Abstract
Debilitating cardiomyocyte loss underlies the progression to heart failure. Although there have been significant advances in treatment, current therapies are intended to improve or preserve heart function rather than regenerate lost myocardium. A major hurdle in implementing a cell-based regenerative therapy is the inefficient differentiation of cardiomyocytes from either endogenous or exogenous stem cell sources. Moreover, cardiomyocytes that develop in human embryonic stem cell (hESC) or human-induced pluripotent stem cell (hIPSC) cultures are comparatively immature, even after prolonged culture, and differences in their calcium handling, ion channel, and force generation properties relative to adult cardiomyocytes raise concerns of improper integration and function after transplantation. Thus, the discovery of natural and novel small molecule synthetic regulators of differentiation and maturation would accelerate the development of stem-cell-based myocardial therapies. Here, we document recent advances in defining natural signaling pathways that direct the multistep cardiomyogenic differentiation program and the development of small molecules that might be used to enhance differentiation as well as the potential characteristics of lead candidates for pharmaceutical stimulation of endogenous myocardial replacement.
Keywords: Cardiomyocyte, Embryonic stem cell, Chemical screening
Introduction
The minimal ability of the adult human heart to regenerate lost or damaged cardiomyocytes has led to an intense effort to direct human embryonic stem cells (hESCs) and, more recently, human-induced pluripotent stem cells (hIPSCs) to form cardiomyocytes in order to model human heart disease and develop therapies [31]. The hESC-derived cardiomyocytes resemble immature human fetal cardiomyocytes by multiple criteria, including electrophysiology [1, 20, 21], calcium handling [1, 12, 28], force generation [12, 21], contractile protein expression, and myofibrillar structure [25], and we find that cardiomyocytes from hIPSCs appear similar [19]. Because hESC-derived cardiomyocytes have the potential to engraft into surgical models of heart disease [26, 37], they have been considered for cardiomyocyte replacement therapy and they might also shed light on regeneration from endogenous stem cell populations in the heart. Despite these encouraging advances, the use of hESC-derived cardiomyocytes for basic developmental research and large-scale applications, such as high-throughput screening, toxicology testing, and large animal studies, has been hindered by their poor yield from typically heterogeneous stem cell cultures.
In this review, we describe the developmental progression from a pluripotent hESC state to cardiomyocyte and discuss the involvement of the signaling pathways that direct the embryonic heart formation. The tight orchestration of diffusible signaling molecules and intracellular mediators that drive progression from an initial cardiac field to a functional heart tube during embryogenesis involves numerous signal transduction proteins and transcription factors that are well conserved across vertebrate and even in some invertebrate species. Judicious testing of naturally occurring, diffusible factors for stimulation of cardiomyogenesis in hESCs has led to optimized, defined conditions for production of cardiomyocytes [26, 41, 43]. Although such advances quantitatively improved the proportion of the cells that differentiate into cardiomyocytes, in most settings the percentage of cardiomyocytes in a final culture still remains less than 10%. A better understanding of the signaling pathways that control differentiation would provide the key insights that are needed to develop reagents and regimens for enhanced differentiation.
Chemical biology offers one means of discovering novel cellular signaling molecules that mediate stem cell cardiogenesis. The development of phenotypic assays that identify compounds based on the ability to stimulate stem cell cardiomyogenesis, coupled with recent advances in image cytometry and the automation of complex cell-based assays, makes it possible to screen chemical libraries in a moderately high-throughput mode. We describe the current state of assay development, screening, and the challenges inherent with identification of cellular targets along with potential strategies for target identification.
