Abstract
The lifestyle of intracellular pathogens has always questioned the skill of a microbiologist in the context of finding the permanent cure to the diseases caused by them. The best tool utilized by these pathogens is their ability to reside inside the host cell, which enables them to easily bypass the humoral immunity of the host, such as the complement system. They further escape from the intracellular immunity, such as lysosome and inflammasome, mostly by forming a protective vacuole-bound niche derived from the host itself. Some of the most dreadful diseases are caused by these vacuolar pathogens, for example, tuberculosis by Mycobacterium or typhoid fever by Salmonella. To deal with such successful pathogens therapeutically, the knowledge of a host-pathogen interaction system becomes primarily essential, which further depends on the use of a model system. A well characterized pathogen, namely Salmonella, suits the role of a model for this purpose, which can infect a wide array of hosts causing a variety of diseases. This review focuses on various such aspects of research on Salmonella which are useful for studying the pathogenesis of other intracellular pathogens.
Keywords: Salmonella, model systems, pathogenicity islands, virulence
Salmonella as a Model Intracellular Pathogen
Salmonella represents a group of Gram-negative facultative anaerobic pathogenic bacteria which costs millions of lives across the world every year. At present, the genus Salmonella is categorized into two species S. bongori and S. enterica, based on the high (96–99%) sequence similarity of the genome. There is only one subspecies under S. bongori namely subspecies V, whereas S. enterica comprises the remaining seven subspecies I, II, IIIa, IIIb, IV, VI and VII.1 Where subspecies I is specific to warm-blooded animals like mammals, others can infect only cold-blooded animals including reptiles. Further division into serovars increases the number of variants to more than 2,500. Out of these, Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Typhi have been discussed here, as they have previously served as tools to study host-pathogen interactions. While S. Typhi infection is strictly limited to humans and higher primates, S. Typhimurium has a wide range of host such as rodents, cattle and mammals.
Intracellular pathogens can either survive in a self-constructed niche in the form of a vacuole or they may choose to live in the cytoplasm of the host cell. Salmonella chooses the most commonly preferred option of forming an intracellular vacuole termed as Salmonella containing vacuole (SCV). SCV arrests the host endosomal pathway at the late endosome stage. It does acquire the late endosome markers, such as vATPases and LAMP1, but loses some of them like mannose-6-phosphate receptor which differentiates it from the late endosome.2 Later the SCV gets juxtaposed to the nucleus by utilizing the microtubule meshwork of the host cell and derives nutrition from the Golgi apparatus.3 Few intracellular pathogens follow an alternate less preferred strategy to survive inside host cells, as they do not form a niche but develop strategies to survive inside the cytoplasm,4 including the examples of Shigella and Listeria.
Other best studied vacuolar pathogens also hijack the endo-phagocytic pathway of the host at various stages bearing the surface markers of that specific stage. Mycobacterium infection involves formation of Mycobacteria pathogen vacuole (MPV) that does not mature after the early endosome stage while being associated with the corresponding markers like EEA1 and Rab5.2 This arrest at early endosome stage prevents the fusion of the MPV with the phagolysosome and hence the clearance of the pathogen. Another example, Brucella containing vacuole (BCV), displays early endosome related markers like EEA1, Rab5, etc. and eventually takes an unconventional route of becoming endoplasmic reticulum (ER) derived autophagosome maturing into ER.2 In case of Legionella infection, Legionella containing vacuole (LCV) bears autophagosome associated markers like Atg7 and Atg8 and further matures into rough ER like organelle.2 Chlamydia form Chlamydia trachomatis inclusion (INC) which moves to the microtubule organizing center (MTOC) like Salmonella.2 Notably, the vacuolar structure INC is segregated from the typical endomembrane pathway unlike other pathogens. Toxoplasma forms a host plasma membrane derived parasitophorous vacuole (PV), which is completely independent of vesicular trafficking of the host cell. The membrane of PV gets incorporated with LDL cholesterol with the help of post-lysosomal vesicles.2
Although a substantial amount of work about the mechanism of establishment of these intracellular structures have been done, there are yet many unanswered questions about the changes induced upon host by the pathogen to maintain the integrity of these vacuoles. Hence understanding the system of a model pathogen will address such questions to great extent.
A faster rate of growth and feasibility of modification of the genome by using recombinant DNA technology makes Salmonella an ideal pathogen to study host-pathogen interaction. Availability of mouse model for typhoid fever as well as gastroenteritis5 and C. elegans for innate immune response during Salmonella infection6 makes it a preferential model pathogen to study. Further, S. Typhimurium alone can be used as a model for two modes of infection, local gastroenteritis as well as systemic typhoid fever. Taking these points in consideration we intend to explain the pathogenic features that render Salmonella eligible to be used as a model intracellular pathogen.
Keys to Success
The key factors behind the success of Salmonella as an intracellular pathogen are described in subsequent sections which cover features specific to Salmonella, making it interesting to venture into the details of Salmonella pathogenesis.
Multiple targets
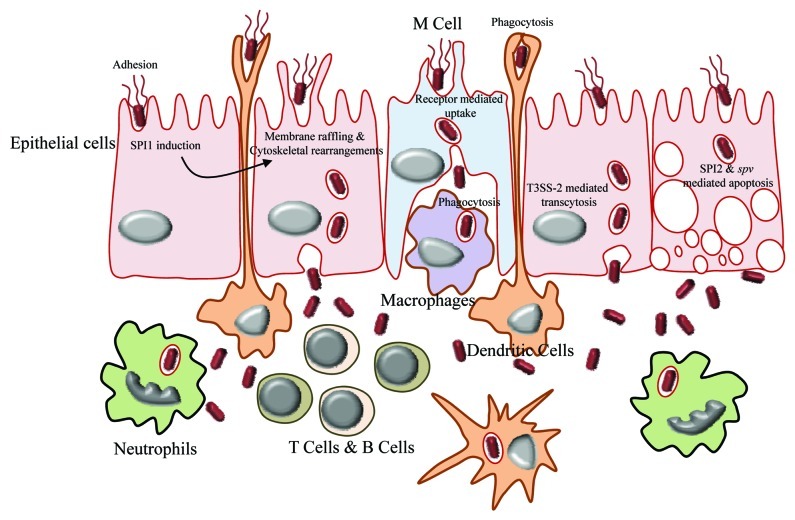
Salmonella possesses extremely versatile strategies to infect different target host cells (Table 1). Interestingly it prefers to proliferate in the usually non-permissive environment of immune cells such as macrophages instead of the much permissive epithelial cells. The mode of entry as well as the strategy followed to survive inside the target cell varies according to the type of cell and depends on the temporal expression of particular genes by Salmonella such as the type three secretion systems. Salmonella is controversially shown to be able to survive and replicate most preferentially inside the microbicidal neutrophils instead of macrophages according to the conventional paradigm.7 Other target cells that encounter Salmonella in different locations include B cells, T cells, monocytes, dendritic cells, granulocytes and gut epithelial cells (Fig. 2). Salmonella displays variety in mechanisms of not only entry and survival but also cytotoxicity, which relies on its virulence factors (Table 1).
Table 1. Multiple cellular targets.
| Cell type | Cell culture model | Mode of internalization | Outcome |
|---|---|---|---|
| M cells |
Mixed culture of CaCo-2 and Raji B cells |
Caveolae mediated endocytosis |
Cell lysis |
| Dendritic cells |
Primary cells |
Phagocytosis |
Apoptosis, cell lysis |
| Macrophages |
RAW 264.7, J774, etc., primary cells |
Phagocytosis |
Apoptosis |
| Epithelial cells | HeLa, CaCo-2, HT-29, Intestine 407, etc. and primary cells | Macropinocytosis | Apoptosis, cell lysis |
Figure 2. Breaching of gut epithelia by Salmonella. The mode of entry of Salmonella in gut lumen varies according to type of cell encountered on the gut epithelium. The M cells take up the bacteria by means of receptor mediated endocytosis, whereas dendritic cells engulf them by phagocytosis. The membrane of epithelial cells is modified by the action of SPI1 to facilitate the entry of bacteria. Once inside the gut lumen, Salmonella is being taken up by macrophages, T cells, B cells, neutrophils, etc.
