Abstract
Methamphetamine (MA) is associated with behavioral and cognitive deficits that may be related to macrostructural abnormalities. Quantitative anatomical comparisons between controls and methamphetamine-dependent individuals have produced conflicting results. We examined local and global differences in brain structure in 61 abstinent methamphetamine-dependent individuals and 44 controls with voxel-based morphometry and tissue segmentation. We related regional differences in gray matter density and whole brain segmentation volumes to performance on a behavioral measure of impulsivity and group membership using multiple linear regression. Within the MA group, we related cortical and subcortical gray matter density to MA use history, length of abstinence and age of first use. Controls had greater density relative to MA in bilateral insula and left middle frontal gyrus. Impulsivity was higher in the MA group and, within all subjects, impulsivity was positively correlated with gray matter density in posterior cingulate cortex and ventral striatum and negatively correlated in left superior frontal gyrus. Length of abstinence from MA was associated with greater amygdalar density. Earlier age of first use of MA (in subjects who initiated use before age 21) was associated with smaller intracranial volume. The findings are consistent with multiple possible mechanisms including neuroadaptations due to addictive behavior, neuroinflammation as well as dopaminergic and serotonergic neurotoxicity.
Introduction
Methamphetamine (MA) dependence has been associated with a range of neuroanatomical findings (Berman et al., 2008). The nature and location of these macrostructural differences between MA dependent individuals and controls are of particular interest because these abnormalities may be clues to the pathology underlying cognitive and behavioral syndromes associated with MA abuse. There is, however, considerable variability in reports of both the location and the nature of anatomical differences between MA dependent patients and control subjects. Using a combination of interactive semiautomatic tissue segmentation and region-of-interest analysis, Jernigan et al. (2005) found larger volume in caudate (CAUD), putamen (PUT), and nucleus accumbens in abstinent MA dependent subjects relative to controls. Chang et al. (2005), also using manual methods, found larger volumes in PUT and globus pallidus in 50 abstinent MA users compared to matched controls. Thompson et al. (2004) found a large diffuse area of gray matter deficit on the right medial surface of the brain, including nearly all of the cingulate cortex, and the hippocampus in 4 - 7 days abstinent MA dependent patients using a novel 3-dimensional, automated landmark analysis. Kim et al. (2006) used voxel-based morphometry (VBM) to investigate gray matter integrity in long term (30.6 months) and short term (2.6 months) abstinent MA dependent subjects compared to controls. They found less dense cortex in bilateral dorsolateral prefrontal cortex (DLPFC) in the abstinent MA patients compared to controls; the largest deficit occurred in short term abstinent users and, though the deficit was smaller in long term abstinent users, cortical density was still significantly below control values. The variability of these findings may be due to a combination of differences in methods, sample size, amount of MA used by subjects or duration of abstinence. Further, the variability of macroscopic group differences may reflect the complexity of mechanisms by which MA causes damage at the neuronal level.
MA exposure causes damage to dopaminergic (DA) (Kuczenski et al., 2007) and serotonergic (Sekine et al., 2006) terminals, loss of striatal DA transporters (DAT) in baboons (Villemagne et al., 1998) and humans (Volkow et al., 2001), neuroinflammation and associated reactive gliosis (Sekine et al., 2008; Thomas et al., 2004) as well as cerebrovascular disease (Berman et al., 2008; Ho et al., 2009). Potential mechanisms for MA toxicity include effects local to DA synapses, such as oxidative stress (Cubells et al., 1994) or mitochondrial damage (Wu et al., 2007). Neuroinflammatory responses (Block et al., 2007) and glutamate-mediated excitotoxicity (Yamamoto and Bankson, 2005) could have both local and more distant downstream effects. It would, therefore, be reasonable to expect that microscopic losses of DA and 5HT terminals would translate into macroscopic regional volume losses while cellular infiltration due to glial activation would lead to macroscopic volume increases. For example, striatal volume increases have been attributed to a more robust inflammatory response in the striatum (Jernigan et al., 2005) while cortical volume losses are attributed to more severe synaptic damage or neuronal apoptosis in the cortex (Krasnova and Cadet, 2009; Thompson et al., 2004).
Although MA dependence is associated with a number of neuropsychiatric and behavioral problems (Scott et al., 2007), impulsive decision making has been studied especially intensely because of its purported importance in initiation and escalation of drug use and the probability of relapse (deWit H., 2009). Delay (or temporal) discounting (DD) probes impulsive behavior by requiring the subject to choose between a smaller immediate and a larger delayed reward (Ainslie, 1974; Rachlin and Green, 1972). In general, a person discounts the value of a delayed monetary reward according to Equation (0), typically referred to as the indifference curve (Bickel and Marsch, 2001). Larger values of the constant, k, associated with a greater preference for immediate rewards, are interpreted as a manifestation of more impulsive decision making. MA users, like alcoholics (Petry, 2001), cocaine users (Coffey et al., 2003; Perry and Carroll, 2008), and smokers (Mitchell, 1999; Reynolds, 2006) prefer smaller immediate rewards.
Impulsivity is correlated with both anatomical and functional findings in both MA dependent patients and normal controls. Bjork et al. (2009) recently reported a negative correlation between impulsivity (represented by the natural logarithm of k) and regional volume of DLPFC and inferolateral prefrontal cortex in a group of normal volunteers. Two studies have used functional magnetic resonance imaging (fMRI) to examine differences in regional activity between MA dependent patients and controls. Monterosso et al. (2007) found that activity increased in posterior parietal cortex (PPC), frontal cortical regions, and insular cortex (INS) when DD was performed in both groups; activity in the control subjects was larger than that in the MA subjects when comparing hard decisions (comparisons in which the subjects picked delayed or immediate reward options with nearly equal probability) to a control task in DLPFC. Hoffman et al. (2008) reported similar results; activity in the controls was greater than in MA subjects in anterior cingulate cortex (ACC), CAUD, and DLPFC when making a difficult discounting decision compared to a control task. The magnitude of discounting was positively correlated with activity in DLPFC, PPC and amygdala (AMYG), suggesting that function in these regions may be linked to impulsive behavior in drug addicted populations.