Results and Discussion
Stem Cell Cardiomyogenesis
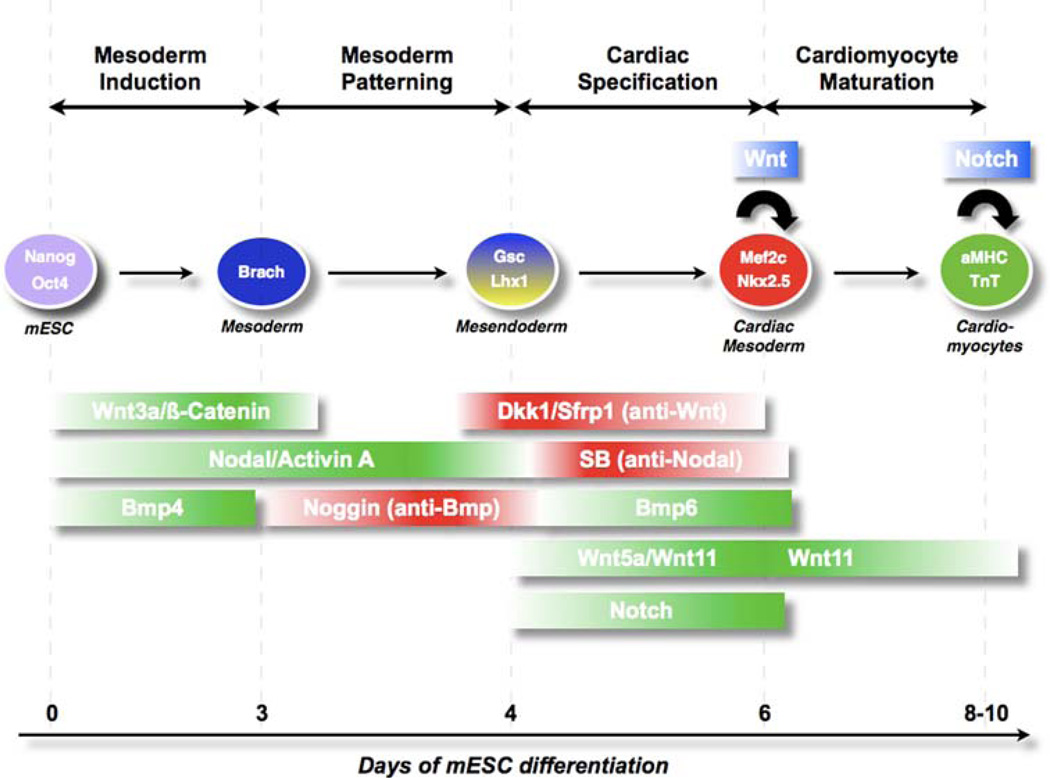
Mimicking embryonic development during ESC differentiation has led to the use of natural inducers to successfully direct specific differentiation programs. Because these factors work optimally during certain time windows and, in some instances, antagonize cardiogenesis during other windows, the timing of the addition to push the ESC in the desired differentiation direction must be carefully worked out. Four main steps (Fig. 1) are required to generate cardiomyocytes from ESCs: (1) formation of mesoderm, (2) the patterning of mesoderm toward anterior mesoderm or cardiogenic mesoderm, (3) formation of cardiac mesoderm, and (4) maturation to early cardiomyocytes. The timing of ESC differentiation to cardiac fate following these steps can be characterized by the expression of stage-specific genetic markers, such as T/Brachyury (T/Bra) for primitive streak mesoderm, Goosecoid (Gsc) and Mesoderm posterior-1 (Mesp1) for cardiogenic mesoderm, and the homeodomain transcription factor Nkx2.5 and the MADS box transcription factor Mef2c for cardiac mesoderm. Maturing cardiomyocytes can be identified by the expression of contractile proteins such as α-myosin heavy chain (αMHC) or the cardiac isoform of Troponin-T (cTnT) for the maturing cardiomyocytes.
Fig. 1.