Horizontally acquired pathogenicity islands
The astonishing variation in pathogenesis of the serovars of the same species is accountable to the acquisition of genes laterally from external sources over the course of million years. One-fourth of the Salmonella genome is estimated to be acquired horizontally. The divergence of Salmonella from E. coli in the process of evolution involved the horizontal transfer of various genes that turned Salmonella into a successful pathogen compared with E. coli. These genes are collectively termed as pathogenicity islands, which are further categorized based on their function. The serovars Typhi and Typhimurium have 11% difference in their genome, which are otherwise 99% similar in their sequences of house-keeping genes. Also the same serovar has variations in their genome within the strains. The genes acquired play roles in pathogenesis (SPI1, SPI2, SPI3, etc.) as well as metabolism (aroA), resistance against antibiotics and many such important functions.8 The horizontal gene transfer could occur by various modes like phage infection, conjugative plasmids, transposition or transformation1 and most commonly by inserting genes within tRNA genes. There are 12 pathogenicity islands known at present and the continuous process of evolution may add up more genes to the list. Apart from gaining external genes, Salmonella may also tend to lose certain genes to maintain virulence, like loss of lac operon during evolution has enhanced the fitness and virulence of Salmonella.9
Two-component systems
The ability of Salmonella to sense the extracellular cues in the surrounding micro-environment and accordingly regulate the expression of genes is dependent majorly upon few two-component systems. The phoP system encodes the sensor PhoQ and response regulator PhoP, whose expression is induced by Mg2+ starvation and low pH, regulates acid tolerance and major virulence genes, such as genes required for invasion,10 intracellular survival11 and resistance to antimicrobial peptides. Another two-component system, ompR, responds to change in osmolarity and regulates invasion12 as well as intracellular survival.13,14 The system pmrAB mediates resistance specifically against the anti-microbial peptide polymyxin B and is further regulated by other two-component systems such as PhoP/PhoQ and PreA/PreB15 reflecting the importance of polymyxin B in Salmonella pathogenesis. Nevertheless, there are many other two-component systems to mediate virulence and adaptation to environmental stresses, like SPI1 induction as well as biofilm formation by SirA/BarA16,17 and SPI2 expression regulation by SsrA/SsrB.18 Some two component systems regulate nutrition uptake too, as seen in the case of ttrRS system which helps in utilization of tetrathionate to produce an alternate electron donor thiosulphate.19 Interestingly these two-component systems are inter-dependent generating a complex network of their activity and regulation.
Global regulators
The success of a pathogen in establishing infection is predicted by the appropriate expression of virulence associated genes tuned by a set of regulators. These regulators control the expression of various genes in favor of the cell in response to certain environmental cues. Global regulators are called so due to their ability to regulate multiple genes simultaneously. To quote some examples, SirA regulates the genes required for gastroenteritis20 as well as biofilm formation.16 Another global regulator HilA, which is itself regulated by SirA, acts as a transcriptional regulator for SPI1, SPI4 and SPI5 that together mediate invasion of the host cells.20 Similarly CsrA is a very well-known global regulator which controls multiple functions including invasion, flagella synthesis, chemotaxis, biofilm formation, vitamin B12 synthesis and maltose operon.16,21 Interestingly, two or more global regulators can dictate the expression of similar genes; for example, Fnr regulates flagellar synthesis and chemotaxis along with CsrA. On the other hand, Fnr is assigned to regulate several important genes including genes for aerobic metabolism, NO˙ detoxification and anaerobic carbon utilization.22
Sentinels of Salmonella: Virulence Factors
Type three secretion systems
Type III secretion systems (T3SS) are present on the cell wall and possess a needle like structure. The major T3SS of Salmonella are encoded by two pathogenicity islands, SPI1 and SPI2. The assembly and functions of these T3SS are coordinated spatially and temporally. The T3SS are dedicated to secrete certain proteins which bring about specific effects in the microenvironment of the cell.
Salmonella pathogenicity island 1 (SPI1)
SPI1 plays pivotal role in both forms of diseases caused by Salmonella, i.e., gastroenteritis as well as systemic infection.23 It carries out multiple functions, which include cytotoxicity of macrophages,24 invasion of epithelial cells,25 inflammation and fluid secretion in ileum26 and cytokine secretion.27,28 SPI1 also induces apoptosis in macrophages24 and executes the exact opposite function in epithelial cells.29 A series of modifications are brought about by SPI1 inside the host to facilitate internalization of Salmonella30 (Table 2). For example, some SPI1 encoded proteins like InvG, InvJ, PrgH, PrgI, PrgK and SpaO assemble the needle complex, whereas others, including SipB, SipC and SipD, translocate effector proteins through this needle.28 Effector proteins may or may not be encoded by SPI1.
Table 2. Functions of the SPI encoded proteins.
| Major event | Proteins encoded |
|---|---|
|
SPI1 |
|
| Assembly of needle and secretion of effector proteins |
SpaO, InvJ, InvG, PrgI, PrgJ, PrgK, SipB, SipC |
| Actin cytoskeletal rearrangement via Rho GTPases and tight junction disruption |
SopE, SopE2, SopB (or SigD) |
| Actin polymerization by decrease in critical concentration |
SipA |
| Modulation of actin cytoskeleton by actin nucleation |
SipC (or SspC) |
| Regaining of cytoskeleton by reversing action of SopE, SopE2 and SopB |
SptP |
| Fluid accumulation in intestine |
SopA, SopD, SopB |
| Modulation of chloride channel to induce diarrhea |
SopB, SopE |
| Inhibition of NFkappaB activity and IL-8 secretion |
AvrA, SspH1 |
| Transmigration of polymorphonuclear leukocytes |
SipA, SopA |
| Activation of caspase 1 and autophagy in macrophages |
SipB (or SspB) |
|
SPI2 |
|
| Needle assembly |
SpiB, SpiC, SpiD etc. (also known as Ssa genes) |
| Effector protein translocation |
SseB, SseC, SseD |
| Interference with endosome trafficking |
SpiC |
| Maintenance of SCV integrity |
SifA |
|
Salmonella induced filament (Sif) formation and microtubule bundling |
SifA, SseF, Sseg, SopD2, PipB2 |
| Inhibition of actin polymerization |
SspH2 |
| Downregulation of Sif formation |
SpvB |
| Host cell dissemination |
SseI |
| Anaerobic respiration by reducing tetrathionate | TtrABC, TtrRS |
Salmonella pathogenicity island 2 (SPI2)
Genes within this pathogenicity island are not essential for gastroenteritis but are indispensable for systemic infection as they support the intracellular survival of Salmonella inside host cells. The formation and maintenance of the SCV involves a number of events controlled by this pathogenicity island30 (Table 2). SPI2 confers protection against reactive oxygen species (ROS)31 as well as reactive nitrogen intermediates (RNI)32 inside macrophages. SPI2 encoded tetrathionate reductase acts on tetrathionate to generate thiosulphate which acts as an alternate electron donor for Salmonella in tetrathionate containing environments like human gut, soil, decomposing carcasses.19,33 An additional T3SS, Spi/Ssa, is encoded by SPI2 during intracellular life of Salmonella. It is regulated by PhoP/PhoQ system and serves as the portal for the exchange of materials within the SCV and host cytoplasm.11