This study was undertaken to investigate anatomical differences between large, well-characterized groups of 61 MA dependent patients and 44 age-matched control subjects (CS), all of whom were evaluated with DD as a behavioral measure of impulsivity. To the extent that MA toxicity is related to dopaminergic mechanisms, we predicted that abnormalities would be found in cortical and subcortical regions receiving heavy dopaminergic innervation. Consistent with this model and previous studies, we hypothesized that we would find volumetric gains in the gray matter of the striatum (possibly associated with neuroinflammation) and losses in frontal cortex (associated with neuronal toxicity). We also hypothesized that there would be correlations between degree of impulsivity and reduced gray matter density in regions identified by functional MRI investigations, such as ventral striatum, DLPFC, ACC and INS. Finally, given the observations of Kim et al. (2006), we hypothesized that length of abstinence would be associated with evidence of recovery from MA-induced changes, e.g., increased gray matter density and gray matter volume.
Materials and Methods
Participants
Sixty one recently abstinent MA dependent patients were recruited from residential treatment programs in Portland, OR and 44 normal controls were recruited through advertisements (Table 1). Age at scan date ranged from 20 to 63 years. Subjects completed a clinical interview (Structured Clinical Interview for DSM-IV [SCID] (First et al., 1998)), the DD task, a urine drug screen and an imaging protocol. All procedures were approved by the Institutional Review Board of the Portland Veterans' Affairs Medical Center and all subjects gave written informed consent. Inclusion criteria for the MA group included DSM-IV criteria for MA dependence, use of at least 0.5 g/day for ≥ 5 days/week for ≥ 2 years, and must have been abstinent for at least 2 weeks and not more than 160 days. MA subjects were excluded for a positive urine drug screen performed at the time of scanning, abuse or dependence within the past 5 years for any other substance (except nicotine and caffeine) or any past or present Axis I psychiatric diagnosis (other than substance dependence or depression). Control subjects were excluded if they had any history of drug abuse or dependence other than nicotine or caffeine, use of any illicit drug other than cannabis or any past or present Axis 1 psychiatric diagnosis (other than a history of depression). No participants were currently taking antipsychotics, benzodiazepines, antiparkinson medications, or anticholinergics; suffered from any past or present medical illnesses that might affect cognition (e.g. stroke, traumatic brain injury, HIV, hepatitis C) or had any contraindications for MRI (including claustrophobia and implanted ferromagnetic objects).
Table 1.
Mean (SD) for demographic characteristics
| Controls (n = 44) | MA Dependent(n = 61) | |
|---|---|---|
| Age (years) | 34.1 (10.7) | 33.4 (8.4) |
| Gender (males/females) | 22/22 | 31/30 |
| Education (years) | 15.7 (2.5) | 11.6 (0.7)* |
| Abstinence length (days) | N/A | 63.7 (32.7) |
| IMP | 0.42 (0.23) | 0.67 (0.23)* |
| Age of first use (AFU, years) ¥ | N/A | 19.1 (7.6) |
| Smokers | 17 | 54 € |
Student's t test, p < 0.001.
Ten MA did not have AFU data.
Pearson's Chi-square test, p < 0.0001.
Delay Discounting
The DD task was administered as described in Hoffman et al. (2008). For each item, subjects were asked to choose between two hypothetical monetary rewards, a smaller amount (in our studies $1 to $99) available immediately or a larger amount (always $100) available after some time, t, between 1 and 365 days. The indifference curve, which gives the values at which individuals have the same preference for the immediate reward I(t) and $100 after time, t, is given by Equation (0). The constant, k, determined from a non-linear fit to the equation, is unique for each individual and is larger in more impulsive persons. We calculated IMP, the overall likelihood that a subject chooses immediate rather than delayed rewards, as the fraction of the total response space above the curve in Equation(0). Higher values of IMP reflect greater preference for immediate rewards and hence more impulsive behavior. Some investigators have used the fractional area under curve (0) (AUC) as a measure of impulsivity (Smith and Hantula, 2008). IMP is, therefore, just 1-AUC and was preferred because larger values of IMP occur in more impulsive individuals.
| (0) |
MRI scans
All imaging data were acquired on a 3T Siemens Trio MRI scanner. A high-resolution T1-weighted anatomical magnetically prepared rapid acquisition gradient echo (MPRAGE) sequence was used (144 slices 1 mm thick, TR = 2.3 s, TE = 4.38 ms, TI = 1200 ms, flip angle = 12°, FOV = 208 × 256 mm, imaging matrix = 208 × 256), yielding 1 mm3 isotropic voxel resolution. Images were reoriented to a left-to-right, posterior-to-anterior, inferior-to-superior coordinate system and compiled into standard NIfTI-1 (Neuroimaging Informatics Technology Initiative, (http://nifti.nimh.nih.gov/nifti-1/) format.
Analysis
Voxel-Wise Voxel-Based Morphometry
Structural data were analyzed with the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library [FSL, specifically, FSL-VBM [(Ashburner and Friston, 2000), (Good et al., 2001), v1.1] carried out with FSL tools [(Smith et al., 2004), v4.1.4]. Structural images were brain-extracted using the Brain Extraction Tool (BET) [(Smith, 2002)]. Tissue-type segmentation was performed using FAST4 (Zhang et al., 2001). The resulting grey-matter images were then aligned to Montreal Neurological Institute 152 subject (MNI-152) standard space using the FMRIB Linear (affine) Image Registration Tool [FLIRT, (Jenkinson et al., 2002; Jenkinson and Smith, 2001)], followed by nonlinear registration using the FMRIB Non-Linear Image Registration Tool [FNIRT (Andersson et al., 2007)]. The resulting images were interpolated to 2 mm3 isovoxels and averaged over all subjects to create a study-specific template, to which the native gray matter images were then nonlinearly re-registered. The registered segmented images were then corrected for local expansion or contraction by dividing by the determinant of the Jacobian of the translated coordinates with respect to the original coordinates. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a full-width at half-maximum of 4 mm. This method yields a value of density for each gray matter voxel in the individual anatomical maps.
In order to examine the relationship of cortical density to group membership, demographic and clinical variables, voxel-wise linear regressions were performed using 3dRegAna (Ward BD, 2006). Although years of education and smoking status both differed significantly between the MA and CS groups, neither variable was correlated with cortical density within either group. Models were explored using education and smoking status as covariates (Results). In addition, for patients who first used MA before age 25, there was a substantial and significant correlation between the age at highest educational achievement and age at first use (AFU) of MA (r = .58, p < .001). Furthermore, the mean difference between age at highest education and AFU was 0.9 ± 2.3 (z(0,2.3) = 2.62, p < .01) with 2/3 of the patients beginning MA use before or at the same time as their age at highest education. That is, initiation of MA use more often preceded school drop-out than the reverse. Therefore, education was included in regressions only after the effect of group membership was considered.