Schematic overview of the sequential steps and the reported growth factors required for obtaining cardiomyocytes from ESCs. Beating cardiomyocytes can be obtained as soon as 8 days after the initiation of differentiation, going through crucial stages such as mesoderm induction, patterning of mesoderm toward the more anterior fate, specification to cardiac mesoderm, and, finally, maturation to beating cardiomyocytes. From the embryo, we know that each step is controlled by specific growth factors and some of these have been successfully applied on mouse embryonic stem cells (mESC) in specific time windows as indicated in green (pathway activation) or red (pathway inhibition) bars. Blue bars represent the ability of these factors to replicate a certain cell population. SB: SB-431542, a specific Nodal signaling inhibitor [18]
The first step (i.e., mesoderm induction) in cardiac differentiation from ESCs has been well characterized. Numerous studies have demonstrated that Wnts, Bmps, and the transforming growth factor (TGF) β-family member Nodal (or Activin A as a mimic of Nodal) efficiently induce mesoderm [16, 27]. In addition, some of the growth factors that pattern embryonic mesoderm to yield cardiogenic mesoderm have been shown to act on mouse and human ESCs, including Nodal [8, 39, 44], mimicking its function in the embryo.
Although some of the mechanisms that control for the next two steps in cardiac differentiation (i.e., patterning to cardiogenic mesoderm and the formation of cardiac mesoderm) have been characterized in embryos, little is known about how these mechanisms might be applied to ESC cardiogenesis. Nodal and Wnt inhibition have been found to regulate formation of cardiomyocytes in Xenopus and chick embryos [13, 14, 29, 34] and appear to be crucial for mESC differentiation to cardiomyocytes [30, 36]. Dickkopf-1 is often used as a Wnt antagonist at this stage of ESC protocols, for instance [43]. Although Wnt inhibition is required, Dkk1 was found to contain a second activity important for cardiogenesis in embryos that functions independently of inhibition of canonical Wnt/β-catenin signaling [22] through a currently uncharacterized pathway. Another important signaling pathway is mediated by the transmembrane receptor Notch, which was shown only recently to drive the induction of a combination of the growth factors Wnt5a, Bmp6, and Sfrp1, which increase the amount of cardiac progenitors from an ESC-derived mesoderm subpopulation [6].
The next and last step in the differentiation cues to cardiomyocytes is the differentiation of committed cardiac progenitors to beating cardiomyocytes, a mechanism that often occurs spontaneously in vitro and is poorly understood but might be controlled by factors such as Wnt11 [36].
It should be noted that in addition to recapitulating signaling that controls early events of cardiogenesis, strategies that might improve cardiomyocyte yields through stimulation of replication of committed progenitors might also be valuable. Toward this end, activation of canonical Wnt signaling was demonstrated to expand the pool of Nkx2.5+, Isl1+ early cardiac progenitors, providing a promising outlook to be able to increase the yields of cardiomyocytes from cardiogenic mesoderm [24, 32]. Additionally, we and others have recently demonstrated that activation of the Notch pathway in immature cardiomyocytes can prolong their period of replicative competence through the regulation of transcription and nuclear localization of D-type cyclins [5, 7], representing another direction to increase the yield of ESC-derived cardiomyocytes.
In summary, very little is known about two crucial steps in cardiogenesis from ESC: (1) how one can promote mesendoderm to form committed cardiac mesoderm and (2) the precise factors that stimulate this tissue to give rise to cardiomyocytes. Evidently, a more in-depth understanding of these mechanisms will improve the yields of ESC-derived cardiomyocytes for large-scale and clinical applications.
Small Molecule Regulators of Cardiomyogenesis
A novel alternative to increase cardiomyocyte yields is the use of small molecules to stimulate cardiomyogenesis. In addition, unbiased screening might lead to the discovery of small molecules that activate signaling pathways that have not been linked to cardiogenesis. Thus, not only would the novel small molecules be reagents to enhance cardiomyogenesis but many might also be probes to identify cellular proteins and signaling pathways that control cardiogenesis. Advantages of small, druglike molecules include their membrane penetration, rapid activation or inhibition of targets, the reversibility of their activity, and the potential for modulating multiple targets of a particular class (e.g., broad-spectrum inhibitors as compared to more selective reagents such as siRNA). Potential targets include modulation of signaling cascades or transcription factor control of gene expression.