On account of their divergent roles, the simultaneous expression of these two type three secretion systems is not expected naturally. But in actual scenario, the spatiotemporal expression of these two pathogenicity islands cannot be demarcated clearly as there is evidence of their overlapping expression. This includes pre-emptive expression of SPI2 in gut lumen before invasion of epithelial cells in order to prepare Salmonella for traversing across basal side of epithelium into lamina propria34 as well as for the upcoming intracellular stress35 and residual SPI1 expression after internalization by macrophage to counteract host immune response by suppressing cytokine expression.36
Other pathogenicity islands
SPI3 encoded mgtC enables Salmonella to survive in Mg2+ starvation conditions, partly controlled by phoP/Q system, and is required for survival within macrophages as well as systemic infection in mouse model.37 SPI4 encodes a type I secretion system and mediates adhesion, whereas SPI5 encodes SopB. SPI7 is exclusively present in the host specific serovar S. Typhi and absent in Typhimurium. The main functions of this pathogenicity island include synthesis as well as the export of Typhi specific Vi antigen. Additionally, genes encoding SPI1 effector SopE and type IVB pilus lie within SPI7.38 Very little information is available about other pathogenicity islands, like Typhi specific SPI639 and SPI1040 encode chaperon-usher fimbrial operon. Similarly SPI8 encodes a pseudo bacteriocin and degenerate integrase, whereas SPI9 codes for a type I secretion system like SPI4.40
Adhesins
The mere attachment of the bacterium to the target cell consists of many steps mediated by various adhesins encoded either by fimbrial genes like type 1 fimbriae (fim),41 plasmid encoded fimbriae (pef),42 long polar fimbriae (lpf)43 and thin aggregative fimbriae (Agf)44 or non-fimbrial genes such as the autotransporters MisL45 and ShdA46 or SPI4 member SiiE.47 Each adhesin belonging to this pool is assigned to mediate adhesion to particular kind of cells due to specificity for the receptors present on the surface of these cells,48 for example, SPI4 is responsible for adhering to polarized cells47 whereas type 1 fimbriae fimH mediates attachment to dendritic cells.49 The same kind of cell can be bound by different adhesins with the progression of adhesion, as described recently in the form of irreversible docking by SPI1 that enhances adhesion mediated by type 1 fimbriae.48 Nevertheless, the flagellum is required to reach the target cell as well as to aid in adhesion in accordance with fimbriae.50
Plasmid encoded virulence genes
Salmonella possesses extrachromosomal genes which are equally important for infection. For example, Spv works in a SPI2 dependent manner and is essential for virulence of S. Typhimurium51 but not for Typhi. Similarly, Pef is a plasmid encoded adhesin to mediate adhesion for infecting gut epithelial cells.42
Counteracting the Worst
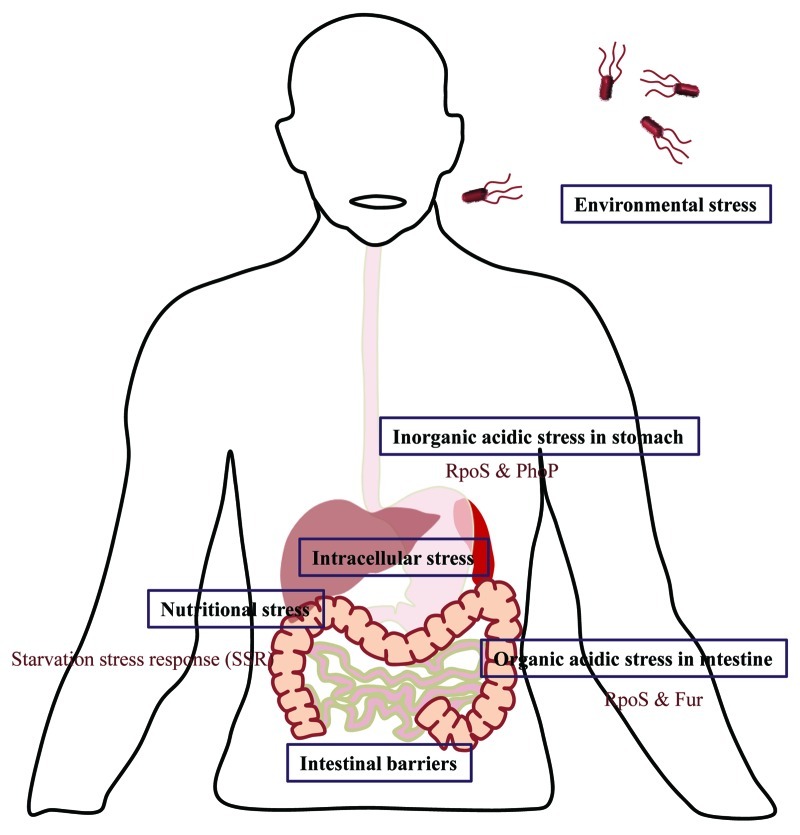
Like any other pathogen, Salmonella has its share of risks while entering the host system. After ingestion along with the contaminated food, Salmonella needs to withstand the highly acidic pH of the stomach. Once it reaches the intestine it establishes the infection in two modes. The invasive mode involves breaching of M cells leading to uptake by phagocytes, whereas the non-invasive mode refers to direct phagocytosis by dendritic cells. The various stresses induced upon Salmonella by the host act as environmental cues to be sensed by response regulators present within Salmonella for the expression of particular set of proteins to sustain the stress.
Acidic stress
The gastric pH acts as the first line of defense against Salmonella infection. The passage through the highly acidic environment of stomach generates the acid tolerance response (ATR) which ensures the escapade of Salmonella from acidic stress (Fig. 1). During ATR, the response regulator PhoP and alternate sigma factor RpoS protect from inorganic acid encountered inside stomach.52 On the other hand RpoS and Fur facilitate the survival in presence of weak organic acids like lactic acid in the intestine.53 Acidic pH also induces expression of certain virulence associated genes. For example, acidity induced STM1485 enables better intracellular replication of Salmonella.54
Figure 1. Challenges encountered by Salmonella. The text boxes represent the various stresses encountered by Salmonella during its life cycle and the open text describes the factors and signals generated by Salmonella in order to combat these stress conditions.
Physical barrier
To begin an intracellular lifestyle Salmonella must cross the gut epithelia (Fig. 2). While M cells allow easy entrance, epithelial cells do not favor passive entry. Salmonella induces membrane ruffling in epithelial cells by modifying actin cytoskeleton, exerted by SPI1, ultimately resulting in macropinocytosis of Salmonella as described in previous sections in this review. Also SPI2 mediated apoptosis helps Salmonella to cross the epithelial lining.55 The alternate path of breaching epithelial barrier includes uptake by CD18+ phagocytes traversing the gap between epithelial cells.56
Evasion of host defense
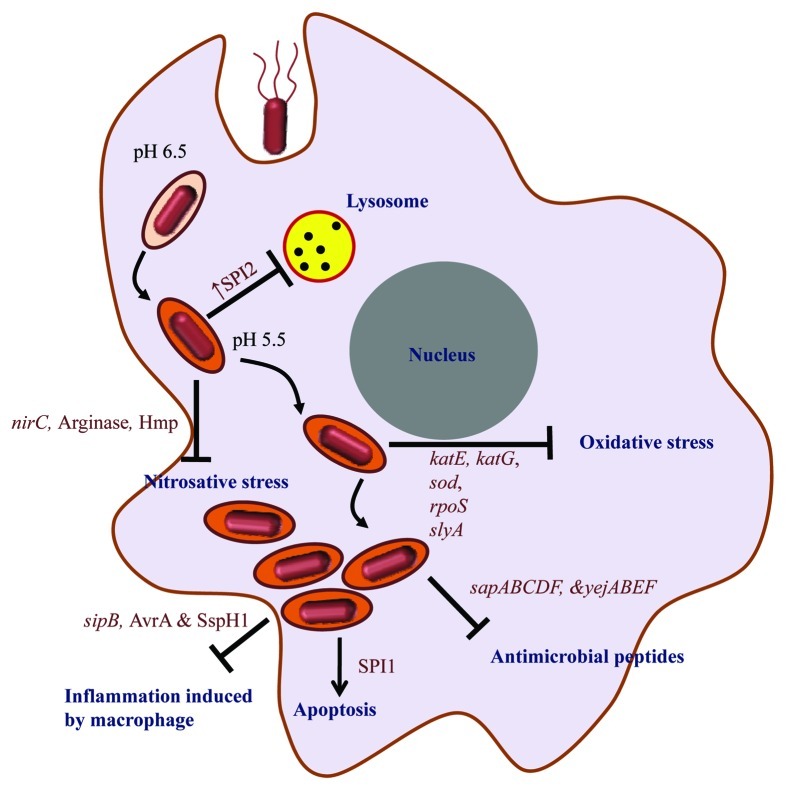
Immune responses generated by host constantly try to eliminate the pathogen (Fig. 3). Anti-microbial peptides produced by Paneth cells of gut epithelia and macrophages can kill extracellular and intracellular Salmonella respectively. To avoid this, Salmonella may undergo changes in the lipidA composition on the cell surface to prevent interactions of the cationic peptides or synthesize proteins like that coded by operons yejABEF57 and sap58 assigned to export these peptides outside cells. The smartness of Salmonella in deviating host defense is reflected in the strategy of retaining one bacterium per SCV to reduce the count of lysosome per SCV.59 Oxidative and nitrosative stresses are two most prominent immune strategies of host which are countered by Salmonella by various mechansims.60 The detrimental nitric oxide generation from arginine by host is counteracted by arginase production by Salmonella that competes with iNOS for arginine.61 Most interestingly, arginine is being transported inside SCV by recruiting host mCAT1 and mCAT2B to the SCV. From the SCV, arginine transporter encoded by ArgT operon is used to transport arginine inside Salmonella.62
Figure 3. Immune evasion strategies of Salmonella. The intracellular life-cycle of Salmonella includes the entry of the bacterium in the host cell, SCV formation (whose pH changes from 6.5 to 5.5 depicted by change in the color of SCV compartment), evasion of host immune response and ultimately host cell death by apoptosis. The text in dark blue shows the immune responses and processes within the host cell that take place during Salmonella infection and text in dark red depicts the factors that help Salmonella to evade these immune responses.