Age and days of abstinence (ABS) had significantly skewed distributions and were square root and natural log transformed, respectively. Within the MA group, a separate regression (controlled for age and gender) was performed for the dependence of cortical density on ABS. The minimum cluster size threshold was calculated using AlphaSim (Ward BD, 2000) to correct for multiple comparisons on all resultant statistical maps (α < 0.05, cluster threshold = 95 and 103 voxels for the between- and within-group (MA users) regressions, respectively).
Whole Brain Volumetric Analysis
Total brain tissue volume, normalized for subject head size, was estimated with Structural Image Evaluation, using Normalisation, of Atrophy (SIENAX) [(Smith et al., 2002), v2.6], part of FSL [(Smith et al., 2004), v4.1.4]. Brain extraction and linear registration of the skull into Montreal Neurological Institute 152 subject atlas space was performed as described in the previous section; this procedure yielded the volumetric scaling factor (VSF), used as a normalization for head size. Next, tissue-type segmentation with volume estimation was carried out in order to calculate total volume of brain tissue (including separate estimates of volumes of total gray matter, white matter, peripheral gray matter (cortical ribbon) and ventricular cerebrospinal fluid [CSF]) (Zhang et al., 2001). Between- and within-group regressions were performed as described in the previous section, except that the dependent variable (gray matter density) was replaced by each whole brain volumetric measure (total gray matter, white matter, peripheral gray, CSF, and VSF).
Results
Demographic and Clinical Characteristics
Two clinical or demographic variables differed between groups
IMP (mean[ CS] = 0.42± 0.23, mean [MA] = 0.67 ± 0.23, t(103) = −5.41, p < 0.001), education (mean[CS] = 15.7 ± 2.5, mean[MA] = 11.61 ± 0.7, t(103) = 10.14, p < 0.001) and smoking status (χ2 = 29.06, p < 0.001). Group explained 22% of the variance in IMP and education explained an additional 4% (t(102) = −2.27, p < .03); smoking status explained an additional (non-significant) 2.1% of the variance over and above group membership. All other relationships between variables were tested and were not significant.
Voxel Based Morphometry
Between Group Analyses
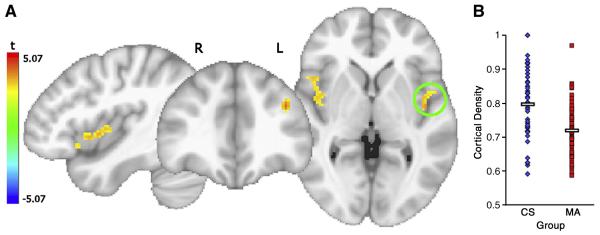
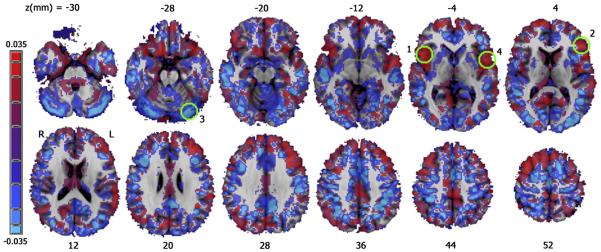
Examination of the effect of group (controlling for age and gender) revealed significantly lower cortical density for MA users than controls in bilateral INS, and left middle frontal gyrus (MFG); there was significantly higher gray matter density relative to CS in left inferior semi lunar lobule (L ISLL), (Figure 1, Figure 2 and Table 2). Relative to control subjects, there was a 9.7% decrease in the MA group in right INS, 9.6% in left INS and a 12.6% decrease in left MFG. There was a 10% density increase in ISLL, part of the cerebellum. Addition of education to the model did not result in any significant increase in explanatory variance in any of the above clusters.
Figure 1.
A. Regions where cortical density for MA was significantly less than for CS, t-statistic corrected for multiple comparisons shown. B. The average cortical density in the significant voxels only in the left insula VOI circled are graphed for each subject, the means and standard errors are 0.72 ± 0.01 and 0.80 ± 0.02 for MA and CS, respectively (t(103) = 4.42, p <.0001).
Figure 2.
A map of the regression coefficient for group membership after controlling for age and gender. CS > MA, red; MA > CS, blue. Green circles indicate significant regions shown in Figure 1, numbered by occurrence in Table 2.
Table 2.
Regions of significance after multiple comparison correction for all voxel-wise regressions
| Group | Predictor | Regions | x | y | z | Average t- value |
Cluster Size (voxels) |
|---|---|---|---|---|---|---|---|
| All Subjects (d.f. = 101) | |||||||
| GRP† | R INS | −44 | −7.8 | −0.4 | 3.0141 | 154 | |
| L MFG | 36.6 | −39 | 32.2 | 2.9786 | 121 | ||
| L ISLL | 2.8 | 64 | −42.1 | −2.8922 | 112 | ||
| L INS | 44.8 | 1.5 | 2.7 | 3.0587 | 101 | ||
| SMK† | L ISLL | 31.7 | 57.2 | −38.6 | −3.0409 | 265 | |
| R PCG | −29.1 | 24.7 | 67.2 | 2.9808 | 187 | ||