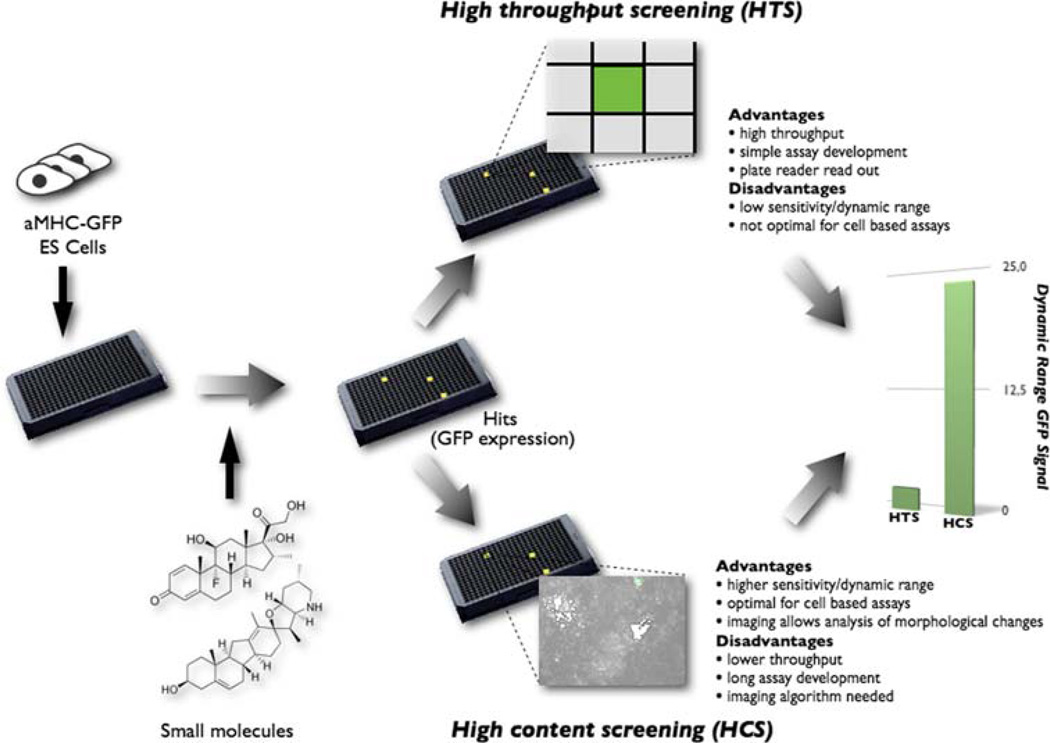
Small molecule screening assays can be directed at identifying compounds that target a single protein, such as modulating activity of a particular enzyme or signaling protein. These biochemical assays are usually designed with the target in solution and a direct biochemical readout (i.e., the visualization of a biological or biochemical process) that reports enzyme activity or protein–peptide interaction. Such high throughput screening (HTS) assays are read out on a plate reader, which is an instrument that measures the intensity of a fluorescent, luminescent or color signal. HTS assays are relatively easy to develop and have typically been the common form of screening in pharmaceutical settings. HTS assays can also be cell based, in which case the plate reader is typically used to measure a reporter protein (e.g., luciferase or a fluorescent protein) that reflects a biological process within the cell, such as the activity of a particular signaling pathway as read out by reporter protein expression (Fig. 2).
Fig. 2.
