Abstract
Phagocytosis is an important component of innate immunity that contributes to the eradication of infectious microorganisms; however, successful bacterial pathogens often evade different aspects of host immune responses. A common bacterial evasion strategy entails the production of toxins and/or effectors that disrupt normal host cell processes and because of their importance Rho-family GTPases are often targeted. Burkholderia cenocepacia, an opportunistic pathogen that has a propensity to infect cystic fibrosis patients, is an example of a pathogenic bacterium that has only recently been shown to disrupt Rho GTPase function in professional phagocytes. More specifically, B. cenocepacia disrupts Rac and Cdc42 seemingly through perturbation of guanine nucleotide exchange factor function. Inactivation of Rac, Cdc42 and conceivably other Rho GTPases seriously compromises phagocyte function.
Keywords: phagocytosis, macrophage, innate immunity, bacterial pathogenesis, guanine nucleotide exchange factor, type VI secretion
Phagocytosis, an essential facet of immunity, normally leads to the eradication of microbial invaders (for a recent review on phagocytosis see ref. 1). However, there is a sizable number of bacterial pathogens that thwart this innate immune response and take residence within professional phagocytes. Generally this is accomplished by disrupting host cell processes through the delivery of effectors and/or toxins that impede the normal function of mammalian proteins. Rho-family GTPases, essential regulators of many actin-dependent processes, are important and common cellular targets of these noxious bacterial proteins. While the mode of action of individual bacterial effectors is varied, they do share a unifying theme: manipulation of Rho GTPase activity (Table 1).
Table 1. Summary of bacterial effector proteins known to disrupt Rho GTPase function.
| Organism | Effector | Target | Mode of action | |
|---|---|---|---|---|
|
Salmonella typhimurium |
SopE |
Rac1, Cdc42 |
Operates as a GEF.22 |
|
|
Yersinia pseudotuberculosis |
YopE |
RhoA, Rac, Cdc42 |
Functions as a GAP.23 |
|
|
Vibrio parahemolyticus |
VopS |
RhoA, Rac, Cdc42 |
Blocks effector binding through covalent modification by adding adenosine 5′-monophosphate.24 |
|
|
Clostridium difficile |
TcdB |
Rho GTPases |
Inactivates through covalent modification by transfer of glucose to most Rho proteins.25 |
|
|
Pseudomonas aeruginosa |
ExoS |
Rac1 |
Possesses GAP activity.26 |
|
|
Clostridium botulinum |
C3-tranferase |
RhoA, RhoB, RhoC |
Inactivates by ADP-ribosylation of Rho GTPases.27 |
|
| Burkholderia cenocepacia | ? | Rac, Cdc42 | Interferes with GEF function.7 | |
Burkholderia cenocepacia, a potentially deadly opportunistic pathogen that commonly infects patients with cystic fibrosis, has now been added to the elite club of bacteria that alter Rho GTPase function. While it has been appreciated for some time that this bacterium can survive within some professional phagocytes (i.e., macrophages and dendritic cells), how the bacteria accomplish this feat is not well understood.2,3 Normally, upon phagocytosis of a bacterium the newly formed vacuole—termed the phagosome—matures into a potent microbicidal organelle that is highly acidic (pH ~4.5–5.0) and degradative.1 In contrast the B. cenocepacia-containing vacuole (BccV) is markedly more alkaline, and the acquisition of late phagosomal markers such as lysosome-associated membrane proteins 1 and 2 (LAMP-1 and 2) is retarded.4 Our laboratory has demonstrated that the BccV does acquire the Rab GTPase Rab7, another late phagosome marker; however, viable B. cenocepacia thwart Rab7 activation to presumably hinder maturation.5 Additionally, Burkholderia limits the production of reactive oxygen species (ROS) by impairing the phagocyte NADPH oxidase, NOX2.6 Despite the description of these phenomena, the bacterial secretion systems and effectors that perturb normal host cell functions have remained an enigma.
Recently we presented new findings demonstrating that B. cenocepacia inactivates the host cell Rho GTPases Rac1 and Cdc42, which in turn disrupts cortical actin, focal contacts and the maintenance of cell shape.7 We demonstrated that overexpression of constitutively active Rac1 or Cdc42 maintains cortical actin despite B. cenocepacia infection indicating that actin disruption is attributable to impaired Rac1 and Cdc42 function. Rho GTPase inactivation is effected through expression of an intact type VI secretion system (T6SS), although the secreted effector is yet to be identified (for a review of T6SS see ref. 8). Interestingly, the elusive factor compromises GTPase activation through interference with guanine nucleotide-exchange factor (GEF) function. This conclusion is inferred from the observation that overexpression of Tiam1, a Rac-GEF, is sufficient to revert cortical actin disruption even after phagocytosis of viable B. cenocepacia. In contrast, actin disruption catalyzed by C. difficile toxin B-dependent glucosylation and inactivation of Rac1 cannot be similarly overcome.7 Ostensibly overexpression of a promiscuous Rho GEF or a Cdc42-specific GEF would produce the same outcome that was observed upon Tiam1 expression provided it can act on Rac and/or Cdc42; however, this has not been demonstrated experimentally. While the precise mechanism employed by the bacteria to impair activation of Rac1, Cdc42 and possibly other host cell Rho GTPases is currently unknown, the effect of Tiam1 provides strong evidence to suggest that B. cenocepacia does not cause Rho GTPase inactivation by means of covalent modification.7
The consequences of B. cenocepacia-induced Rho GTPase inactivation are anticipated to be extensive, and in our study we demonstrated that two actin dependent processes, macropinocytosis and phagocytosis, were severely compromised upon macrophage infection.7 Published in parallel with our manuscript, an independent study recapitulated our observations with respect to Rho GTPase inactivation and, in addition, attributed diminished function of the NOX2 phagocyte oxidase to Rac inactivation.9 Taken together, these effects ensure that disruption of Rac and Cdc42 by B. cenocepacia infection will be manifested as a functionally incompetent phagocyte that will serve as a bacterial safe haven. Some of the physiological consequences of Rho GTPase corruption by B. cenocepacia are considered further below.
