Homologs of the Bacillus subtilis DivIVA protein can be found in a wide variety of Gram-positive bacteria. DivIVA proteins are coiled-coil proteins that bind to the cytosolic face of the cytoplasmic membrane and accumulate at membrane regions with higher curvature. By directly interacting with downstream proteins they serve as spatial regulators of other cellular processes. Initial DivIVA studies focused on its role as a topological determinant for the MinCDJ division inhibiting complex in B. subtilis, but recent evidence suggests that DivIVA can fulfill more diverse roles in different species of the firmicutes and actinomycetes and can even be relevant for virulence.
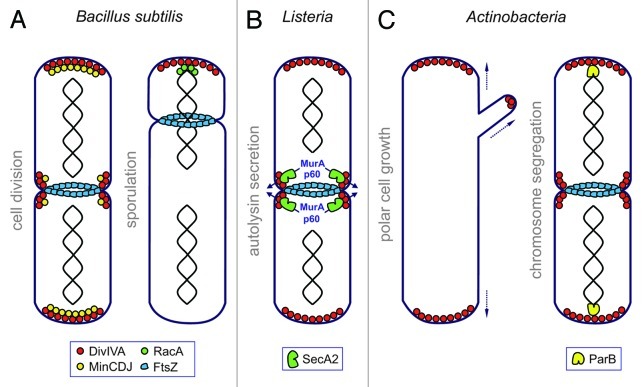
DivIVA proteins are highly conserved among different Gram-positive bacteria. They are lipid binding proteins sensitive to membrane curvature and accumulate at areas of the cytoplasmic membrane that are more strongly bent (concave) (Lenarcic et al., EMBO J 2009; Ramamurthi and Losick, Proc Natl Acad Sci U S A 2009). Zones with higher membrane curvature occur at the cell poles of non-coccoid bacteria and establish along the invaginating membrane ring at the site of cell division. DivIVA orthologs of different bacteria were shown to localize to these subcellular regions even when expressed heterologously in other taxa suggesting that membrane binding and curvature sensitivity is an intrinsic feature of DivIVA and does not require the assistance of other factors (Edwards et al., EMBO J 2000). As an autonomously localizing protein, DivIVA acts as a topological determinant for different cellular processes. DivIVA of Bacillus subtilis functions as a spatial regulator of the FtsZ-inhibiting MinCD proteins and restricts their activity to the polar and septal regions of the cell, preventing division in the chromosome-free spaces near the poles and in the vicinity of an existing Z-ring (Marston et al., Genes Dev 1998; Fig. 1A). During sporulation of B. subtilis however, polar localized DivIVA tethers the origin region of the spore chromosome to the distal pole of the prespore. This polar origin recruitment is vital for the correct transfer of one chromosome copy from the mother cell into the developing spore compartment and is thus essential for sporulation (Thomaides et al., Genes Dev 2001; Fig. 1A). Both functions involve direct protein-protein interactions of DivIVA to specific binding partners: The trans-membrane protein MinJ is the molecular bridge mediating the contact between DivIVA and MinD to ensure polar and septal recruitment of MinCD (Bramkamp et al., Mol Microbiol 2008; Patrick and Kearns, Mol Microbiol 2008), whereas RacA acts as a sporulation specific DivIVA binding partner (Ben-Yehuda et al., Science 2003; Wu and Errington, Mol Microbiol 2003). This protein binds to 14 bp inverted repeats scattered around the chromosomal origin and also binds directly to DivIVA (Ben-Yehuda et al., Mol Cell 2005; Lenarcic et al., EMBO J 2009).
Figure 1. Models illustrating the diverse DivIVA functions in different Gram-positive bacteria. (A) B. subtilis DivIVA recruits the Z-ring inhibitors MinCDJ away from mid-cell in order to ensure Z-ring formation in the chromosome free space at mid-cell. During sporulation the prespore chromosome is tethered to the prespore pole via RacA, which binds to polar DivIVA. (B) L. monocytogenes DivIVA is required for efficient protein secretion via the accessory secretion ATPase SecA2. DivIVA recruits the autolysins p60 and MurA to the division septum from where they are secreted in a SecA2-dependent manner. Please note that septal localization of SecA2 is independent of DivIVA. (C) In different actinobacteria, DivIVA marks the site of cell wall synthesis at the poles and the hyphal tips indicating that DivIVA recruits cell wall biosynthetic proteins to these subcellular regions. DivIVA of several actinobacteria furthermore interacts with ParB, an origin binding protein, in order to recruit the origin to the cell poles. It is believed that this event is required for efficient chromosome segregation.