| L CING | 0.5 | 12.4 | 36.5 | −2.9840 | 157 | ||
| IMP† | L PUT/VS | 23.8 | −5.4 | −1.5 | 2.9577 | 344 | |
| PCC | 5.5 | 45.2 | 31 | 2.9842 | 276 | ||
| L SFG | 9.7 | −60.6 | 24.8 | −3.0734 | 107 | ||
| R PUT | −27.7 | 0.6 | 7.3 | 3.1198 | 106 | ||
| AGE† | L THAL | 0.8 | −2.4 | 4.2 | −7.7881 | 6083 | |
| L MFG | 9.4 | −25.4 | 35.6 | −5.6074 | 2920 | ||
| L PCING | 2.5 | 56.5 | 36.3 | −6.0687 | 1276 | ||
| L M1 | 41.3 | 28.3 | 42.4 | −4.0404 | 605 | ||
| L CUL | −11.2 | 57.1 | −23 | −5.4688 | 591 | ||
| R IPL | −38.9 | 39.6 | 44.2 | −5.2326 | 493 | ||
| R M1 | −31 | 23.5 | 53.8 | −4.9509 | 423 | ||
| L Ton | 41.1 | 55.1 | −35.7 | −4.1407 | 295 | ||
| L MFG | 39.9 | −13.3 | 28.1 | −5.3647 | 206 | ||
| L IFG | 39.3 | −33.3 | −12.3 | −4.5678 | 181 | ||
| L PRECUN | 26.5 | 62.8 | 39.7 | −3.9263 | 179 | ||
| R SFG | −24 | −8.6 | 53.9 | −3.6879 | 173 | ||
| R IFG | −11.4 | −37 | −21.4 | −3.5648 | 163 | ||
| R MFG | −24.6 | −26.2 | 40.8 | −3.9943 | 145 | ||
| R ACC | −16.7 | −27 | 7.7 | 6.624 | 130 | ||
| R LING | −12 | 81 | 7.1 | −4.0292 | 129 | ||
| R MFG | −39.5 | −18.6 | 28 | −3.6007 | 113 | ||
| L ACC | 17.4 | −27 | 8.3 | 5.6233 | 110 | ||
| SEX† | L PHIPP | 19.6 | 39.9 | −16.5 | −5.0668 | 782 | |
| L CUN | −0.4 | 88.5 | 18.7 | 6.0361 | 699 | ||
| R THAL | 0.2 | 17 | 8.5 | −5.2924 | 598 | ||
| R CAUD | −12.4 | −12.9 | 8.8 | −5.8252 | 541 | ||
| L IPL | 35 | 39.1 | 47 | −4.5125 | 341 | ||
| R CING | −3.2 | −21.1 | 26.7 | −3.9018 | 277 | ||
| L M1 | 39.4 | 19.5 | 47.9 | −4.6328 | 272 | ||
| L CAUD | 12 | −9.8 | 10 | −4.304 | 260 | ||
| L ISLL | 5.2 | 71.5 | −41.7 | −4.0882 | 258 | ||
| R CUN | −29.6 | 74.3 | 31.8 | −4.467 | 238 | ||
| R PHIPP | −25.8 | 19.9 | −22 | −5.127 | 236 | ||
| L PRECUN | 10.8 | 37.4 | 47 | −4.2025 | 199 | ||
| R IPL | −35.3 | 49.1 | 40.9 | −5.1504 | 191 | ||
| L MOG | 30.9 | 80.5 | 19.9 | −4.0391 | 173 | ||
| L MFG | 39.7 | −7.5 | 29.4 | −4.8308 | 167 | ||
| R M1 | −44.6 | 13.3 | 36.9 | −3.5618 | 121 | ||
| L PRECUN | 27.7 | 67.5 | 41.7 | −4.0361 | 116 | ||
| R OFG | −13.8 | −36.6 | −22.6 | −3.9989 | 111 | ||
| L IFG | 24.5 | −21.1 | −26.3 | 4.5329 | 101 | ||
| R STG | −46.8 | 42.1 | 7.3 | −4.6134 | 100 | ||
| MA | |||||||
| ABS¥ (d.f. = 57) | L AMYG | 21.9 | 0.8 | −35.9 | 3.1154 | 335 | |
| R AMYG/R PUT | −19.3 | 3.5 | −27.3 | 2.9653 | 323 | ||
| R Ton | −28.9 | 63.2 | −37.3 | 3.0033 | 234 | ||
| L FG | 35.7 | 42 | −25.1 | 3.0236 | 167 | ||
| R MFG | −35.1 | −47.9 | 23.2 | −3.2066 | 151 | ||
| AGE¥ | L THAL | 7.4 | 3.9 | −0.6 | −7.0466 | 1772 | |
| L MFG | 24.3 | −26.8 | 44.1 | −5.9569 | 668 | ||
| L CING | −0.5 | −32.6 | 24.2 | −4.0739 | 549 | ||
| R MFG | −36.5 | −45.3 | 12.8 | −5.5696 | 330 | ||
| L Ton | 44.6 | 55.5 | −45.7 | −3.5705 | 273 | ||
| L CAUD | −0.9 | −11.2 | 17.1 | 5.5105 | 226 | ||
| R MFG | −22.3 | −7.3 | 54.4 | −4.0086 | 175 | ||
| L STG | 42.9 | −15.1 | −25.4 | −4.5625 | 162 | ||
| L MTG | 40 | 76.3 | 13.1 | −4.7776 | 151 | ||
| L MedFG | 8.3 | 9 | 43.4 | −4.6653 | 125 | ||
| R CULMEN | 1.2 | 51.5 | −18.9 | −5.3636 | 117 | ||
| L MTG | 43.8 | 59.1 | 15.3 | −4.0466 | 117 | ||
| L MedFG | 7.9 | −46.1 | 9.7 | −4.1279 | 108 | ||
| R M1 | −21.8 | 28.1 | 57.1 | −3.9531 | 106 | ||
| SEX¥ | L PRECUN | 1.4 | 36.3 | 51.5 | −4.3269 | 374 | |
| L CUN | 0.2 | 91.4 | 22.8 | 4.8306 | 305 | ||
| L CUL | 14 | 43.4 | −11.5 | −4.688 | 226 | ||
| L MOG | 31.9 | 82 | 16.8 | −4.6825 | 173 | ||
| L IFG | 39.1 | −8.1 | 30.7 | −4.7275 | 103 |
L = Left, R = Right, AMYG = Amygdala, PUT = Putamen, Ton = Tonsil, FG = Fusiform gyrus, MFG = Middle frontal gyrus, ISLL = Inferior semi-lunar lobule (cerebellum), INS = Insula, VS = Ventral striatum, PCING = Posterior cingulate cortex, SFG = Superior frontal gyrus, MCING = Middle cingulate cortex, PRECUN = Precuneus, MOG = Middle occipital gyrus, IFG = Inferior frontal gyrus, CUL = Culmen, CUN = Cuneus, M1 = Primary motor cortex, MedFG = Medial frontal gyrus, MTG = Middle temporal gyrus, STG = Superior temporal gyrus, CAUD = Caudate, OFG = Orbital frontal gyrus, PCC = Posterior cingulate cortex, THAL = Thalamus, IPL = Inferior parietal lobule, PHIPP = Parahippocampal gyrus, CING = Cingulate gyrus, LING = Lingual gyrus, PCS = Postcentral Gyrus.
t(101) > 2.627, p < 0.01, MCS = 95, α < 0.05.
t(57) > 2.666, p < 0.01, MCS = 103, α < 0.05.
