from the Centers for Disease Control and Prevention (CDC) age-, sex-, and cause-specific data [29, 30]. HIV-related mortality, defined as in the CDC life tables [29, 30], depended on CD4 count and whether the patient was on treatment [33, 34]. We added the background and HIV-related mortality rates to the ART-related cardiac mortality rate, and converted that total rate into 6-month probability of death for each time step.
Treatment Benefits
Given the lack of consensus on the benefits of ART at a CD4 count of >500 cells/mm3 [15, 23], we considered 3 scenarios:
(1) “All benefits”: ART lowers HIV-related mortality at any CD4 count, and also leads to a rise in CD4 that delays HIV progression regardless of the CD4 count when ART is initiated. (2) “Partial benefits”: ART leads to an increase in CD4 count, just as in the “all benefits” case, but CD4-specific mortality is identical for those on and off ART above 500 cells/mm3. (3) “No benefits above 500 cells/mm3”: At CD4 counts >500 cells/mm3, CD4 decline and CD4-specific HIV-related mortality are the same regardless of ART status (ART is still associated with slower disease progression and lower mortality at CD4 counts <500 cells/mm3) (Table 1).
Patient Preferences
Personal preferences and the quality of life (QOL) associated with receiving ART play an important consideration in treatment decisions, especially at high CD4 counts. We considered 3 patient preference patterns for taking ART at high CD4 counts (Figure 1 and Table 1): (1) “ART-induced decrement”: Taking ART is associated with lower QOL than not taking ART at CD4 counts >650 cells/mm3, and improves QOL at lower CD4 counts. (2) “ART dominating”: Taking ART is associated with the same QOL as not taking ART at CD4 counts >650 cells/mm3, and improves QOL at lower CD4 counts. (3) “Life-years maximizing”: Taking ART makes no difference for QOL regardless of CD4 count. We chose the “life-years maximizing” quality-of-life estimates as our base case.
Figure 1.
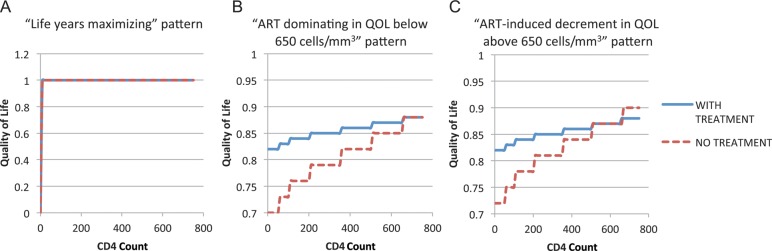
Health utility patterns considered (see also Table 1): A, “Life-years maximizing”; B, “ART dominating in QOL below 650 cells/mm3”; and C, “ART-induced QOL decrement above 650 cells/mm3.” Abbreviations: ART, antiretroviral therapy; QOL, quality of life.
Calibration and Model Analyses
We calibrated our model such that the non-HIV-related mortality in the absence of ART matches that in the United States for the HIV-negative population (Supplementary Data).
We used the model to examine optimal treatment initiation strategies for infected individuals aged >20 years. We compared the QALE from following the optimal strategy determined by our model to that from starting ART only when the patient's CD4 drops below 500 cells/mm3 [26], and to that from a “test and treat” strategy in which treatment is always initiated as soon as the patient is evaluated, regardless of the patient's age, sex, or CD4 count (Supplementary Data).
RESULTS
Baseline Results
Figure 2 shows the optimal actions for males (Figure 2A) and females (Figure 2B) for one representative scenario: life-years-maximizing health utilities; ART causes a constant 100% increase above the age- and sex-matched baseline cardiovascular mortality rate; and initiating ART brings both progression and HIV-mortality benefits.
Figure 2.

Optimal actions for males and females assuming “life-years maximizing” health utilities, the “all benefits” case, and a constant relative increase of 100% in cardiovascular death rate due to antiretroviral therapy above the age- and sex-matched cardiovascular mortality rate. Abbreviation: ART, antiretroviral therapy.
