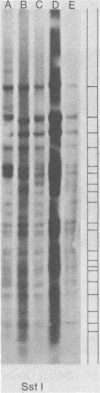
Abstract
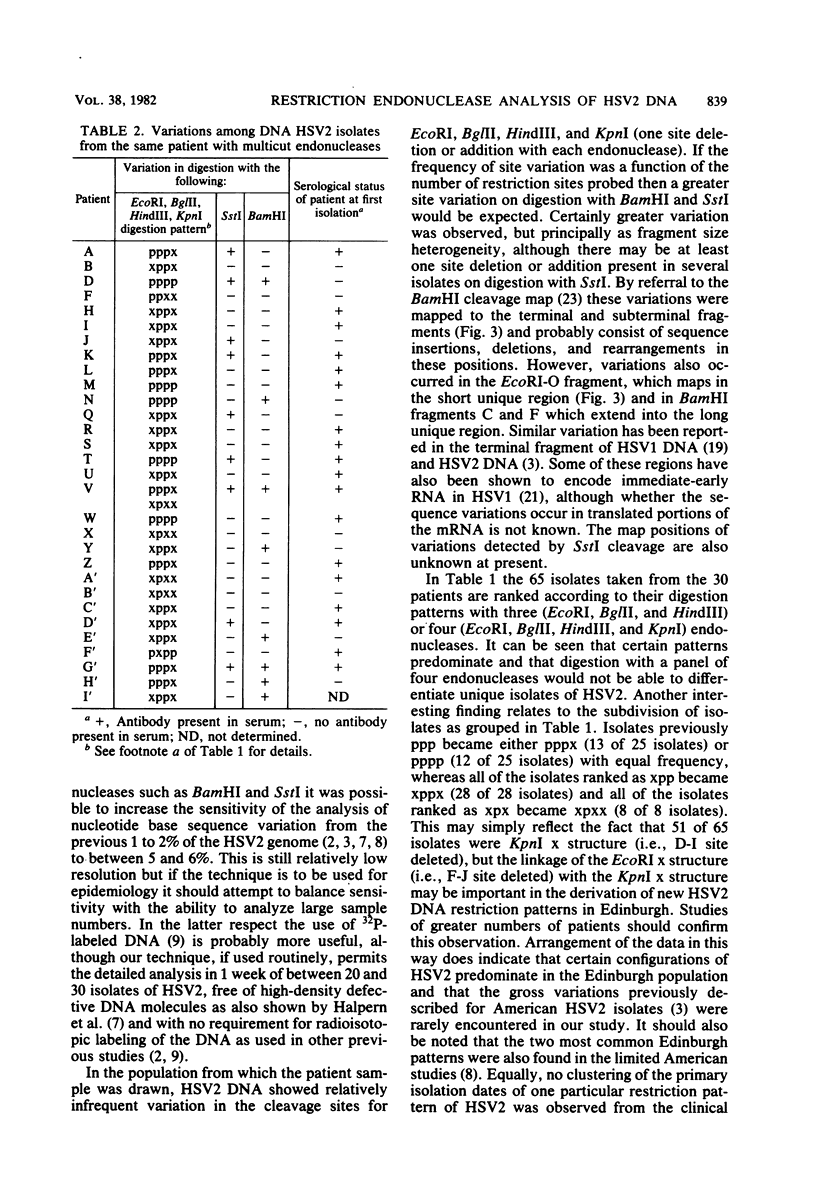
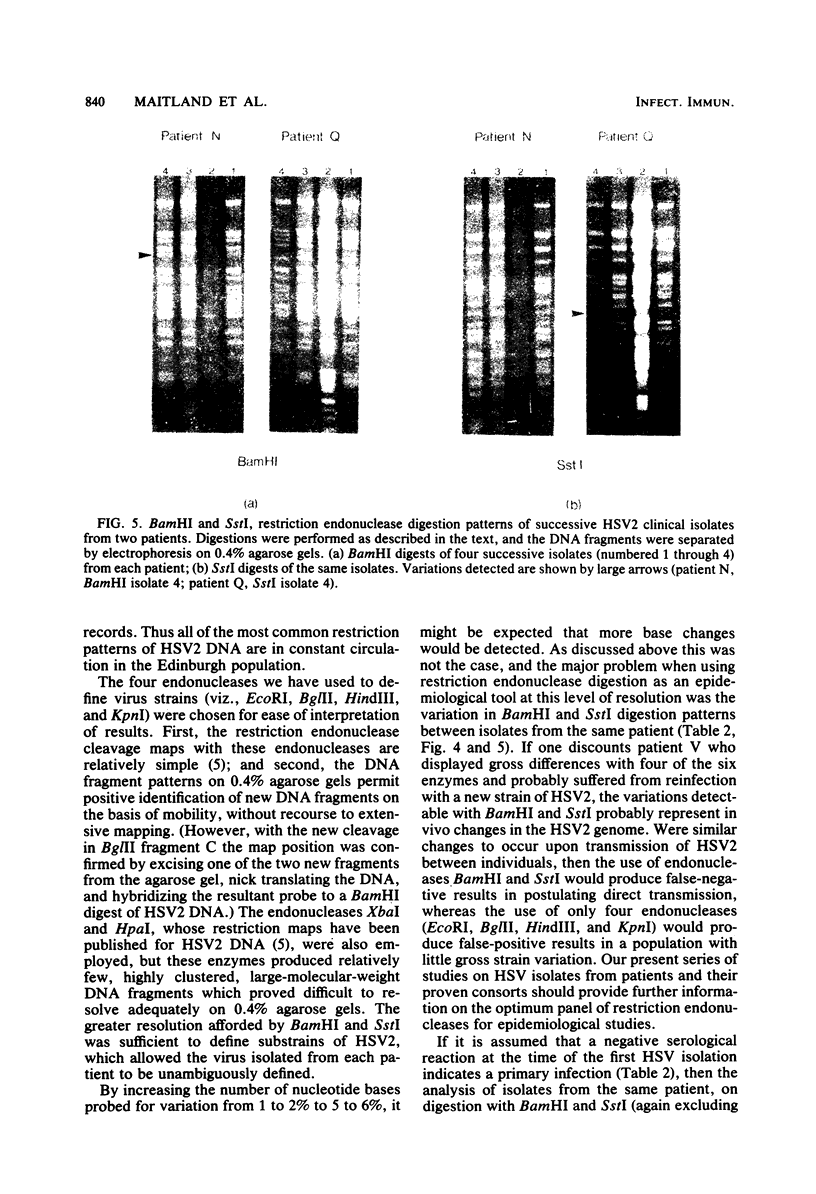
High-resolution restriction endonuclease analysis of DNA from multiple herpes simplex virus type 2 isolates from 30 patients at a single clinical center has indicated that herpes simplex virus type 2 DNA sequences are highly conserved. However, the use of six endonucleases, EcoRI, BglII, HindIII, KpnI, BamHI, and SstI, established that each patient was infected by a unique virus. Comparison of virus isolates from the same patient show that the most sequence variation occurred in the terminal and subterminal BamHI fragments. The results suggest that each individual may induce specific variation in the herpes simplex virus type 2 genome and that the results of epidemiological studies based on restriction endonuclease analyses of herpes simplex virus type 2 DNA must be interpreted with care.
Full text
PDF
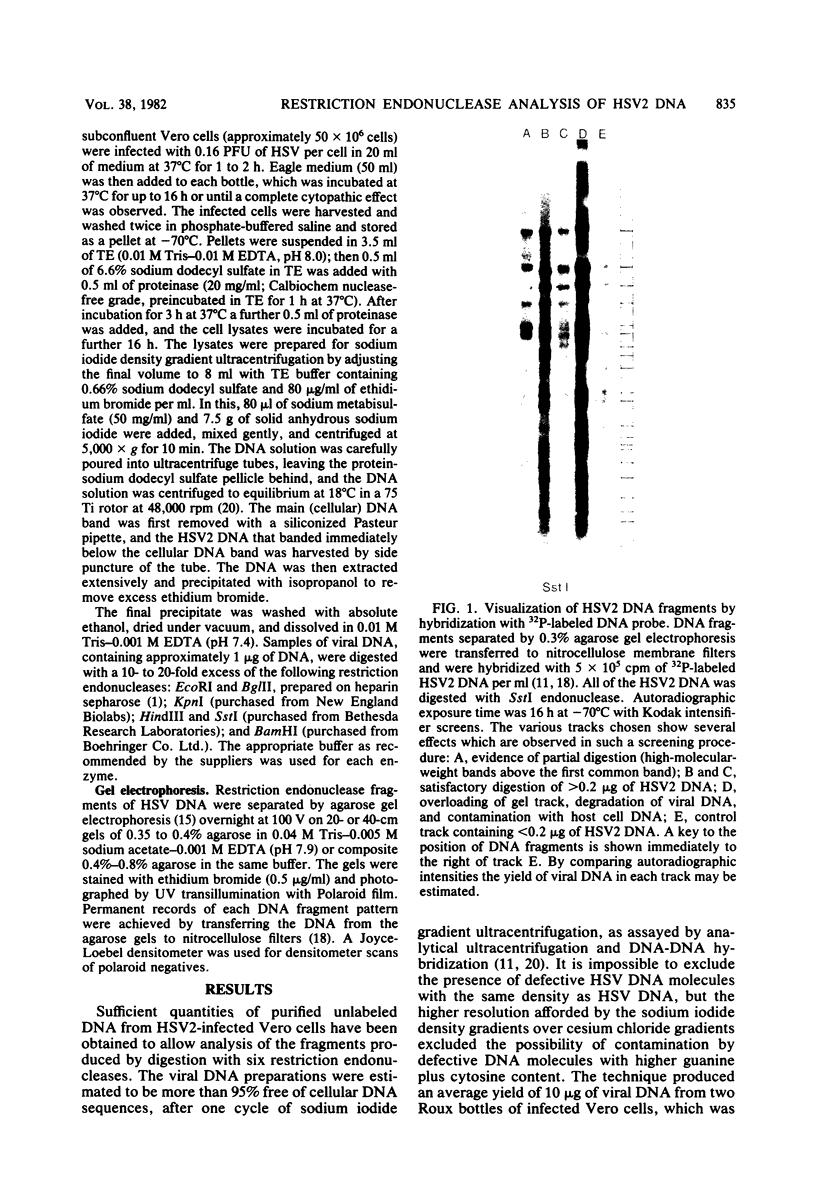
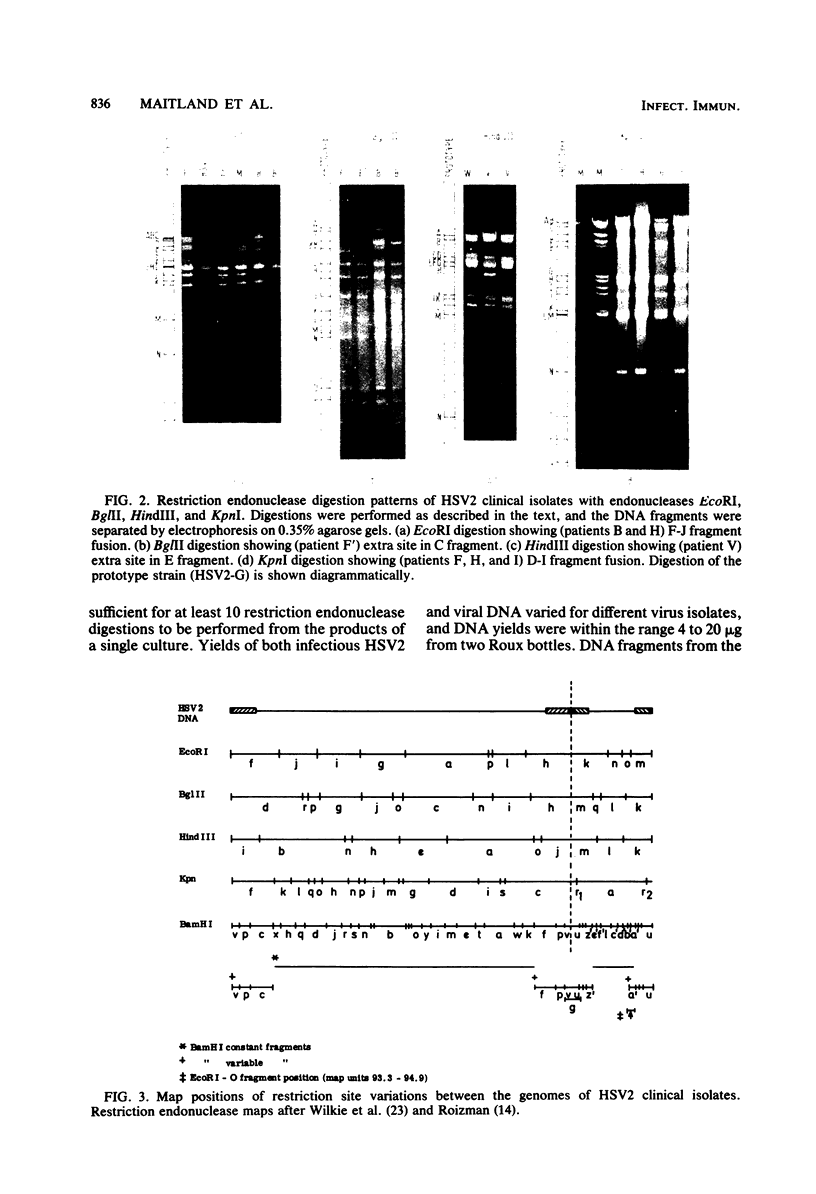




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman T. G., Roizman B., Adams G., Stover B. H. Restriction endonuclease fingerprinting of herpes simplex virus DNA: a novel epidemiological tool applied to a nosocomial outbreak. J Infect Dis. 1978 Oct;138(4):488–498. doi: 10.1093/infdis/138.4.488. [DOI] [PubMed] [Google Scholar]