When smoking status was added to the group regression (controlled for age and gender) the statistical map associated with MA dependence identified volumes of lower cortical density in the MA group identical to those listed above. Two additional volumes associated with higher cortical density in MA subjects relative to CS were identical to regions in which the map of the regression coefficient of smoking status indicated lower density in smokers than non-smokers (middle cingulate and rostral precentral gyrus [premotor cortex], Table 2). More detailed examination of the relationship of mean cortical density and mean regression coefficients in the two volumes associated with both smoking and MA use revealed that the smoking effect was due to substantially lower density in CS smokers compared to CS non-smokers. Cortical density in these two regions was not significantly lower in MA abusing smokers than MA abusing non-smokers and did not differ between the MA group and CS when smoking status was ignored. Addition of an interaction term between smoking and MA status did not identify any significant volumes, likely because the number of non-smoking MA users and smoking CS were small (Table 1). As the addition of smoking to the model did not affect the regions associated with MA status, a voxel-wise regression was performed that investigated the effect of smoking controlled only for age and gender. This analysis identified regions distinct from those associated with MA group membership (Table 2).
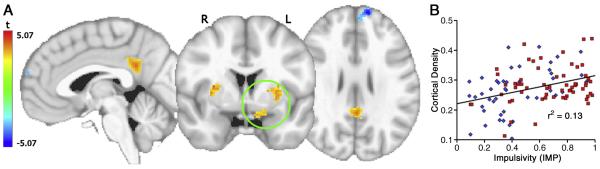
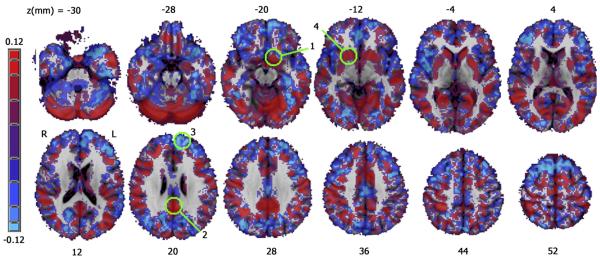
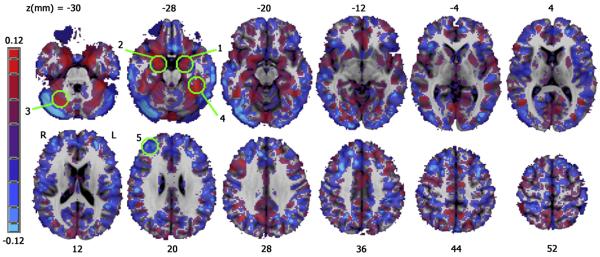
IMP (controlling for age and gender) correlated with increased gray matter density in posterior cingulate cortex (PCC) and bilateral PUT extending inferiorly into ventral striatum on the left. There was a significant negative correlation between IMP and cortical density in the left superior frontal gyrus (SFG) near the frontal pole (Figures 3, 4 and Table 2). Rates of change in gray matter density (normalized to unit IMP) were 29% for the left PUT/ventral striatum, 23% in the PCC, 53% in the right PUT, and −28% ( a decrease) in the left SFG. The effect of IMP on cortical density did not reach significance within either the MA or CS group alone.
Figure 3.
A. Map of the significant t-statistic map for the regression coefficient of IMP, corrected for multiple comparisons. B. Average cortical density value in significant voxels within circled VOI vs IMP. Blue diamonds CS; red squares MA. Line (n = 105) represents the ordinary least squares fit (F(1,104) = 15.37, p < .001).
Figure 4.
A map of the regression coefficient for IMP after controlling for age and gender. Positive correlations are shown in red, negative correlations are shown in blue. Green circles indicate significant regions shown in Figure 3, numbered by occurrence in Table 2.
Age was associated with decreased cortical density on the medial brain surface, large bilateral regions over much of the DLPFC, bilateral CAUD, bilateral INS, and bilateral inferior parietal lobule. No cortical region showed a significant density increase with age. In general, females had greater gray matter density than males in widespread cortical and subcortical regions while males had greater cortical density only in cuneus and the lower portion of the left inferior frontal gyrus. Details of the statistical maps for all variables are given in Table 2.
Within Group Analysis: MA Subjects
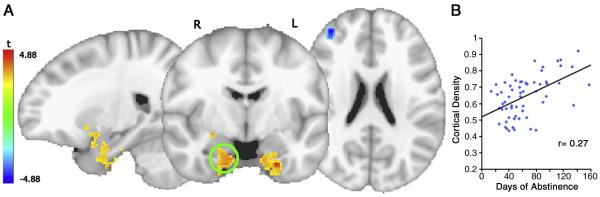
Gender, age and ABS were each associated with significant main effects on gray matter density after correction for multiple comparisons (minimum voxel cluster threshold [MCS] = 103 2 mm3 voxels, Table 2). ABS (controlling for age and gender) was associated with significantly greater gray matter density in the bilateral AMYG, extending up into right PUT/ventral striatum, left fusiform gyrus, and right cerebellar tonsil; ABS was negatively correlated with cortical density in the right MFG (Figures 5, 6 and Table 2). Gray matter density increase per day of abstinence for the right AMYG was 0.3%, for the left AMYG, 0.5%, for left fusiform gyrus, 0.2%, and for cerebellar tonsil, 0.3%. Cortical density decreased 0.2% per day of abstinence in the right MFG. The maps of age and gender in the MA group did not qualitatively differ from the maps in the combined sample. The differences in Table 2 are reflections of the smaller number of subjects (and hence decreased power and increased cluster threshold) in the patient group. Furthermore, there were no regions in which there was a significant interaction between group and either age or gender.
Figure 5.
A. Regions of significance for the t-statistic associated with the regression coefficient of ABS in MA group, corrected for multiple comparisons. B. Days of abstinence versus average cortical density in the significant voxels in right amygdalar/right putamen volume (circled). Line (n = 61) represents the ordinary least squares fit (F(1,60) = 21.81, p < .001).
Figure 6.
A map of the regression coefficient for ABS after controlling for age and gender. Positive correlations are shown in red, negative correlations are shown in blue. Green circles indicate significant regions shown in Figure 5, numbered by occurrence in Table 2.
Whole Brain Volumetric Analysis
Combined Groups
Of the whole brain volumetric measures, only volumetric scaling factor (controlling for age and gender) differed between groups (t(104) = −2.57, p = .01, MA > CS). Group accounted for 3.8% of the variance in VSF and education added a (non-significant) 0.6% to the total; similarly, the inclusion of smoking status contributed only and additional (non-significant) 0.5%. IMP (controlling for age and gender) was not significantly correlated with any global volumetric variable.