Schematic comparison between HTSs and HCSs of cell-based assays using fluorescent proteins. Cells carrying a fluorescent reporter are plated after which small molecules are added at a time point of interest. Hits will be identified by the expression of GFP, which can be quantified by using plate readers (HTS) or automated imagers (HCS). Plate readers quantify the GFP signal over the entire well, whereas, with automatic imaging, GFP is quantified by masking the GFP-specific areas alone. The dynamic range of the GFP signal can be about 10-fold greater in HCSs than in HTSs [4]
In contrast to HTS, cellular screens can also be developed with end points that are quantified by image analysis. Image analysis offers the possibility of monitoring diverse cellular processes and is therefore used in assays to discover compounds that modulate complex phenomena that cannot be reduced to a simple readout (Fig. 2). Termed high content screening (HCS) because of the high information content that can be derived from image analysis, these assays generally are more difficult to develop because they require automated microscopy instrumentation and image algorithm development expertise. Examples of assays that are not readily quantified by plate reader measurement but are well suited to HCS include direct visualization of protein localization or translocation, alterations in the morphology of cells or subcellular structures, phagocytosis, and so forth. Automated microscopy and image analysis used in HCS also offers greater dynamic range and sensitivity than plate-reader-based HTS, even if the assay could be run on a plate reader. For instance, we compared the ability of commonly used plate readers versus an automated microscope to quantify cellular expression of fluorescent proteins and cellular immunostaining and found the dynamic range of plate readers to be about one-tenth that of the automated microscope in this common application [4].
Molecules, or hits, that are active in the primary screening in the Burnham Center for Chemical Genomics (http://bccg.burnham.org), the Human Biomolecular Research Institute (http://www.hbri.org), and the Torrey Pines Institute for Molecular Studies (http://www.tpims.org) are confirmed through a dose range and prioritized according their effective concentration (EC50) and selectivity in secondary assays that rule out artifactual hits—for instance, molecules that appeared active since they affected the reporter system. Desirable compounds are taken through iterations of medicinal chemistry optimization to improve chemical properties, potency, and selectivity that increase their value as selective reagents and probes. Furthermore, should the molecules target processes in the in vitro stem cell cardiogenic assays that resemble cardiogenic differentiation of endogenous stem cells, the compounds can be optimized for in vivo administration in anticipation of testing their efficacy in stimulating endogenous regeneration in animal models of heart injury. Finally, the molecules can be developed into affinity reagents for biochemical target identification in a process that has been termed chemical genomics [2, 9, 42].
Only a few examples of chemical compound mediators of cardiogenesis have been reported, including Cardiogenols, ascorbic acid, isoxazolyl-serines, sulfonyl hydrazones, and DMSO [11, 33, 35, 38, 40] and are summarized in Table 1. All of these molecules were identified based on their ability to upregulate late-stage markers of cardiogenesis. Whereas some clearly have many effects on cells such as DMSO and ascorbic acid [10], others are probably more selective. Of the published compounds, only the sulfonyl hydrazones have been characterized as to their timing and potential biological mechanism of action. These appear to act early in the differentiation program and upregulate the expression of the mesoderm marker T/Bra, which was presumed to reflect an increase in the amount of mesoderm in the cultures that will ultimately lead to an increased number of cardiac cells. Not included in Table 1 are Wnt pathway agonists, such as QS11 [45], that would be expected to induce T/Bra+ mesoderm.
Table 1.