Nutritional stress
Starvation for nutrients inside the host is very common which leads to starvation stress response that enable Salmonella to withstand the stress as well as to counteract other environmental stresses.63 An excellent example of Salmonella induced host manipulation to meet its own nutritional requirements is presented by tetrathionate production induced inflammation during Salmonella infection.33 The excess of tetrathionate aids in competing with gut micro-flora for electron source and hence better survival.
Availability of Tools
Cell-culture model/in vitro model
M cells
M cells, present in Peyer’s patches, serve as the gateway for Salmonella to enter host reticuloendothelial system. As M cells lack glycocalyx, Salmonella can conveniently enter these cells to be further taken up by the underlying macrophages. The adhesion of Salmonella to M cells is believed to be mediated by the fimbrial assembly chaperone and the invasion is receptor mediated64 independently of SPI1 and SPI2.65 Although the cytotoxicity of M cells by Salmonella is not clearly understood, the regulator SlyA seems to play a role in damaging M cells and strains defective in invasion are attenuated in killing M cells.64 The caveolae mediated entry in M cells, was deduced by using a co-culture of Caco-2 cells and Raji B cells.66,67
Epithelial cells
Epithelial cells beside M cells or within an organ like gall bladder engulf Salmonella by macropinocytosis, in SPI1 dependent manner. In cell-culture model, after internalization the monolayer epithelial cells are directed toward caspase 3 mediated apoptosis depending upon the effector proteins encoded by SPI2 and spv loci,55 whereas polarized enterocytes are lysed due to lipid peroxidation by Salmonella induced ROS generation.68 To name few, HeLa, CaCo-2, HT-29, etc. serve as very good model cell-lines for studying invasion of epithelial cell by Salmonella and henceforth its proliferation.
Dendritic cells
Salmonella can breach gut epithelia by an alternate mechanism by being engulfed by dendritic cells (DCs). These DCs are also major antigen presenting cells like macrophages which phagocytose Salmonella and present antigen to the specific CD4+T and CD8+T cells. Although, they do not provide hospitable environment for the survival of the pathogen, they act as steady carrier of Salmonella for its passive dissemination to systemic sites. Also Salmonella induces caspase-1 mediated cytotoxicity in DCs depending upon SPI1 needle assembly and the expression of SPI1 effector protein SipB.30 The killing is possibly mediated by stimulation of P2X7 receptor or pore forming property of SPI1 which leads to leakage of cytoplasmic matter.69 Primary cells isolated from bone marrow for animal models or healthy humans are used as in-vitro model for dendritic cells.
Macrophages
They act as reservoir for Salmonella and play the most vital role in the dissemination as well as the antigen presentation of Salmonella. The macrophages present in the gut associated lymphoid tissue phagocytose Salmonella as soon as the intestinal epithelium is breached and harbor them until they undergo apoptosis induced by SPI1.24 The route of macrophages through reticuloendothelial system, while carrying Salmonella, is believed to be one major reason behind systemic site infections. A murine macrophage like cell line RAW 264.7 is the most useful model cell line to study intracellular survival of Salmonella within macrophages. Other cell lines like murine macrophages J774-A.1, most preferred by S. Typhimurium, can also be used.
Monocytes and granulocytes
The idea of dendritic cells being the major antigen presenting cells vanished when it was discovered that transport and antigen presentation of Salmonella in lymph is mainly performed by monocytes and granulocytes instead of dendritic cells.70 The example of survival within immature granulocytes in association with malaria71 presents a special situation of survival of Salmonella in unconventional targets. The model used for such non-DC myeloid cells are primary cells isolated from animal models. The human monocyte cell line THP-1 can provide for the in-vitro model for monocytes.
Recently it was discovered that pathogenesis of Salmonella varies based on the polarization status of the cells. Polarized cells allowed easier internalization of Salmonella than non-polarized cells and phagocytes and intracellular survival in polarized cells was found to be independent of SPI2, which is otherwise essential for surviving inside other cells types.72
Animal models/in vivo model
Salmonella infects a wide range of animal hosts and the causative agent of human infection usually comes from livestock in the form of meat, eggs and similar products. Hence animal models are essential to improvise understanding of pathogenesis as it helps to extrapolate the results to humans. Animal models are used for the two major forms of diseases that occur in humans namely enteritis and systemic typhoid. There are suitable models for each kind of infection. The susceptible mouse strain BALB/c, lacking Nramp1 protein, is used most commonly for Salmonella Typhimurium infections, as the manifestation of disease in this model resembles closely to that of humans. C57BL/6 is another common strain of mouse used as a model system. In comparison to mouse, bovine model is considerably more suitable to study enteritis.5 Rhesus monkeys are also used for enteritis.5 Unfortunately, there is no ideal animal model available for S. Typhi infection. The mouse model for typhoid varies from that of human typhoid fever. For example, in case of Typhimurium infection of mice, few genes, such as spv operon, are essential that are not required by Typhi to infect humans. Hence it remains a challenge to study the pathogenesis of Typhi infection at physiological level. Although there is provision of artificial systems like iron-treated mice for Typhi infection,73 these are not preferred over the natural mouse model for Typhimurium infection. Introduction of humanized mice has generated some hope. One such example, namely the humanized immune system (HIS) mouse model, has the incorporation of human immune cells into the reticuloendothelial system of mouse.74 Typhoid can also be induced in chimpanzees by oral infection.5 Also, C. elegans can serve as a potential animal model for studying Salmonella pathogenicity.75
Different Outcomes of Infections
The peculiarity of Salmonella infection is depicted by its ability to bring about different outcomes in different targets during the course of infection. As described previously, each cell type targeted by Salmonella meets a different fate. Similar effects are implied in organs harboring these cells. In spleen, the highest population of cells containing Salmonella are found to be monocytes and neutrophils, whereas liver shows accumulation of macrophages, both resulting in splenomegaly and hepatomegaly respectively.5,7 The gall bladder harbors Salmonella within the favorable environment of the epithelial cells.76 Mesenteric lymph node (MLN) prohibits dissemination by restricting the trafficking of Salmonella containing DCs.77 The fact that B cells carry Salmonella to bone marrow, gives a possible explanation for Salmonella induced osteomyelitis.7 On the other hand, some regions like gall stones serve as a platform for biofilm formation.78 Occasionally Salmonella crosses the blood brain barrier and causes meningitis, mainly in infants.79 The ultimate stage of infection is reached in the form of bacteremia which describes the presence of bacteria in blood circulation.