MA group
ABS (after controlling for age and gender) was not significantly correlated with any of the dependent variables. Inspection of the plot of vs AFU revealed that there appeared to be a linear relationship between the variables for earlier ages of first use, but no relationship at later ages of initiation. Therefore, a two stage linear regression was performed of the form,
| (2) |
where ζ represents the age at first use at which the dependency of VSF on AFU changes from linear to constant. The regression identified ζ = 21 as the value that gave the best fit. Earlier AFU was a significant predictor of lower VSF (t(38) = −2.03, p = 0.050) in subjects with AFU < 21 but not in subjects with AFU > 21 (Fig 7). Two subjects were clearly outliers (AFU > 40), but the finding was qualitatively the same even if these subjects were omitted from the regression.
Figure 7.
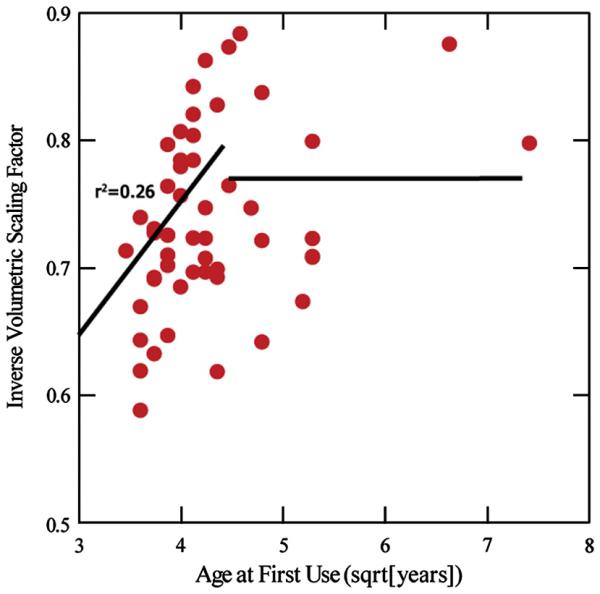
Square root of Age of First Use (AFU) vs Inverse Volumetric Scaling Factor (1/VSF). Smaller 1/VSF is equivalent to smaller head size. Pearson's r2 is shown for the left (first stage) regression line.
Discussion
Effect of MA Dependence on Cortical Density
The present study found that MA dependent subjects had reduced gray matter density in the bilateral INS and the left MFG. The INS has not previously been reported as a site of reduced cortical density in MA dependent patients, although this result is consistent with findings of reduced glucose metabolism in the INS of MA dependent subjects during an auditory vigilance task and reduced grey to white matter volume ratio in the INS of chronic cocaine users (Franklin et al., 2002). These results are also in general agreement with previous volumetric studies finding reduced gray matter density in MA users in the MFG (Kim et al., 2006) the cingulate/paralimbic cortex (Thompson et al., 2004), and the hippocampus (London et al., 2005; Thompson et al., 2004).
The insula plays a crucial role in “conscious drug urges and in translating interoceptive signals into conscious feelings and behavioral biases” (Naqvi and Bechara, 2009). For example, high levels of insular activation have been shown to predict relapse in MA dependent patients (Paulus et al., 2005). Furthermore, insular lesions can cause the spontaneous extinction of tobacco addiction in humans (Naqvi et al., 2007) and amphetamines in rats (Contreras et al., 2007). The INS may mediate withdrawal phenomena through translation of physiological symptoms into conscious dysphoria (Naqvi and Bechara, 2009). It is possible, although speculative, that thinner insular cortex is related to the narrowed behavioral range and motivational repertoire associated with addiction. That is, as drug seeking and self administration consume more of the addicted persons thoughts and behavior, the insular cortex may lose synaptic contacts related to competing non-drug related rewards and activities.
The MFG has been implicated in the valuation system that is modulated by the DLPFC in order to exert self control (Hare et al., 2009) and is an integral part of the cerebral circuit that underlies cognitive control (Cole and Schneider, 2007). Cognitive control is necessary to choose among competing stimuli and has been shown to be deficient in MA dependent individuals (Hoffman et al., 2008; Salo et al., 2008). For example, Kim et al. (2006) found that reduced gray matter density in the right MFG of MA users correlated with errors on the Wisconsin Card Sorting Test, as did reduced glucose metabolic rate in the right frontal white matter in the same subjects. Therefore, a reduction in MFG gray matter is consistent with cognitive control deficits in patients with MA dependence. Although our design precludes confirmation of the precise mechanisms that contribute to MA associated volumetric deficits, one hypothesis is that, although a single MA administration causes an acute elevation in extracellular DA, the central nervous system adapts to chronic MA use, resulting in the observed long-term down-regulation of DA and serotonin receptors, reduced tyrosine hydroxylase and tryptophan hydroxylase activity, altered vesicular monoamine transporter-2 function, and decreased extracellular DA and serotonin concentrations in chronic MA users (Krasnova and Cadet, 2009). As has been shown in rodent models of MA abuse, this chronic DA and serotonin depletion results in poor modulation of glutamate activity, contributing to neuronal injury in glutamate-rich regions such as the frontal cortex, perhaps due to glutamate excitotoxicity (Marshall et al., 2007). These findings would be consistent with reduced cortical density in the frontal regions of humans. Alternatively, rodent models of amphetamine abuse have been used to demonstrate that chronic amphetamine use is associated with reduced plasticity in the neocortex and nucleus accumbens (Belcher et al., 2005) and reduced learning (Kolb et al., 2003) in response to new experiences. That is, amphetamines may hamper the capacity for new axonal branching and growth in response to new learning experiences, which would be consistent with our findings of reduced cortical volumes in the frontal cortex, as well as previous findings of reduced hippocampal volumes (London et al., 2005; Thompson et al., 2004) in human MA addicts.