Synthetic cardiogenic compounds from screening
| Molecule | Assay readout |
EC50 (µM) |
Representative structure | Activity note | Timing (biological mode) of action |
Ref. |
|---|---|---|---|---|---|---|
| DMSO Butyric acid | Beating | nd | 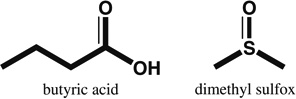 |
Chromatin remodeling; broadly increases transcriptional activity in cells | nd; unselective | [11] |
| Ascorbic acid | αMHC –eGFP reporter | 10.0 | 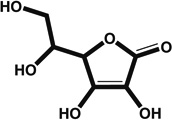 |
Antioxidant; cosubstrate for oxoacid-dependent dioxygenases; potential signaling pathways affected include hypoxia/HIF1α and signaling by EGF repeat domain (e.g., Notch receptors) | D2–6 (not precisely determined) | [35] |
| Cardiogenols | ANF–eGFP reporter | 0.1–1 | 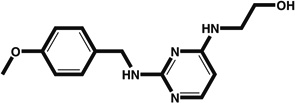 |
Induces expression of GATA4 and MEF2c | <D6 (not precisely determined) | [40] |
| Isoxolyl-serines | αMHC–eGFP reporter | 10.0 | 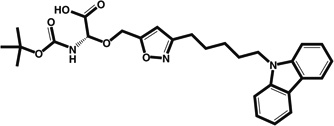 |
Designed as PPAR ligand but lacks PPAR agonist activity; thought to act on cardiogenesis pathways | D2–6 (not precisely determined) | [38] |
| Sulfonyl hydrazones | Nkx2.5–Luc reporter | 0.95* | 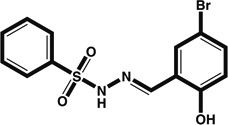 |
Increases T/Bra+ mesoderm and possibly thereby expands the number of cardiogenic precursors | <D3 (mesoderm induction) | [33] |
| BIMR1 | αMHC–eGFP reporter | 2.34 | Unpublished | Unknown | D2–4 (mesoderm to cardiogenic mesendoderm) | Unpublished |
| BIMR2 | αMHC–eGFP reporter | 1.35 | Unpublished | Unknown | D2–4 (mesoderm to cardiogenic mesendoderm) | Unpublished |
| BIMR3 | αMHC–eGFP reporter | 1.75 | Unpublished | Unknown | D4–6 (cardiogenic mesoderm to cardiac tissue) | Unpublished |
Note: The table is an overview of reported synthetic cardiogenic compounds identified using HCS indicating the assay read out used, the EC50 value if available, as well as the compound’s biological mode of action, window of action, and the report reference. Timing is based on the expression of other cardiac markers as studied in the respective reports and <D6 corresponds to induction of genes such as ANF or Nkx2.5.
Abbreviations/symbols: <, before; nd, not determined;
EC50 estimated based on published data
Clearly, we are at the early days of chemical biology of cardiogenesis. Little information is available regarding the biochemical or cell signaling mechanism of action of these molecules, and the actual pathways or mechanisms that they modulate to induce cardiomyocytes remain unknown. Second, if studied at all, these molecules appear to activate the early mesoderm specification program, a step that can be easily mimicked with available natural inducers [16, 33].
A HCS for Inducers of ESC Cardiogenesis
We recently described the use of HCS to identify compounds that promote mouse ESC (mESC) cardiogenesis [3] using a modified CGR8 cell line that had been engineered to express eGFP (enhanced green fluorescent protein) under control of the αMHC promoter [35]. In this screen, images were acquired on a GE InCell1000 or a Beckman Coulter IC100 automated microscope. An image analysis algorithm was devised to quantify the integrated pixel intensity of the eGFP fluorescence within an intensity thresholded mask. The development of an imaging method to quantify eGFP+ cardiomyocytes was critical because it extended the dynamic range of the assay, overcoming the limitations of relatively high well-to-well variation in cardiomyocyte differentiation and background fluorescence in the green (eGFP) channel. This screen revealed a number of confirmed hits (to be described elsewhere), of which three structurally distinct compounds are referred in Table 1 as BIMR1–3 as examples. The compounds have been subjected to several iterations of medicinal chemistry optimization resulting in testing of several hundred analogues that yielded improvements in chemical properties, such as solubility, stability, and selectivity. The hits also appear active at early stages of differentiation and synergize with Activin/Nodal signaling to enhance cardiogenesis in a serum-free assay that has the ability to assess the activity of the molecules alone and in combination with known inducers without the confounding effects of serum factors. At least two of the hits potentiate known signaling pathways, as seen with the use of specific transcription factor response element–luciferase reporter assays that will be described elsewhere. Comparing the pathway activity and cardiogenic activity profiles across many analogues can assess the relevance of a particular activity to cardiogenesis. A concordant structure–activity relationship (SAR) profile between the pathway and cardiogenic activities would suggest that the pathway activity is indeed relevant, whereas discordant SAR profiles would suggest that the pathway activity is not important for the cardiogenic activity. In this way, we were able to rule out Wnt pathway synergy as relevant for one of the compounds (BIMR-1), as analogues could be found that have substantial cardiogenic activity with no Wnt synergist activity, demonstrating that the two activities can be separated by chemistry.