Other Facets
Salmonella and cancer
It was observed more than 100 y ago that bacterial infections can reduce tumor growth and since then several studies have been conducted to use bacteria as a vector for cancer therapy including Shigella flexneri, Listeria monocytogenes, Lactococcus lactis, E. coli, etc.80 Researchers showed specific interest in Salmonella because of their preferential colonization in solid tumors and the retardation of the tumor growth.81,82 Avirulent strains of Salmonella can reduce the tumor size directly83 or through the expressed therapeutic proteins84 in mouse models. Different attenuated strains of Salmonella have been used for cancer therapy85-90 and some of them made it to phase I clinical trials9,52,53,57 as in case of metastatic melanoma in human patients, attenuated Salmonella strain VNP20009 was used. Although it showed only moderate tumor targeting.91 Type 3 secretion system of Salmonella (T3SS) has been exploited for cancer therapy as well as cancer vaccination.92 The siRNA against MDR1 gene that codes for P-glycoprotein (ATP binding cassette transporters), has been delivered through attenuated Salmonella Typhi to revert multidrug-resistant tumor cells. Salmonella could retard the tumor growth and the tumor cells have responded to the chemotherapy after siRNA delivery through Salmonella.82 Obligate anaerobic bacteria are restricted to anaerobic region of tumor and hence cannot target the vascular system, whereas Salmonella, which is a facultative anaerobe, can target the vascular system as well as cause vessel destruction and tumor retardation.93
There are few reports addressing the specificity of Salmonella toward tumor.94,95 High-throughput screening of Salmonella mutants for their preferential growth in tumors showed that STM3120 is even more efficient than aroA for the tumor colonization and targeting.95 Twelve elements which are expressed only in tumor but not in normal tissues have been predicted using promoter trap library in Salmonella Typhimurium. These elements are very specific and can be used to express therapeutic proteins only in the tumor cells.96 From the phase I human trials, it was observed that the bacterial strain could not localize in the tumor cells in human, whereas the ability and colonization in the tumor was very efficient in murine model.91 These studies clearly show that there are specific host pathogen interactions which are not well understood.
Salmonella and vaccine delivery
Apart from the tumor therapy, Salmonella has also been known as a vector for vaccination because of the ability to induce immune response.97 Salmonella T3SS is widely exploited to deliver the antigen and elicit immune response against cancer.92,98-100 Apart from cancer Salmonella was also tested as a vaccine candidate for pneumonia by delivering pneumococcal PspA antigen101 and against Helicobacter pylori by delivering A and B subunits of H. pylori urease.102 Although, attenuated Salmonella induces cell mediated immune response (Th1 cells and IgG2a class switching) in cancer immunity,92,99 mixed Th1 and Th2 responses have also been reported in case of Salmonella mediated vaccination against H. pylori102 and Streptococcus pneumonia.101
Mathematical Models of Salmonella Infection
A number of mathematical models are developed in the field of infection biology that help researchers to look into the infectious diseases virtually. It is an interdisciplinary approach where biological experiments are translated into equations used to analyze and interpret the data easily.
Mathematical models for intracellular distribution and population dynamics at whole cell level of Salmonella enterica have been developed from experimental data. Wild-type isogenic tagged strains were used to infect the same animal simultaneously to check the spread of the bacteria in vivo and based on that the model has been developed.103,104 The heterogeneous behavior of Salmonella infection (early rapid replication of bacteria and local spread of bacteria in later stage) was used in this model to understand Salmonella spread in vivo.103 Mathematical model for Salmonella infection of macrophages at single cell level explains the possibility of two populations of macrophages. The variation in infection at single cell level may be due to the heterogeneity in the cell population.105 Apart from infection models, a mathematical model for Salmonella T3SS (SPI1) regulation by SirA through HilA and HilC was developed and this model can be used to predict the virulence and intermediate components in the regulation in accordance with the experimental results.106
Mathematical models can provide some clue for antiviral therapy against HIV by understanding the basic mechanism of viral spread and immunity.107,108 Few models are only available for bacterial infection and diseases and a number of models have been developed for Salmonella dynamics.109-112 In future more accurate mathematical models can be used for a better prediction and treatment of the diseases.
Model for Other Intracellular Pathogens
Despite having stark differences in pathogenesis, many intracellular pathogens resemble Salmonella in various aspects of infection and strategies for survival within the host. Additionally, it is more feasible to perform genetic engineering in Salmonella as compared with other pathogenic bacteria. This virtue lets one to exploit Salmonella to generate information for the comparatively fastidious pathogens. For example, the gene noxR3 was shown to be required by Mycobacterium for combating oxidative and nitrosative stress by expressing noxR3 in S. Typhimurium.113 The process of vacuole formation in case of intravacuolar pathogens is similar to SCV formation up to certain stage, for instance, Mycobacterium, Brucella, Legionella and Chlamydia exploit endosomal pathway and avoid fusion with lysosome by various strategies, one of them being the accumulation of cholesterol in vacuolar membrane, as seen in Salmonella.2 Recently it was shown that Salmonella effector protein SipC interacts with host protein syntaxin6 to recruit LAMP1 on SCV in order to stabilize the vacuole and prevent LAMP1 recruitment on lysosome,114 which may hint at similar mechanism adapted by other intracellular pathogens too, to maintain their intracellular niche. To quote example of extrapolation of information obtained from work on Salmonella to other pathogens, the characterization of arylamine N-acetyltransferases (NATs) in Mycobacterium in inactivating the antitubercular drug isoniazid was done based on the knowledge of NATs in Salmonella.115 Thus it is plausible to utilize the information obtained from the studies on SCV formation and integrity for studying intracellular life of other intravacuolar pathogens, hence fulfilling the purpose of Salmonella being a model pathogen.
Challenges
Although Salmonella represents the group of well-studied intracellular pathogens, certain challenges do exist in the research dealing with pathogenesis of Salmonella. The non-availability of an animal model for S. Typhi presents the best example. On the other hand, there is no mouse epithelial cell-line available for studying intracellular life of S. Typhimurium within epithelial cells. Hence extrapolation of results of S. Typhimurium to S. Typhi is not possible in all cases. Moreover, the ability of Salmonella to survive in various extreme conditions within cells like macrophages, neutrophils and dendritic cells with the help of numerous virulence proteins generates a complex network of functioning of all these effector proteins. As a result, deciphering the underlying mechanisms becomes quite challenging. Further, complete understanding of the mechanism of evasion of lysosomal fusion to SCV remains as one of the biggest challenges, pointing at the requirement of better detection of intracellular Salmonella by means of appropriate markers as well as better imaging techniques. The development of such tools would certainly lead us to the answers of many questions related to Salmonella pathogenesis.