Immediate treatment initiation provided the greatest benefits for younger patients in our model. For males up to age 49 years, immediate ART initiation yielded the highest QALE. According to our model, these individuals experienced the highest remaining QALE when ART was initiated even at very high CD4 counts, because their baseline cardiovascular risk was low and the additional risks associated with ART were outweighed by the HIV survival benefits. At older ages, however, with greater cardiovascular disease risk and shorter remaining life expectancy (resulting in smaller lifetime ART benefits), delaying treatment initiation was favored in many circumstances. The model suggests that a 65-year-old man, for instance, should initiate treatment only when his CD4 count is <500 cells/mm3. For females, the optimal policy generally mandates initiating treatment earlier than for males, as evidenced by the dark area under the curve (Figure 2A and 2B).
Varying the Benefits of ART Above 500 Cells/mm3
Figure 3 presents the optimal actions, as per our model, for the 3 cases of benefits we considered. Immediate treatment initiation remains optimal for younger patients unless we assume the “no benefits above 500 cells/mm3” case, in which case ART should never be started at CD4 counts >500 cells/mm3. For 45-year-old men, immediate treatment initiation is optimal under the “all benefits” case, whereas treatment initiation is optimal below 650 cells/mm3 under the “partial benefits” case, and below 500 cells/mm3 for the “no benefits above 500 cells/mm3” case. For 65-year-old men, the optimal policy determined by our model was to initiate treatment at 500 cells/mm3 under the “all benefits” and “partial benefits” scenarios, and at 350 cells/mm3 under the “no benefits above 500 cells/mm3” scenario.
Figure 3.
Optimal actions for males (A, C, and E ) and females (B, D, and F ) assuming ”life-years maximizing” health utilities, for the 3 types of antiretroviral therapy benefits considered: A and B, “All benefits” pattern; C and D, “Partial benefits above 500 cells/mm3” pattern; and E and F, “No benefits above 500 cells/mm3” pattern. Abbreviation: ART, antiretroviral therapy.
For patients of the same sex, the greatest change in our model's optimal policy toward delayed treatment initiation was brought by the cancellation of the progression and mortality benefits of ART at CD4 counts >500 cells/mm3, as seen in the “no benefits above 500 cells/mm3” panels (Figure 3E and 3F) compared with “partial benefits” (Figure 3C and 3D) and “all benefits” (Figure 3A and 3B). In the “no benefits above 500 cells/mm3” case, initiating ART above 500 cells/mm3 is never optimal (because no benefit is realized in this case for such CD4 counts), whereas initiating ART above 350 cells/mm3 is optimal only for men <63 years of age and women <70 years of age.
Patient Preferences
We examined the impact of patient preferences by considering alternative QOL values. As shown in Figure 4, patient preference patterns can have a significant impact on the optimal strategy determined by our model. The optimal strategy for the “life-years maximizing” pattern favors waiting until lower CD4 counts than the “ART dominating” pattern and the “ART-induced decrement” pattern.
Figure 4.

Optimal actions for males and various health utility functions, assuming a constant 100% increase in cardiovascular risk above baseline due to treatment and that antiretroviral therapy brings both progression and mortality benefits from all CD4 counts. Abbreviation: ART, antiretroviral therapy.
DISCUSSION
We model the important clinical and policy decision of initiating HIV treatment under the possibility of an increase in cardiovascular mortality associated with ART [35]. Our analysis suggests that under the “all benefits” assumption, starting ART immediately is optimal for young patients regardless of CD4 count under a wide range of assumptions. This is because the baseline cardiovascular risk is low and increases very slowly at younger ages, while the decline in CD4 without treatment is much more rapid. More than 41 000 new HIV infections are identified every year in the United States in patients <50 years of age [36]. We estimate that >9900 quality-adjusted life-years could be gained every year by following the optimal strategy compared with starting ART at a CD4 cell count of 500 cells/mm3.