Conclusion
The compounds in Table 1 represent the emerging chemical biology of stem cell cardiogenesis. There are two major challenges for advancing the field. The first is the development of focused assays that target specific steps in cardiomyogenesis. Differentiation is a complex, multistep process and small molecules are likely to target single pathways relevant to differentiation. Thus, complete coverage of the developmental program will require assays that focus on stimulating the progression from individual progenitors to the next step in the program. Therefore, elucidation of the progenitors through biomarkers, combined with the incorporation of these cells in new assays that focus on the critical steps in the differentiation program, will improve the likelihood of obtaining the most valuable molecules. The second challenge is to identify the cellular targets of the molecules. Affinity as well as systems biology approaches are ongoing in our laboratory to map the signaling pathways affected by our compounds.
Finally, a promise of chemical screening is that certain of the hits might be developed into drugs to enhance endogenous regeneration. Although endogenous cardiomyocyte regeneration is limited (see, for instance, [15, 17, 23]), certain of the pathways that control the size or differentiation potential of endogenous progenitor pools might overlap with those that regulate ESC cardiomyogenesis— in particular, the later steps involving committed precursors. In this regard, it is encouraging that we observe structural similarities between some of our hits and known drugs that might be exploited in the development of drug leads. For example, one of the compounds found to stimulate cardiogenesis in our screens is itself a Food and Drug Administration-approved drug, and its desirable pharmacological and pharmaceutical properties can be exploited in developing the compound for the ability to stimulate endogenous regeneration.
Acknowledgments
Our research cited in this review was conducted in collaboration with the laboratories of Dr. Marcia I. Dawson (Burnham Institute), Dr. John Cashman (Human Biomolecular Research Institute), Dr. Clemencia Pinilla, and Dr. Richard Houghten (Torrey Pines Institute for Molecular Studies) and supported by research grants from the NIH/NHLBI and California Institute for Regenerative Medicine. This work was funded by grant support from the California Institute for Regenerative Medicine (CIRM) (RC1-00132-1), the NIH National Heart, Lung and Blood Institute (R37HL059502, R33HL088266), and Mather’s Charitable Foundation to MM and CIRM fellowship (T2-00004) to EW.
References
- 1.Binah O, Dolnikov K, Sadan O, Shilkrut M, Zeevi-Levin N, Amit M, Danon A, Itskovitz-Eldor J. Functional and developmental properties of human embryonic stem cells-derived cardiomyocytes. J Electrocardiol. 2007;40:S192–S196. doi: 10.1016/j.jelectrocard.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff HI, Dowd CS. Chemical genetics: an evolving toolbox for target identification and lead optimization. Prog Drug Res. 2007;64(49):51–77. doi: 10.1007/978-3-7643-7567-6_3. [DOI] [PubMed] [Google Scholar]
- 3.Bushway PJ, Mercola M. High-throughput screening for modulators of stem cell differentiation. Methods Enzymol. 2006;414:300–316. doi: 10.1016/S0076-6879(06)14017-3. [DOI] [PubMed] [Google Scholar]
- 4.Bushway PJ, Mercola M, Price JH. A comparative analysis of standard microtiter plate reading versus imaging in cellular assays. Assay Drug Dev Technol. 2008;6:557–567. doi: 10.1089/adt.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campa VM, Gutierrez-Lanza R, Cerignoli F, Diaz-Trelles R, Nelson B, Tsuji T, Barcova M, Jiang W, Mercola M. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J Cell Biol. 2008;183:129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen VC, Stull R, Joo D, Cheng X, Keller G. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat Biotechnol. 2008;26:1169–1178. doi: 10.1038/nbt.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collesi C, Zentilin L, Sinagra G, Giacca M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J Cell Biol. 2008;183:117–128. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 9.Darvas F, Dorman G, Krajcsi P, Puskas LG, Kovari Z, Lorincz Z, Urge L. Recent advances in chemical genomics. Curr Med Chem. 2004;11:3119–3145. doi: 10.2174/0929867043363848. [DOI] [PubMed] [Google Scholar]