Conclusion
Salmonella displays most elegant mechanisms of manipulation of the host. The diversity in the modes of evasion of Salmonella from host immune system gives an overall view of major strategies followed by most of the intracellular pathogens makes it a model pathogen. Difference in pathogenesis of different serovars, Typhi and Typhimurium, demonstrate the complicated lifestyle of a pathogen that can be tuned according to the type of host. Adaptation of such variable lifestyles is attributable to acquisition of numerous virulence associated genes over millions of years. The orchestrated action of these virulence proteins result in two major modes of Salmonella infection, local gastroenteritis or systemic typhoid. The latter imparts minimal damage to host providing optimal conditions for survival of pathogen. Recent contradictions of many established facts, such as spatiotemporal overlapping expression of SPI1 and SPI2, survival within hostile environment of neutrophils and dendritic cell mediated dissemination, describe the challenges lying in future of Salmonella related studies. Apart from being a successful pathogen, Salmonella has served to be a useful system for various therapeutic and biotechnological applications. This further paves the path for extensive research to dissect the unknown aspects of Salmonella infection.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/21087
References
- 1.Porwollik S, McClelland M. Lateral gene transfer in Salmonella. Microbes Infect. 2003;5:977–89. doi: 10.1016/S1286-4579(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 2.Kumar Y, Valdivia RH. Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe. 2009;5:593–601. doi: 10.1016/j.chom.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deiwick J, Salcedo SP, Boucrot E, Gilliland SM, Henry T, Petermann N, et al. The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect Immun. 2006;74:6965–72. doi: 10.1128/IAI.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009;7:333–40. doi: 10.1038/nrmicro2112. [DOI] [PubMed] [Google Scholar]
- 5.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Bäumler AJ. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 2001;3:1335–44. doi: 10.1016/S1286-4579(01)01495-2. [DOI] [PubMed] [Google Scholar]
- 6.Tenor JL, McCormick BA, Ausubel FM, Aballay A. Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr Biol. 2004;14:1018–24. doi: 10.1016/j.cub.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 7.Geddes K, Cruz F, Heffron F. Analysis of cells targeted by Salmonella type III secretion in vivo. PLoS Pathog. 2007;3:e196. doi: 10.1371/journal.ppat.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groisman EA, Ochman H. Pathogenicity islands: bacterial evolution in quantum leaps. Cell. 1996;87:791–4. doi: 10.1016/S0092-8674(00)81985-6. [DOI] [PubMed] [Google Scholar]
- 9.Eswarappa SM, Karnam G, Nagarajan AG, Chakraborty S, Chakravortty D. lac repressor is an antivirulence factor of Salmonella enterica: its role in the evolution of virulence in Salmonella. PLoS One. 2009;4:e5789. doi: 10.1371/journal.pone.0005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas RL, Lostroh CP, DiRusso CC, Spector MP, Wanner BL, Lee CA. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar typhimurium. J Bacteriol. 2000;182:1872–82. doi: 10.1128/JB.182.7.1872-1882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bijlsma JJ, Groisman EA. The PhoP/PhoQ system controls the intramacrophage type three secretion system of Salmonella enterica. Mol Microbiol. 2005;57:85–96. doi: 10.1111/j.1365-2958.2005.04668.x. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj V, Hwang C, Lee CA. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–27. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim CC, Falkow S. Delineation of upstream signaling events in the salmonella pathogenicity island 2 transcriptional activation pathway. J Bacteriol. 2004;186:4694–704. doi: 10.1128/JB.186.14.4694-4704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beuzón CR, Méresse S, Unsworth KE, Ruíz-Albert J, Garvis S, Waterman SR, et al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–49. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merighi M, Carroll-Portillo A, Septer AN, Bhatiya A, Gunn JS. Role of Salmonella enterica serovar Typhimurium two-component system PreA/PreB in modulating PmrA-regulated gene transcription. J Bacteriol. 2006;188:141–9. doi: 10.1128/JB.188.1.141-149.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teplitski M, Al-Agely A, Ahmer BM. Contribution of the SirA regulon to biofilm formation in Salmonella enterica serovar Typhimurium. Microbiology. 2006;152:3411–24. doi: 10.1099/mic.0.29118-0. [DOI] [PubMed] [Google Scholar]
- 17.Johnston C, Pegues DA, Hueck CJ, Lee A, Miller SI. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–27. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 18.Garmendia J, Beuzón CR, Ruiz-Albert J, Holden DW. The roles of SsrA-SsrB and OmpR-EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology. 2003;149:2385–96. doi: 10.1099/mic.0.26397-0. [DOI] [PubMed] [Google Scholar]
- 19.Hensel M, Hinsley AP, Nikolaus T, Sawers G, Berks BC. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol Microbiol. 1999;32:275–87. doi: 10.1046/j.1365-2958.1999.01345.x. [DOI] [PubMed] [Google Scholar]
- 20.Ahmer BM, van Reeuwijk J, Watson PR, Wallis TS, Heffron F. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol. 1999;31:971–82. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 21.Lawhon SD, Frye JG, Suyemoto M, Porwollik S, McClelland M, Altier C. Global regulation by CsrA in Salmonella typhimurium. Mol Microbiol. 2003;48:1633–45. doi: 10.1046/j.1365-2958.2003.03535.x. [DOI] [PubMed] [Google Scholar]
- 22.Fink RC, Evans MR, Porwollik S, Vazquez-Torres A, Jones-Carson J, Troxell B, et al. FNR is a global regulator of virulence and anaerobic metabolism in Salmonella enterica serovar Typhimurium (ATCC 14028s) J Bacteriol. 2007;189:2262–73. doi: 10.1128/JB.00726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen-Wester I, Hensel M. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 2001;3:549–59. doi: 10.1016/S1286-4579(01)01411-3. [DOI] [PubMed] [Google Scholar]
- 24.Chen LM, Kaniga K, Galán JE. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–15. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 25.Galán JE. Interaction of Salmonella with host cells through the centisome 63 type III secretion system. Curr Opin Microbiol. 1999;2:46–50. doi: 10.1016/S1369-5274(99)80008-3. [DOI] [PubMed] [Google Scholar]
- 26.Galyov EE, Wood MW, Rosqvist R, Mullan PB, Watson PR, Hedges S, et al. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:903–12. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- 27.Hobbie S, Chen LM, Davis RJ, Galán JE. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–9. [PubMed] [Google Scholar]
- 28.Galán JE. Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 29.Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–64. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- 30.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 31.Janssen R, van der Straaten T, van Diepen A, van Dissel JT. Responses to reactive oxygen intermediates and virulence of Salmonella typhimurium. Microbes Infect. 2003;5:527–34. doi: 10.1016/S1286-4579(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 32.Chakravortty D, Hansen-Wester I, Hensel M. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med. 2002;195:1155–66. doi: 10.1084/jem.20011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–9. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller AJ, Kaiser P, Dittmar KE, Weber TC, Haueter S, Endt K, et al. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe. 2012;11:19–32. doi: 10.1016/j.chom.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Brown NF, Vallance BA, Coombes BK, Valdez Y, Coburn BA, Finlay BB. Salmonella pathogenicity island 2 is expressed prior to penetrating the intestine. PLoS Pathog. 2005;1:e32. doi: 10.1371/journal.ppat.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavlova B, Volf J, Ondrackova P, Matiasovic J, Stepanova H, Crhanova M, et al. SPI-1-encoded type III secretion system of Salmonella enterica is required for the suppression of porcine alveolar macrophage cytokine expression. Vet Res. 2011;42:16. doi: 10.1186/1297-9716-42-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanc-Potard AB, Solomon F, Kayser J, Groisman EA. The SPI-3 pathogenicity island of Salmonella enterica. J Bacteriol. 1999;181:998–1004. doi: 10.1128/jb.181.3.998-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seth-Smith HM. SPI-7: Salmonella’s Vi-encoding Pathogenicity Island. J Infect Dev Ctries. 2008;2:267–71. doi: 10.3855/jidc.220. [DOI] [PubMed] [Google Scholar]