In a recent study (Halbedel et al., Mol Microbiol 2012) we have analyzed the function of DivIVA in the facultative human pathogen Listeria monocytogenes, which is closely related to B. subtilis. To our surprise, deletion of the divIVA gene in L. monocytogenes caused a phenotype that was different from the classical filamentous and mini-cell producing phenotype of a B. subtilis ΔdivIVA mutant. In contrast, ΔdivIVA mutants of L. monocytogenes grew as long chains of cells that had clearly completed cell division, which however remained attached even after completion of cross wall synthesis. Electron microscopy revealed that the daughter cell cytoplasms of the ΔdivIVA mutant were precisely separated at the division site and a cross wall was made in-between them. But whereas wild-type cells degrade this cross wall to physically separate the daughter cells, cross walls of the ΔdivIVA mutant showed no signs of degradation. Obviously, deletion of divIVA had affected the post-divisional separation of daughter cells and not cell division per se. Since L. monocytogenes encodes two autolysins, p60 and MurA, that play a role in cross wall turnover (Pilgrim et al., Infect Immun 2003; Carroll et al., J Bacteriol 2003), we tested their production in the ΔdivIVA background. We found that secretion of p60 and MurA was severely impaired in ΔdivIVA cells and this result led us to the accessory secretion ATPase SecA2. Accessory SecA2 ATPases are encoded by a number of Gram-positive pathogens in addition to the canonical housekeeping SecA and specifically help to translocate a small subset of secretory proteins across the cytoplasmic membrane. Both listerial autolysins are known substrates of this secretion route (Lenz et al., Proc Natl Acad Sci U S A 2003) and therefore it appeared plausible that DivIVA somehow is involved in this. We tested this concept by comparing the secretion defect of the ΔsecA2 deletion strain with the ΔdivIVA mutant. And indeed, both mutants were similarly deficient in p60 and MurA secretion and accumulated the precursors of p60 and MurA inside their cytoplasms to the same extent. A microscopic analysis of the subcellular localization of p60, MurA and SecA2 gave us the first hint as to how DivIVA might act at the molecular level. GFP-tagged versions of p60, MurA and SecA2 localized to the division sites, suggesting that SecA2-dependent secretion of p60 and MurA occurs at the septum. In a ΔdivIVA mutant however, p60-GFP and MurA-GFP are diffuse whereas the subcellular distribution of SecA2 was unaffected. One possible interpretation is that DivIVA might recruit the pre-proteins of p60 and MurA to the invaginating membrane ring that occurs at the division site as soon as the divisome starts to constrict (Fig. 1B). Septal autolysin secretion might be one mechanism to restrict the activity of p60 and MurA to the freshly synthesized cross wall peptidoglycan that is the substrate of both autolysins. Cells lacking DivIVA fail to target the secretion substrates to the translocon, hence secretion of autolysins cannot occur. This role of DivIVA in SecA2-dependent autolysin secretion had pleiotropic implications for the biology of L. monocytogenes: likewise to naturally occurring secA2 mutants or deletion mutants in secA2 (Lenz and Portnoy, Mol Microbiol 2002), ΔdivIVA cells formed rough colonies. They cannot grow as a biofilm and were non-motile even though flagella were clearly assembled on the cell surface. In vitro infection models revealed that ΔdivIVA cells were severely attenuated in terms of invasion and cell-to-cell spread and the severity of these effects was similar to those seen with a ΔsecA2 mutant strain. Most likely, the formation of long cell chains impairs the ability of L. monocytogenes to enter eukaryotic host cells and prevents coordinated intracellular motility and thus bacterial spreading in host tissues.
These data already indicated that the function of DivIVA proteins might be quite diverse even in closely related species. However, another recent publication (Donovan et al., Mol Microbiol 2012) further illustrates the diversity of DivIVA functions. In the actinomycete Corynebacterium glutamicum, DivIVA is supposed to act as a polar origin tethering factor during chromosome segregation (Fig. 1C). This was deduced from experiments where DivIVA and the centromere-binding protein ParB were expressed as fluorescently tagged versions in Escherichia coli. Polar and septal localization of ParB-CFP clearly required the presence of DivIVA in the heterologous E. coli background. ParB binds to 16 bp direct repeats, called parS sites, near the origin of the C. glutamicum chromosome, and was found to accumulate close to the cell poles in C. glutamicum cells (Donovan et al., J Bacteriol 2010). The divIVA gene could not be deleted from the C. glutamicum genome, suggesting that it codes for an essential cellular function (Ramos et al., Microbiology 2003) but depletion of DivIVA resulted in coccoid cells, which normally would grew as rods (Letek et al., J Bacteriol 2008). Whether polar localization of ParB is lost and chromosomes are less efficiently segregated under this condition has so far not been addressed experimentally. But expression of a mutant ParB protein that no longer binds to DivIVA (ParBR21A) in cells of C. glutamicum resulted in a clear chromosome segregation defect as many anucleate cells were produced. In line with these results, ParBR21A fused to CFP was less frequently recruited to the cell poles (Donovan et al., Mol Microbiol 2012). Taken together these data support a model in which polar localized DivIVA is used to attach the chromosomal origins to the cell pole via the centromere-binding protein ParB and this event facilitates chromosome segregation in C. glutamicum (Fig. 1C). The same study provided evidence for similar interactions between the DivIVA homolog of Mycobacterium tuberculosis Wag31 and its cognate ParB protein, as well as between DivIVA and ParB from Streptomyces coelicolor (Donovan et al., Mol Microbiol 2012). Hence, the role of DivIVA in polar recruitment of ParB likely represents a conserved feature of the actinobacteria.