- Buchman T. G., Roizman B., Nahmias A. J. Demonstration of exogenous genital reinfection with herpes simplex virus type 2 by restriction endonuclease fingerprinting of viral DNA. J Infect Dis. 1979 Sep;140(3):295–304. doi: 10.1093/infdis/140.3.295. [DOI] [PubMed] [Google Scholar]
- Buchman T. G., Simpson T., Nosal C., Roizman B., Nahmias A. J. The structure of herpes simplex virus DNA and its application to molecular epidemiology. Ann N Y Acad Sci. 1980;354:279–290. doi: 10.1111/j.1749-6632.1980.tb27972.x. [DOI] [PubMed] [Google Scholar]
- Cortini R., Wilkie N. M. Physical maps for HSV type 2 DNA with five restriction endonucleases. J Gen Virol. 1978 May;39(2):259–280. doi: 10.1099/0022-1317-39-2-259. [DOI] [PubMed] [Google Scholar]
- Halperin S. A., Hendley J. O., Nosal C., Roizman B. DNA fingerprinting in investigation of apparent nosocomial acquisition of neonatal herpes simplex. J Pediatr. 1980 Jul;97(1):91–93. doi: 10.1016/s0022-3476(80)80140-5. [DOI] [PubMed] [Google Scholar]
- Linnemann C. C., Jr, Buchman T. G., Light I. J., Ballard J. L. Transmission of herpes-simplex virus type 1 in a nursery for the newborn. Identification of viral isolates by D.N.A. "fingerprinting". Lancet. 1978 May 6;1(8071):964–966. doi: 10.1016/s0140-6736(78)90251-9. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M. A rapid technique for distinguishing herpes-simplex virus type 1 from type 2 by restriction-enzyme technology. Lancet. 