- 39.Townsend SM, Kramer NE, Edwards R, Baker S, Hamlin N, Simmonds M, et al. Salmonella enterica serovar Typhi possesses a unique repertoire of fimbrial gene sequences. Infect Immun. 2001;69:2894–901. doi: 10.1128/IAI.69.5.2894-2901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413:848–52. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 41.Lockman HA, Curtiss R., 3rd Isolation and characterization of conditional adherent and non-type 1 fimbriated Salmonella typhimurium mutants. Mol Microbiol. 1992;6:933–45. doi: 10.1111/j.1365-2958.1992.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 42.Bäumler AJ, Tsolis RM, Bowe FA, Kusters JG, Hoffmann S, Heffron F. The pef fimbrial operon of Salmonella typhimurium mediates adhesion to murine small intestine and is necessary for fluid accumulation in the infant mouse. Infect Immun. 1996;64:61–8. doi: 10.1128/iai.64.1.61-68.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bäumler AJ, Heffron F. Identification and sequence analysis of lpfABCDE, a putative fimbrial operon of Salmonella typhimurium. J Bacteriol. 1995;177:2087–97. doi: 10.1128/jb.177.8.2087-2097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grund S, Weber A. A new type of fimbriae on Salmonella typhimurium. Zentralbl Veterinarmed B. 1988;35:779–82. doi: 10.1111/j.1439-0450.1988.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 45.Dorsey CW, Laarakker MC, Humphries AD, Weening EH, Bäumler AJ. Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol Microbiol. 2005;57:196–211. doi: 10.1111/j.1365-2958.2005.04666.x. [DOI] [PubMed] [Google Scholar]
- 46.Kingsley RA, Santos RL, Keestra AM, Adams LG, Bäumler AJ. Salmonella enterica serotype Typhimurium ShdA is an outer membrane fibronectin-binding protein that is expressed in the intestine. Mol Microbiol. 2002;43:895–905. doi: 10.1046/j.1365-2958.2002.02805.x. [DOI] [PubMed] [Google Scholar]
- 47.Gerlach RG, Jäckel D, Stecher B, Wagner C, Lupas A, Hardt WD, et al. Salmonella Pathogenicity Island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell Microbiol. 2007;9:1834–50. doi: 10.1111/j.1462-5822.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 48.Misselwitz B, Kreibich SK, Rout S, Stecher B, Periaswamy B, Hardt WD. Salmonella enterica serovar Typhimurium binds to HeLa cells via Fim-mediated reversible adhesion and irreversible type three secretion system 1-mediated docking. Infect Immun. 2011;79:330–41. doi: 10.1128/IAI.00581-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo A, Lasaro MA, Sirard JC, Kraehenbühl JP, Schifferli DM. Adhesin-dependent binding and uptake of Salmonella enterica serovar Typhimurium by dendritic cells. Microbiology. 2007;153:1059–69. doi: 10.1099/mic.0.2006/000331-0. [DOI] [PubMed] [Google Scholar]
- 50.Dibb-Fuller MP, Allen-Vercoe E, Thorns CJ, Woodward MJ. Fimbriae- and flagella-mediated association with and invasion of cultured epithelial cells by Salmonella enteritidis. Microbiology. 1999;145:1023–31. doi: 10.1099/13500872-145-5-1023. [DOI] [PubMed] [Google Scholar]
- 51.Browne SH, Hasegawa P, Okamoto S, Fierer J, Guiney DG. Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol Med Microbiol. 2008;52:194–201. doi: 10.1111/j.1574-695X.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- 52.Bearson BL, Wilson L, Foster JW. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J Bacteriol. 1998;180:2409–17. doi: 10.1128/jb.180.9.2409-2417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baik HS, Bearson S, Dunbar S, Foster JW. The acid tolerance response of Salmonella typhimurium provides protection against organic acids. Microbiology. 1996;142:3195–200. doi: 10.1099/13500872-142-11-3195. [DOI] [PubMed] [Google Scholar]
- 54.Allam AS, Krishna MG, Sen M, Thomas R, Lahiri A, Gnanadhas DP, et al. Acidic pH induced STM1485 gene is essential for intracellular replication of Salmonella. Virulence. 2012;3:122–35. doi: 10.4161/viru.19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paesold G, Guiney DG, Eckmann L, Kagnoff MF. Genes in the Salmonella pathogenicity island 2 and the Salmonella virulence plasmid are essential for Salmonella-induced apoptosis in intestinal epithelial cells. Cell Microbiol. 2002;4:771–81. doi: 10.1046/j.1462-5822.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 56.Vazquez-Torres A, Jones-Carson J, Bäumler AJ, Falkow S, Valdivia R, Brown W, et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–8. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- 57.Eswarappa SM, Panguluri KK, Hensel M, Chakravortty D. The yejABEF operon of Salmonella confers resistance to antimicrobial peptides and contributes to its virulence. Microbiology. 2008;154:666–78. doi: 10.1099/mic.0.2007/011114-0. [DOI] [PubMed] [Google Scholar]
- 58.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8:11–26. [PubMed] [Google Scholar]
- 59.Eswarappa SM, Negi VD, Chakraborty S, Chandrasekhar Sagar BK, Chakravortty D. Division of the Salmonella-containing vacuole and depletion of acidic lysosomes in Salmonella-infected host cells are novel strategies of Salmonella enterica to avoid lysosomes. Infect Immun. 2010;78:68–79. doi: 10.1128/IAI.00668-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lahiri A, Lahiri A, Iyer N, Das P, Chakravortty D. Visiting the cell biology of Salmonella infection. Microbes Infect. 2010;12:809–18. doi: 10.1016/j.micinf.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Das P, Lahiri A, Lahiri A, Chakravortty D. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 2010;6:e1000899. doi: 10.1371/journal.ppat.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das P, Lahiri A, Lahiri A, Sen M, Iyer N, Kapoor N, et al. Cationic amino acid transporters and Salmonella Typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth. PLoS One. 2010;5:e15466. doi: 10.1371/journal.pone.0015466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spector MP, Cubitt CL. Starvation-inducible loci of Salmonella typhimurium: regulation and roles in starvation-survival. Mol Microbiol. 1992;6:1467–76. doi: 10.1111/j.1365-2958.1992.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 64.Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect. 2001;3:1183–90. doi: 10.1016/S1286-4579(01)01478-2. [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Argudo I, Jepson MA. Salmonella translocates across an in vitro M cell model independently of SPI-1 and SPI-2. Microbiology. 2008;154:3887–94. doi: 10.1099/mic.0.2008/021162-0. [DOI] [PubMed] [Google Scholar]
- 66.Gullberg E, Leonard M, Karlsson J, Hopkins AM, Brayden D, Baird AW, et al. Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem Biophys Res Commun. 2000;279:808–13. doi: 10.1006/bbrc.2000.4038. [DOI] [PubMed] [Google Scholar]
- 67.Lim JS, Na HS, Lee HC, Choy HE, Park SC, Han JM, et al. Caveolae-mediated entry of Salmonella typhimurium in a human M-cell model. Biochem Biophys Res Commun. 2009;390:1322–7. doi: 10.1016/j.bbrc.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 68.Mehta A, Singh S, Ganguly NK. Role of reactive oxygen species in Salmonella typhimurium-induced enterocyte damage. Scand J Gastroenterol. 1998;33:406–14. doi: 10.1080/00365529850171044. [DOI] [PubMed] [Google Scholar]
- 69.van der Velden AW, Velasquez M, Starnbach MN. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J Immunol. 2003;171:6742–9. doi: 10.4049/jimmunol.171.12.6742. [DOI] [PubMed] [Google Scholar]
- 70.Bonneau M, Epardaud M, Payot F, Niborski V, Thoulouze MI, Bernex F, et al. Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node. J Leukoc Biol. 2006;79:268–76. doi: 10.1189/jlb.0605288. [DOI] [PubMed] [Google Scholar]
- 71.Cunnington AJ, de Souza JB, Walther M, Riley EM. Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med. 2012;18:120–7. doi: 10.1038/nm.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hölzer SU, Hensel M. Divergent roles of Salmonella pathogenicity island 2 and metabolic traits during interaction of S. enterica serovar typhimurium with host cells. PLoS One. 2012;7:e33220. doi: 10.1371/journal.pone.0033220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Brien AD. Innate resistance of mice to Salmonella typhi infection. Infect Immun. 1982;38:948–52. doi: 10.1128/iai.38.3.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mian MF, Pek EA, Chenoweth MJ, Coombes BK, Ashkar AA. Humanized mice for Salmonella typhi infection: new tools for an old problem. Virulence. 2011;2:248–52. doi: 10.4161/viru.2.3.16133. [DOI] [PubMed] [Google Scholar]
- 75.Aballay A, Yorgey P, Ausubel FM. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol. 2000;10:1539–42. doi: 10.1016/S0960-9822(00)00830-7. [DOI] [PubMed] [Google Scholar]
- 76.Menendez A, Arena ET, Guttman JA, Thorson L, Vallance BA, Vogl W, et al. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J Infect Dis. 2009;200:1703–13. doi: 10.1086/646608. [DOI] [PubMed] [Google Scholar]