A key question in the decision to initiate ART is whether ART reduces mortality at CD4 counts >500 cells/mm3. Several observational studies have addressed this question with mixed conclusions [6, 15, 16]. Given this uncertainty, we considered 3 cases of ART benefits. An important finding of our analysis is the difference in the predicted optimal policy depending on ART's benefits above 500 cells/mm3. Importantly, in the “no benefits above 500 cells/mm3” case, treatment initiation was never optimal above 500 cells/mm3.
Our analysis highlights trade-offs in treatment initiation thresholds for older individuals and those at higher cardiovascular risk. The exact age at which it is no longer optimal to initiate ART at any CD4 count depends on the true relationship between ART and cardiovascular risk. Studies that combine HIV-specific and non-HIV-specific mortality into all-cause mortality bypass the ambiguities in defining HIV-related mortality and serve an important role. However, the absence of cause-specific mortality by age and CD4 count makes it difficult to estimate the competing risks critical for decisions about treatment initiation. Understanding these risks, and in particular the risk association between ART and cardiovascular mortality, is especially important for older HIV-infected patients.
Our use of ART throughout this analysis refers primarily to those regimens that may increase cardiovascular risk, mostly protease inhibitors and possibly abacavir. One immediate implication of our findings is that agents with greater cardiovascular toxicity profile should be avoided in older individuals, especially those with greater baseline cardiovascular risk. This is not always possible with transmitted drug resistance to other important antiretrovirals. Thus, delaying treatment until the patient reaches a CD4 count of 350 cells/mm3 may be optimal if protease inhibitors are a principal component of ART for older individuals.
Patient preferences play an important role in the timing of ART initiation. Our analysis shows that optimal treatment initiation is at lower CD4 counts for those whose quality of life is unchanged by taking ART compared with those whose quality of life is improved with ART. However, even among patients whose quality of life is not improved by ART, immediate treatment initiation is optimal at ages <32 years provided treatment is associated with benefits at all CD4 counts.
Although we find improved health outcomes with immediate treatment under a broad range of conditions, we do not explore the cost-effectiveness implications of such strategies. A strategy with the highest remaining QALE may not be preferable from a societal perspective if the associated costs suggest that the strategy is not an efficient use of resources.
Our analysis has several limitations. We did not consider other potential toxicities associated with ART such as kidney damage or liver injury. These are important considerations, though they are less morbid than cardiovascular disease. Our results are stable even when the cardiovascular risk associated with ART is high; that is, the results are less likely to change substantively with inclusion of additional ART-related toxicities. In addition, our analysis extrapolates beyond the available evidence, especially since experience with ART is short compared with life expectancies. The best available evidence contains great uncertainty over the association of ART with cardiovascular mortality. For instance, no patients in the D:A:D cohort took darunavir because it was not available at the time [10]. If the cardiac risk from newer agents is on the low end of our range, the optimal policy would trend toward earlier treatment initiation. We explored this uncertainty in cardiovascular risk throughout the analysis, and considered other forms of cardiovascular risk, including a CD4 count–dependent increase in cardiovascular risk <500 cells/mm3 (Supplementary Data) [18]. We also assumed that ART-associated cardiovascular risk is stable over a patient's lifetime. In reality, physicians may switch high-risk individuals to antiretroviral drugs with fewer cardiovascular risks. Such behavior will serve to increase the benefits of immediate treatment initiation. Finally, we only considered the therapeutic benefits of ART for individuals without accounting for its preventive benefits. Coupled with our finding that the loss in QALE is relatively small for most patients, the preventive benefits of ART could make immediate ART initiation upon diagnosis a very appealing strategy from a societal perspective.
Our analysis can inform decisions about when to initiate ART. We provide age thresholds for men and women for which immediate ART is associated with the best health outcomes after accounting for possible cardiac toxicities. Our analysis highlights the potential risks of immediate ART initiation in older patients, as well as the important role that patient preferences play in determining the optimal policy, particularly at CD4 counts >650 cells/mm3.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number RC1-AI086927) and the National Institute on Drug Abuse (grant number R01 DA15612–016). D. M. N. is supported by a Stanford Graduate Fellowship. D. K. O. is supported by the Department of Veterans Affairs. E. B. is supported by the National Institute of Allergy and Infectious Diseases (K01-AI084582).