If dopaminergic alterations are indeed the primary or upstream mechanism contributing to anatomical changes in MA dependence, as is often proposed (Krasnova and Cadet, 2009), it is curious that we found no changes in gray matter density in dopamine-rich regions such as the striatum. Jernigan et al. (2005) and Chang et al. (2005) reported increased volumes in the basal ganglia in MA dependent patients, and both speculated that neuroinflammatory processes may have contributed to these enlargements. Striatal changes, however, were not observed in this study nor that of Thompson et al. (2004) or Kim et al. (2006). Although we cannot definitively determine the cause for this inter-study variability, factors could include differences in methodologies and sample characteristics. The present study includes patients who are all relatively early into recovery (mean length of abstinence = 63.7 ±32.7 days, range = 14-160 days) compared to previous studies (Jernigan et al., 2005, mean length of abstinence = 93.6 ± 89.2 days, range = 10-330 days; Chang et al., (2005), mean length of abstinence = 4.0 ± 6.2 months, range = .25 – 36 months). Unfortunately, these previous studies did not report analyses to determine whether length of abstinence was associated with increases or decreases in gray matter density, and neither our study nor these previous studies included actively using patients. Thus, it is unclear whether different regions of the brain adapt to and then recover from MA abuse at different rates. For example, chronic MA abuse may cause basal ganglia injury due to DA toxicity that would correspond to reduced grey matter volumes (or neuronal cell counts) in active users. Consistent with our results, however, group differences in gray matter density during early recovery may be non-significant in the basal ganglia because human volumetric methods do not allow us to distinguish the relative contribution of various cell types (e.g., neurons versus microglia versus astroglia) and reduced neuronal grey matter may actually be masked by neuroinflammation in these regions. Finally, consistent with previous studies (Chang et al., 2005; Jernigan et al., 2005), neuronal cell counts and/or neuroinflammation in late recovery may have increased to the point that group differences become significant. While these speculative hypotheses are consistent with our results (e.g., correlations between increased length of abstinence and increased volumes in the AMYG and PUT), longitudinal studies including active users followed into early and late recovery are needed to clarify the progression of change.
Smoking
This study was not designed to study the effects of smoking on cortical density and the numbers of smokers in the control group and non-smokers in the MA group are too few to reliably separate the effects statistically. Volumes characterized by decreased cortical density in the MA group are unchanged after statistically controlling for smoking status. Furthermore, smoking and MA dependence identify distinct brain regions in both combined and separate analyses, suggesting that the addictions may independently affect cortical density. Although the interaction of smoking with MA dependence did not identify any significant clusters in a voxel-wise regression, the limited number of smoking controls and non-smoking MA abusers restricted the power to detect an interaction.
Although this study is underpowered to robustly investigate the effect of smoking per se, our observation of decreased density in smokers in the middle cingulate gyrus is generally consistent with those of other investigators. For example, Gallinat et al. (2006) reported cortical density deficits in smokers in the middle cingulate and Brody et al. (2004) found that smokers had volume decrements in dorsal ACC. Further investigation of the effects of the interaction of tobacco and MA addiction will require identification of larger numbers of non-smoking MA dependent individuals.
Impulsivity and VBM
The present finding of higher impulsivity in MA as measured by a delay discounting task is consistent with a large body of evidence (deWit H., 2009; Hoffman et al., 2008). There were significant positive correlations between gray matter density and impulsivity in the bilateral PUT, left ventral striatum and the PCC and a negative correlation in the left SFG. This latter finding in the SFG is generally consistent with two previous studies that examined the relationship between impulsivity and gray matter volumes. Specifically, Bjork et al. (2009) used an automated segmentation technique to demonstrate that increased dorsolateral and inferolateral cortical grey matter volume was correlated with lower impulsivity. Matsuo et al. (2009) reported that a higher impulsivity (score on Barrett's Impulsivity Scale) was correlated with reduced bilateral orbitofrontal cortex and left ACC cortical density derived from VBM. In contrast, we identified other regions in which higher gray matter density was correlated with increased IMP (bilateral PUT, left ventral striatum and PCC). These regions have not been reported in previous anatomical studies, possibly because the methods lacked the regional sensitivity of VBM or the range of impulsivity was restricted in normal study populations. The regions have, however, been identified in functional studies of impulsivity. In fMRI studies of DD, the ventral striatum and PUT have been implicated in encoding the delay to the reception of a reward (Wittmann et al., 2007) and the PUT has further been implicated in reward processing and encoding the subjective value of a delayed reward (Kable and Glimcher, 2007; McClure et al., 2007). PCC is thought to play a role in the integration of magnitude and delay information (Ballard and Knutson, 2009). The causal relationship between these findings remain unclear, as it cannot be determined whether volumetric changes precede or are the result of differences in impulsivity that distinguish addicts from non-addicts.
Abstinence and VBM
Length of abstinence was positively correlated with gray matter density in the bilateral AMYG and PUT and the left fusiform gyrus and negatively correlated with cortical density in the right MFG. There is significant evidence in the literature that these regions are functionally relevant to the process of abstaining from drugs, particularly the AMYG and MFG. The amygdala is an integral part of the brain's reward circuit which must be altered in order to deviate from the drug-seeking behavior that characterizes addiction (Bechara, 2005; Ernst and Paulus, 2005). Bilateral excitotoxic lesions of the basolateral AMYG in cocaine self-administering rodents prevent drug associated stimuli from inducing reinstatement following extinction sessions (Meil and See, 1997).
Previous research has found decreased amygdalar volumes in cocaine addicts to be associated with drug craving (Makris et al., 2004) and amygdalar volume deficits in alcoholics to be associated with craving and predictive of relapse (Wrase et al., 2008). Brain-derived neurotrophic factor levels in the AMYG progressively increased on a time course parallel to increases in craving during cocaine withdrawal (Grimm et al., 2003). Systemic or central amygdalar injections of LY379168, which decreases glutamate release, prevented increases in cue-induced sucrose seeking after prolonged sucrose abstinence in rats (Uejima et al., 2007), and exposure to cocaine cues increased ERK phosphorylation in rats in the central AMYG after 30 days of abstinence but not 1 day (Lu et al., 2005). Finding larger amygdalar size in subjects with longer abstinence is consistent with these studies if subjects with larger amygdalae are less likely to relapse and thus more likely to be available for study enrollment later into abstinence.
The MFG, in addition to the AMYG, is also part of the cortical system that underlies craving. The region has been implicated, using fMRI, as an area in which the common value of a goal-directed decision is encoded. The modulation of this value by the DLPFC is necessary for exerting cognitive control over impulsive behavior (Cole and Schneider, 2007; Hare et al., 2009).
In summary, the correlations between gray matter density and length of abstinence are consistent with alternative, but not mutually exclusive, hypotheses: (1) abstinence from MA may result in gradual volumetric changes or (2) the volumes of these regions may be predictive of the ability to maintain abstinence. It is not possible to distinguish between these hypotheses with the data in this study both because this study is cross-sectional and we are naïve as to whether (and when) subjects relapsed following participation in the study.