- 77.Griffin AJ, Li LX, Voedisch S, Pabst O, McSorley SJ. Dissemination of persistent intestinal bacteria via the mesenteric lymph nodes causes typhoid relapse. Infect Immun. 2011;79:1479–88. doi: 10.1128/IAI.01033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prouty AM, Schwesinger WH, Gunn JS. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun. 2002;70:2640–9. doi: 10.1128/IAI.70.5.2640-2649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srifuengfung S, Chokephaibulkit K, Yungyuen T, Tribuddharat C. Salmonella meningitis and antimicrobial susceptibilities. Southeast Asian J Trop Med Public Health. 2005;36:312–6. [PubMed] [Google Scholar]
- 80.Ryan RM, Green J, Lewis CE. Use of bacteria in anti-cancer therapies. Bioessays. 2006;28:84–94. doi: 10.1002/bies.20336. [DOI] [PubMed] [Google Scholar]
- 81.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–44. [PubMed] [Google Scholar]
- 82.Bermudes D, Low B, Pawelek J. Tumor-targeted Salmonella. Highly selective delivery vectors. Adv Exp Med Biol. 2000;465:57–63. doi: 10.1007/0-306-46817-4_6. [DOI] [PubMed] [Google Scholar]
- 83.Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V, et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65:3920–7. doi: 10.1158/0008-5472.CAN-04-3002. [DOI] [PubMed] [Google Scholar]
- 84.Weyel D, Sedlacek HH, Müller R, Brüsselbach S. Secreted human beta-glucuronidase: a novel tool for gene-directed enzyme prodrug therapy. Gene Ther. 2000;7:224–31. doi: 10.1038/sj.gt.3301072. [DOI] [PubMed] [Google Scholar]
- 85.Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg SA, Spiess PJ, Kleiner DE. Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium. J Immunother. 2002;25:218–25. doi: 10.1097/00002371-200205000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jia LJ, Wei DP, Sun QM, Huang Y, Wu Q, Hua ZC. Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci. 2007;98:1107–12. doi: 10.1111/j.1349-7006.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin Cancer Res. 2008;14:1905–12. doi: 10.1158/1078-0432.CCR-07-2050. [DOI] [PubMed] [Google Scholar]
- 89.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106:992–8. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffman RM. Tumor-targeting amino acid auxotrophic Salmonella typhimurium. Amino Acids. 2009;37:509–21. doi: 10.1007/s00726-009-0261-8. [DOI] [PubMed] [Google Scholar]
- 91.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–52. doi: 10.1200/JCO.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panthel K, Meinel KM, Sevil Domènech VE, Geginat G, Linkemann K, Busch DH, et al. Prophylactic anti-tumor immunity against a murine fibrosarcoma triggered by the Salmonella type III secretion system. Microbes Infect. 2006;8:2539–46. doi: 10.1016/j.micinf.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle. 2010;9:4518–24. doi: 10.4161/cc.9.22.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arrach N, Zhao M, Porwollik S, Hoffman RM, McClelland M. Salmonella promoters preferentially activated inside tumors. Cancer Res. 2008;68:4827–32. doi: 10.1158/0008-5472.CAN-08-0552. [DOI] [PubMed] [Google Scholar]
- 95.Arrach N, Cheng P, Zhao M, Santiviago CA, Hoffman RM, McClelland M. High-throughput screening for salmonella avirulent mutants that retain targeting of solid tumors. Cancer Res. 2010;70:2165–70. doi: 10.1158/0008-5472.CAN-09-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leschner S, Deyneko IV, Lienenklaus S, Wolf K, Bloecker H, Bumann D, et al. Identification of tumor-specific Salmonella Typhimurium promoters and their regulatory logic. Nucleic Acids Res. 2012;40:2984–94. doi: 10.1093/nar/gkr1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiang R, Luo Y, Niethammer AG, Reisfeld RA. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev. 2008;222:117–28. doi: 10.1111/j.1600-065X.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 98.Roider E, Jellbauer S, Köhn B, Berchtold C, Partilla M, Busch DH, et al. Invasion and destruction of a murine fibrosarcoma by Salmonella-induced effector CD8 T cells as a therapeutic intervention against cancer. Cancer Immunol Immunother. 2011;60:371–80. doi: 10.1007/s00262-010-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Panthel K, Meinel KM, Sevil Domènech VE, Trülzsch K, Rüssmann H. Salmonella type III-mediated heterologous antigen delivery: a versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int J Med Microbiol. 2008;298:99–103. doi: 10.1016/j.ijmm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 100.Xiong G, Husseiny MI, Song L, Erdreich-Epstein A, Shackleford GM, Seeger RC, et al. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int J Cancer. 2010;126:2622–34. doi: 10.1002/ijc.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang HY, Srinivasan J, Curtiss R., 3rd Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar typhimurium vaccine. Infect Immun. 2002;70:1739–49. doi: 10.1128/IAI.70.4.1739-1749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corthésy-Theulaz IE, Hopkins S, Bachmann D, Saldinger PF, Porta N, Haas R, et al. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–6. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grant AJ, Restif O, McKinley TJ, Sheppard M, Maskell DJ, Mastroeni P. Modelling within-host spatiotemporal dynamics of invasive bacterial disease. PLoS Biol. 2008;6:e74. doi: 10.1371/journal.pbio.0060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mastroeni P, Grant A, Restif O, Maskell D. A dynamic view of the spread and intracellular distribution of Salmonella enterica. Nat Rev Microbiol. 2009;7:73–80. doi: 10.1038/nrmicro2034. [DOI] [PubMed] [Google Scholar]
- 105.Gog JR, Murcia A, Osterman N, Restif O, McKinley TJ, Sheppard M, et al. Dynamics of Salmonella infection of macrophages at the single cell level. J R Soc Interface. 2012 doi: 10.1098/rsif.2012.0163. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ganesh AB, Rajasingh H, Mande SS. Mathematical modeling of regulation of type III secretion system in Salmonella enterica serovar Typhimurium by SirA. In Silico Biol. 2009;9:S57–72. [PubMed] [Google Scholar]
- 107.Bonhoeffer S, May RM, Shaw GM, Nowak MA. Virus dynamics and drug therapy. Proc Natl Acad Sci U S A. 1997;94:6971–6. doi: 10.1073/pnas.94.13.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wodarz D, Nowak MA. HIV therapy: managing resistance. Proc Natl Acad Sci U S A. 2000;97:8193–5. doi: 10.1073/pnas.97.15.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sheppard M, Webb C, Heath F, Mallows V, Emilianus R, Maskell D, et al. Dynamics of bacterial growth and distribution within the liver during Salmonella infection. Cell Microbiol. 2003;5:593–600. doi: 10.1046/j.1462-5822.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 110.Chapagain PP, van Kessel JS, Karns JS, Wolfgang DR, Hovingh E, Nelen KA, et al. A mathematical model of the dynamics of Salmonella Cerro infection in a US dairy herd. Epidemiol Infect. 2008;136:263–72. doi: 10.1017/S0950268807008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Setta A, Barrow PA, Kaiser P, Jones MA. Immune dynamics following infection of avian macrophages and epithelial cells with typhoidal and non-typhoidal Salmonella enterica serovars; bacterial invasion and persistence, nitric oxide and oxygen production, differential host gene expression, NF-κB signalling and cell cytotoxicity. Vet Immunol Immunopathol. 2012;146:212–24. doi: 10.1016/j.vetimm.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 112.Setta AM, Barrow PA, Kaiser P, Jones MA. Early immune dynamics following infection with Salmonella enterica serovars Enteritidis, Infantis, Pullorum and Gallinarum: Cytokine and chemokine gene expression profile and cellular changes of chicken cecal tonsils. Comp Immunol Microbiol Infect Dis. 2012;35:397–410. doi: 10.1016/j.cimid.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Ruan J, St John G, Ehrt S, Riley L, Nathan C. noxR3, a novel gene from Mycobacterium tuberculosis, protects Salmonella typhimurium from nitrosative and oxidative stress. Infect Immun. 1999;67:3276–83. doi: 10.1128/iai.67.7.3276-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Madan R, Rastogi R, Parashuraman S, Mukhopadhyay A. Salmonella acquires lysosome-associated membrane protein 1 (LAMP1) on phagosomes from Golgi via SipC protein-mediated recruitment of host Syntaxin6. J Biol Chem. 2012;287:5574–87. doi: 10.1074/jbc.M111.286120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Payton M, Auty R, Delgoda R, Everett M, Sim E. Cloning and characterization of arylamine N-acetyltransferase genes from Mycobacterium smegmatis and Mycobacterium tuberculosis: increased expression results in isoniazid resistance. J Bacteriol. 1999;181:1343–7. doi: 10.1128/jb.181.4.1343-1347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]