- 10.De Tullio MC, Arrigoni O. Hopes, disillusions and more hopes from vitamin C. Cell Mol Life Sci. 2004;61:209–219. doi: 10.1007/s00018-003-3203-8. (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinsmore J, Ratliff J, Deacon T, Pakzaban P, Jacoby D, Galpern W, Isacson O. Embryonic stem cells differentiated in vitro as a novel source of cells for transplantation. Cell Transplant. 1996;5:131–143. doi: 10.1177/096368979600500205. [DOI] [PubMed] [Google Scholar]
- 12.Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Itskovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–245. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 13.Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foley AC, Korol O, Timmer AM, Mercola M. Multiple functions of Cerberus cooperate to induce heart downstream of Nodal. Dev Biol. 2007;303:57–65. doi: 10.1016/j.ydbio.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 19.Kan N, Talantova M, Chen HSV, Mercola M. Selection of cardiomyocytes from differentiating human induced pluripotent stem cells. 2008 (in review) [Google Scholar]
- 20.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002;91:659–661. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 21.Kita-Matsuo H, Barcova M, Prighozhina N, Salomonis N, Wei K, Jacot JG, Nelson B, Spiering S, Haverslag R, Kim C, Talantova M, Terskikh A, McCulloch AD, Price JH, Conklin BR, Chen HSV, Mercola M. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS ONE. 2009 doi: 10.1371/journal.pone.0005046. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korol O, Gupta RW, Mercola M. A novel activity of the Dickkopf-1 amino terminal domain promotes axial and heart development independently of canonical Wnt inhibition. Dev Biol. 2008;324:131–138. doi: 10.1016/j.ydbio.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci USA. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–671. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 27.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–3044. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 29.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 32.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Sadek H, Hannack B, Choe E, Wang J, Latif S, Garry MG, Garry DJ, Longgood J, Frantz DE, Olson EN, Hsieh J, Schneider JW. Cardiogenic small molecules that enhance myocardial repair by stem cells. Proc Natl Acad Sci USA. 2008;105:6063–6068. doi: 10.1073/pnas.0711507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 36.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Laake LW, Passier R, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–1010. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 38.Wei ZL, Petukhov PA, Bizik F, Teixeira JC, Mercola M, Volpe EA, Glazer RI, Willson TM, Kozikowski AP. Isoxazolylserine-based agonists of peroxisome proliferator-activated receptor: design, synthesis, and effects on cardiomyocyte differentiation. J Am Chem Soc. 2004;126:16714–16715. doi: 10.1021/ja046386l. [DOI] [PubMed] [Google Scholar]
- 39.Willems E, Leyns L. Patterning of mouse embryonic stem cell-derived pan-mesoderm by Activin A/Nodal and Bmp4 signaling requires fibroblast growth factor activity. Differentiation. 2008;76(7):745–759. doi: 10.1111/j.1432-0436.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Ding S, Ding Q, Gray NS, Schultz PG. Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc. 2004;126:1590–1591. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- 41.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 44.Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T, Nishikawa S. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, Major MB, Takanashi S, Camp ND, Nishiya N, Peters EC, Ginsberg MH, Jian X, Randazzo PA, Schultz PG, Moon RT, Ding S. Small-molecule synergist of the Wnt/beta-catenin signaling pathway. Proc Natl Acad Sci USA. 2007;104:7444–7448. doi: 10.1073/pnas.0702136104. [DOI] [PMC free article] [PubMed] [Google Scholar]