1979 Apr 21;1(8121):849–852. doi: 10.1016/s0140-6736(79)91265-0. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Brown S. M., Lang J., Subak-Sharpe J. H., Koprowski H., Warren K. G. Variations in herpes simplex virus isolated from human ganglia and a study of clonal variation in HSV-1. Ann N Y Acad Sci. 1980;354:291–308. doi: 10.1111/j.1749-6632.1980.tb27973.x. [DOI] [PubMed] [Google Scholar]
- Maitland N. J., Kinross J. H., Busuttil A., Ludgate S. M., Smart G. E., Jones K. W. The detection of DNA tumour virus-specific RNA sequences in abnormal human cervical biopsies by in situ hybridization. J Gen Virol. 1981 Jul;55(Pt 1):123–137. doi: 10.1099/0022-1317-55-1-123. [DOI] [PubMed] [Google Scholar]
- Peutherer J. F., Smith I. W., Robertson D. H. Genital infection with herpes simplex virus type I. J Infect. 1982 Jan;4(1):33–35. doi: 10.1016/s0163-4453(82)90930-6. [DOI] [PubMed] [Google Scholar]
- Peutherer J. F. The specificity of rabbit antisera to Herpesvirus hominis and its dependence on the dose of virus inoculated. J Med Microbiol. 1970 May;3(2):267–272. doi: 10.1099/00222615-3-2-267. [DOI] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smith I. W., Maitland N. J., Peutherer J. F., Robertson D. H. Restriction enzyme analysis of herpesvirus-2 DNA. Lancet. 1981 Dec 19;2(8260-61):1424–1424. doi: 10.1016/s0140-6736(81)92840-3. [DOI] [PubMed] [Google Scholar]
- Smith I. W., Peutherer J. F., Robertson D. H. Characterization of genital strains of Herpesvirus hominis. Br J Vener Dis. 1973 Aug;49(4):385–390. doi: 10.1136/sti.49.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Summers W. C. Structure of the joint region and the termini of the DNA of herpes simplex virus type 1. J Virol. 1978 Aug;27(2):374–387. doi: 10.1128/jvi.27.2.374-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Sullivan M., Vande Woude G. F. Structures of two spliced herpes simplex virus type 1 immediate-early mRNA's which map at the junctions of the unique and reiterated regions of the virus DNA S component. J Virol. 1981 Jan;37(1):431–444. doi: 10.1128/jvi.37.1.431-444.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Davison A., Chartrand P., Stow N. D., Preston V. G., Timbury M. C. Recombination in herpes simplex virus: mapping of mutations and analysis of intertypic recombinants. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):827–840. doi: 10.1101/sqb.1979.043.01.089. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M. Physical maps for Herpes simplex virus type 1 DNA for restriction endonucleases Hind III, Hpa-1, and X. bad. J Virol. 1976 Oct;20(1):222–233. doi: 10.1128/jvi.20.1.222-233.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]