Disclaimer. The funding sources had no role in the design, conduct, and reporting of the study, nor in the decision to submit the manuscript for publication.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ho D. Time to hit HIV, early and hard. N Engl J Med. 1995;333:450–1. doi: 10.1056/NEJM199508173330710. [DOI] [PubMed] [Google Scholar]
- 2.Cozzi Lepri A, Phillips N, d'Arminio Monforte A, et al. When to start highly active antiretroviral therapy in chronically HIV-infected patients: evidence from the ICONA study. AIDS. 2001;15:983–90. doi: 10.1097/00002030-200105250-00006. [DOI] [PubMed] [Google Scholar]
- 3.Harrington M, Carpenter CC. Hit HIV-1 hard, but only when necessary. Lancet. 2000;355:2147–52. doi: 10.1016/S0140-6736(00)02388-6. [DOI] [PubMed] [Google Scholar]
- 4.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–15. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd BE, Jenkins C, Rebeiro PF, et al. Estimating the optimal CD4 count for HIV-infected persons to start antiretroviral therapy. Epidemiology. 2010;21:698–705. doi: 10.1097/EDE.0b013e3181e97737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterne JC, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Vlahov D, Galai N, et al. Mortality in HIV-seropositive versus -seronegative persons in the era of highly active antiretroviral therapy: implications for when to initiate therapy. J Infect Dis. 2004;190:1046–54. doi: 10.1086/422848. [DOI] [PubMed] [Google Scholar]
- 8.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babiker AG, Emery S, Fatkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2012 doi: 10.1177/1740774512440342. Available at:http://ctj.sagepub.com/content/early/recent . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis-Møller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 11.Friis-Møller N, Sabin C, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 12.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–30. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 13.Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011;25:1289–98. doi: 10.1097/QAD.0b013e328347fa16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Sadr W, Lundgren JD, Neaton J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 15.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–9. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achhra AC, Amin J, Law MG, et al. Immunodeficiency and the risk of serious clinical endpoints in a well studied cohort of treated HIV-infected patients. AIDS. 2010;24:1877–86. doi: 10.1097/QAD.0b013e32833b1b26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–47. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 19.Mocroft A, Reiss P, Gasiorowski J, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55:262–70. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- 20.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55:615–9. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schackman BR, Freedberg KA, Weinstein MC, et al. Cost-effectiveness implications of the timing of antiretroviral therapy in HIV-infected adults. Arch Intern Med. 2002;162:2478–86. doi: 10.1001/archinte.162.21.2478. [DOI] [PubMed] [Google Scholar]
- 22.Braithwaite RS, Roberts MS, Chang CCH, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy: a decision model. Ann Intern Med. 2008;148:178–85. doi: 10.7326/0003-4819-148-3-200802050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piroth L, Fournel I, Mahy S, et al. A decision tree to help determine the best timing and antiretroviral strategy in HIV-infected patients. Epidemiol Infect. 2011;139:1835–44. doi: 10.1017/S0950268810002980. [DOI] [PubMed] [Google Scholar]
- 24.Shechter SM, Bailey MD, Schaefer AJ, Roberts MS. The optimal time to initiate HIV therapy under ordered health states. Oper Res. 2008;56:20–33. [Google Scholar]
- 25.Palella FJ, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 26.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 28 July 2011. [Google Scholar]
- 27.Bertsekas DP. Dynamic Programming and Optimal Control. 3rd ed. Belmont, MA: Athena Scientific; 2005. [Google Scholar]
- 28.Mellors JW, Muñoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Arias E. United States life tables, 2006. Natl Vital Stat Rep. 2010;58:1. [PubMed] [Google Scholar]
- 30.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1. [PubMed] [Google Scholar]
- 31.Sabin CA, Worm SW, et al. D:A:D Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–26. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Law M, Friis-Møller N, El-Sadr WM, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D study. HIV Med. 2006;7:218–30. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 33.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 34.Phillips AN, Gazzard B, Gilson R, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21:1717–21. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- 35.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 36.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