Morphological aberrations upon DivIVA depletion are also seen in other actinobacteria in which the divIVA gene is essential such as S. coelicolor and Mycobacterium smegmatis. The reason for this is that cell growth in actinobacteria is brought about by polar cell wall elongation, which is mediated by the DivIVA-dependent recruitment of the peptidoglycan biosynthetic apparatus to the poles of the cell (Flärdh, Mol Microbiol 2003; Kang et al., Microbiology 2008; Letek et al., J Bacteriol 2008; Hempel et al., J Bacteriol 2008). It is presently unknown which exact peptidoglycan biosynthetic proteins are recruited to the cell poles by actinobacterial DivIVA proteins. However, two emerging candidates are the cellulose synthase like protein CslA of S. coelicolor and the penicillin-binding protein PBP3 of M. smegmatis. CslA mainly accumulates at the hyphal tips of the S. coelicolor mycelium and is involved in the deposition of β-1,4-linked polysaccharides at the tips of the hyphae. CslA interacts with DivIVA in a bacterial two-hybrid assay (Xu et al., J Bacteriol 2008) but evidence that polar recruitment of CslA requires DivIVA is still lacking. Such analyses are hampered in actinobacteria by the severe morphological deformations in DivIVA depleted cells which make it hard to conclude whether a possible protein localization defect is due to the lack of DivIVA or caused by alterations of cell shape. The interaction between PBP3 and the DivIVA-homolog Wag31 from mycobacteria was shown by a multitude of approaches including co-immunoprecipitation, bacterial two-hybrid analysis, and surface plasmon resonance experiments (Mukherjee et al., Mol Microbiol 2009). Likewise to the situation in S. coelicolor, both PBP3 and Wag31 can be found at the cell poles (Datta et al., Mol Microbiol 2006; Nguyen et al., J Bacteriol 2007) but the interdependence of their subcellular localization patterns still has to be addressed experimentally. Interestingly, Wag31 needs to be in the periplasm to interact with PBP3 since mutations in the extracellular transpeptidase domain of PBP3 abolished the Wag31-PBP3 interaction. Wag31 was indeed detected in the extracytosolic fraction of M. smegmatis (Mukherjee et al., Mol Microbiol 2009). These findings, however, challenge our current view on DivIVA proteins that are supposed to be non-secreted cytosolic factors. The exciting question as to how a cytosolic protein that lacks a signal sequence can be transported across the membrane, still awaits further clarification.
What the DivIVA proteins from all the mentioned species have in common is their unique localization to curved membrane regions at the cell poles and the division site, as well as their conserved domain arrangement. What distinguishes them, however, is the physiological context they work in and the proteins they bind to. Their ability to attach to membranes is mediated by a highly conserved N-terminal lipid binding domain (LBD) that is believed to interact with membranes via the insertion of surface exposed hydrophobic amino acid side chains into the likewise hydrophobic core of the phospholipid bilayer (Lenarcic et al., EMBO J 2009; Oliva et al., EMBO J 2010). Via a flexible linker of varying length, the LBD is connected to an α-helical C-terminal domain (CTD) that is rich in coiled coils (Oliva et al., EMBO J 2010). Monomers of B. subtilis DivIVA assemble into a parallel dimer by means of a coiled coil in the LBD and due to the coiled coil regions in the CTD. Based on analytical ultracentrifugation it was concluded that DivIVA forms tetramers in solution (Oliva et al., EMBO J 2010). Consistently, crystallographic data revealed that formation of the DivIVA tetramer arises from dimerization of two DivIVA dimers that are arranged in an end-to-end orientation. It is tempting to speculate that this tetramer represents the basic building block of higher order DivIVA aggregates since assembly into string- and network-like ultrastructures was indeed observed in cryo-negative stain transmission electron microscopy experiments with a mutant variant (E162K) of B. subtilis DivIVA (Stahlberg et al., Mol Microbiol 2004). But this mutant protein was described as a 6- to 8-mer in solution and thus behaved different from wild-type DivIVA. All in all, however, DivIVA homologs are two-domain proteins that consist of an invariant N-terminal membrane-binding module fused to variable, species-specific CTDs. It therefore appears plausible that these regions are important for the interaction of DivIVA homologs with their species-specific binding partners and it will be interesting to determine the structural basis of this diversity.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/20747