Age of Onset and Head Size
The only significant group difference from the SIENAX segmentation after sex and age were taken into account was VSF (p < 0.01), i.e., MA users had significantly smaller intracerebral volume than controls. This result was unexpected and no previous report of this type of finding in a drug-dependent population was found. While there is not any information about the effect of MA abuse on brain (or body) growth rates in adolescents, both smaller subcortical volumes (Chang et al., 2004) and decreased fractional anisotropy (Cloak et al., 2009) have been reported in children of MA dependent mothers. A recent study found growth deficits in children with Attention Deficit Hyperactivity Disorder continuously taking prescribed stimulants (Swanson et al., 2007). Another found that growth rate deficits associated with prescribed stimulants correlated with dosage (Charach et al., 2006). Therefore, it is possible that the MA group had significantly smaller skulls due to growth retardation secondary to early onset MA abuse. These findings are also consistent with the observation that MA use may co-vary with socioeconomic status and poor nutrition, both of which may also contribute to smaller head size.
Limitations
The principle limitation of the study is the cross-sectional design which restricts its ability to determine whether MA dependence is a cause or effect of the volumetric, behavioral (IMP) and demographic (education or smoking status) differences from the control group. The question could be answered more definitively and quantitatively with prospective quasi-experimental studies of adolescents who have not initiated drug use. Additionally, determining the cause of cortical or subcortical density (and volume) differences is not feasible given the current resolution of MRI at 3 Tesla. Thus, we cannot, for example, distinguish whether a larger regional volume in one group is due to an increase in glial infiltration in that group or loss of neurons and neuronal projections in the other.
Furthermore, the results obtained from the methods used in anatomical studies (in this paper and the previous literature) may vary depending on the particular mechanism underlying MA-induced damage. Consider, for example, automatic segmentation of tissue type (white from gray matter), the first step in nearly all of the in vivo structural human imaging studies. While this process has become increasingly reliable since its earliest use, the mechanisms of damage in MA use and abuse may differentially blur the line between the two tissue types, confounding a priori classification. Furthermore, some mechanisms of damage are likely to be anatomically specific; for instance, a loss of dopaminergic (DA) terminals will primarily affect downstream regions that are innervated by these neurons (e.g., striatum and frontal pole). This may lead to anatomically selective failures to successfully delineate the gray-white boundary, and may exacerbate or minimize effects in this area. Due to the resolution at which many of the studies have been performed (~ 1mm isotropic voxels at 3T), there is no way to identify and isolate the effects of specific mechanisms and the localized confounds that they might introduce to the method; as such, discussions of the causes of such changes and associated absolute effect sizes should be met with skepticism.
Causal ambiguities extend to the relationship between MA dependence and education. Adolescents and young adults could be at higher risk for initiation of MA dependence if they drop out of school. It is also possible that initiation of MA abuse precedes and contributes to the likelihood of school dropout. We favor the latter explanation, as the MA subjects in this study tended to start use before dropping out of school. Similarly, there may be an independent contribution of smoking to regional decreases in cortical density that is difficult to separate from the effect of MA dependence in this sample. The bias towards cigarette smoking and low completed years of education in the MA dependent population prevents us from investigating these effects in this report; resolution of the many cause and effect conundra identified in this report must await future studies for disambiguation.
Supplementary Material
Acknowledgments
Supported by the, Department of Veterans Affairs Merit Review Program (WFH), Stanley Medical Research Institute and Department of Veterans Affairs Career Development Program (MSH), NIH grants P50DA018165 (WFH, SHM, BHM), and DA015543 (SHM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ainslie GW. Impulse control in pigeons. Journal of the Experimental Analysis of Behavior. 1974;21:485–489. doi: 10.1901/jeab.1974.21-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. 2007 [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat.Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O'Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann.N.Y.Acad.Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. Delay discounting correlates with proportional lateral frontal cortex volumes. Biol.Psychiatry. 2009;65:710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat.Rev.Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol.Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol.Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Charach A, Figueroa M, Chen S, Ickowicz A, Schachar R. Stimulant treatment over 5 years: effects on growth. J.Am.Acad.Child Adolesc.Psychiatry. 2006;45:415–421. doi: 10.1097/01.chi.0000199026.91699.20. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp.Clin.Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J.Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deWit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict.Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of Decision Making: A Selective Review from a Neurocognitive and Clinical Perspective. Biol.Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Computer-Assisted SCID-Clinician Version (CAS-CV) Multi-Health Systems, Inc, American Psychiatric Press, Inc.; North Tonawanda, NY: 1998. [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol.Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur.J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J.Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Ho EL, Josephson SA, Lee HS, Smith WS. Cerebrovascular complications of methamphetamine abuse. Neurocrit.Care. 2009;10:295–305. doi: 10.1007/s12028-008-9177-5. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl) 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med.Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am.J.Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat.Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon SY, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int.J.Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc.Natl.Acad.Sci.U.S.A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res.Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp.Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol.Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat.Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Jr., Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Belcher AM, Feinstein EM, O'Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2007;102(Suppl 1):61–69. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum.Brain Mapp. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav.Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum.Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch.Gen.Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. Journal of the Experimental Analysis of Behavior. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. The Experiential Discounting Task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behav.Pharmacol. 2006;17:133–142. doi: 10.1097/01.fbp.0000190684.77360.c0. [DOI] [PubMed] [Google Scholar]
- Salo R, Leamon MH, Natsuaki Y, Moore C, Waters C, Nordahl TE. Findings of preserved implicit attention in methamphetamine dependent subjects. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2008;32:217–223. doi: 10.1016/j.pnpbp.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol.Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J.Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch.Gen.Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Smith CL, Hantula DA. Methodological considerations in the study of delay discounting in intertemporal choice: A comparison of tasks and modes. Behav.Res.Methods. 2008;40:940–953. doi: 10.3758/BRM.40.4.940. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum.Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De LM, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De SN, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De SN. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, Hechtman L, Epstein JN, Pelham WE, Abikoff HB, Newcorn JH, Molina BS, Hinshaw SP, Wells KC, Hoza B, Jensen PS, Gibbons RD, Hur K, Stehli A, Davies M, March JS, Conners CK, Caron M, Volkow ND. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J.Am.Acad.Child Adolesc.Psychiatry. 2007;46:1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci.Lett. 2004;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J.Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav.Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J.Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am.J.Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for FMRI Data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Ward BD. Multiple Linear Regression Analysis across FMRI 3d Datasets. 2006 http://afni.nimh.nih.gov/pub/dist/doc/manual/3dRegAnam.pdf.
- Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp.Brain Res. 2007 doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am.J.Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Wu CW, Ping YH, Yen JC, Chang CY, Wang SF, Yeh CL, Chi CW, Lee HC. Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol.Appl.Pharmacol. 2007;220:243–251. doi: 10.1016/j.taap.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit Rev.Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans.Med.Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.