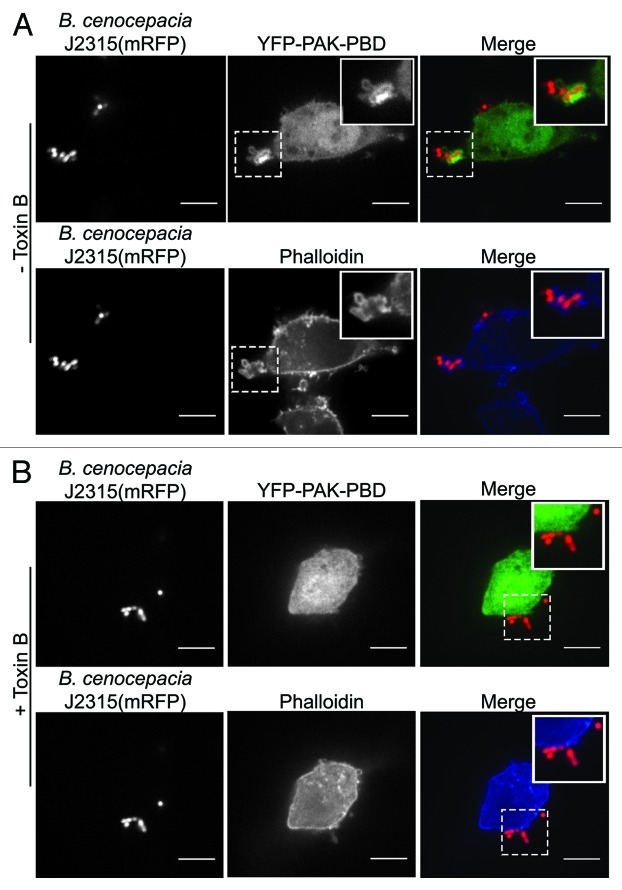
Phagocytosis is an extremely dynamic process that is absolutely depent on actin re-organization that in turn requires Rho GTPase activation.10,11 Indeed, inhibition of Rac or Cdc42 ablates even the early stages of phagocytic cup formation.11 In our in vitro models of cellular infection B. cenocepacia is rapidly engulfed and, within minutes, phagosomes harboring live bacteria are completely formed. Because B. cenocepacia is readily internalized, it should be immediately apparent that the inactivation of Rac and Cdc42 and the ensuing disruption of the actin cytoskeleton induced by the bacteria are delayed, at least until the bacteria have taken residence in the confines of the phagosome. Accordingly, we find that a biosensor for active GTP-bound Rac and Cdc42, the PBD domain of p21-activated kinase (PBD-PAK), robustly accumulates at sites of B. cenocepacia entry (Fig. 1A, top middle panel). The apparent accumulation of YFP-PBD-PAK on the limiting membrane of the forming phagosome indicates that the cytosolic face of the phagosomal membrane is decorated with GTP-bound Rac, Cdc42 or in all likelihood both. Concomitant with PAK-PBD recruitment is what appears like normal accumulation of F-actin at the phagocytic cup (Fig. 1A, bottom panels). Not surprisingly, the accumulation of actin and PAK-PBD during B. cenocepacia phagocytosis are both Rho GTPase-dependent, as pre-treatment of macrophages with C. difficile toxin B ablates these phenomena (Fig. 1B). In fact, toxin B pre-treatment eliminates uptake altogether, emphasizing the need for Rho GTPase activation for the initial phagocytic event that leads to bacterial uptake. Together, these observations indicate that B. cenocepacia delivers the secreted effector(s) that disrupt GEF function only after phagosome formation is completed. To verify this assertion, we expended considerable effort in our study to ensure that, at the time when Rac and Cdc42 inactivation became apparent, B. cenocepacia was in fact confined inside a membrane-bound vacuole.7 At present, we do not know if intraphagosomal bacteria deliver the effector to the lumen of the phagosome in which they reside—meaning that the effector would have to cross the phagosomal membrane to gain access to the cytosol—or if the bacteria deliver the Rho GTPase-inactivating effector directly to the cytosol, perhaps via an injection system or through a pore. Regardless, the notion that the bacteria “wait” to inhibit Rho GTPases makes teleological sense, considering that the door to the “safe haven” should not be locked before the pathogen secludes itself. This behavior contrasts with that of other bacterial pathogens, such as Pseudomonas aeruginosa, which prefer to evade phagocytosis altogether, rather than confront the inhospitable environment of the phagosome (Table 1). We are currently undertaking a careful determination of the timing of Rac and Cdc42 inactivation by B. cenocepacia; when host cell GTPases are inactivated will have important consequences affecting the earliest innate immune functions of the phagocyte.
Figure 1. Recruitment of Rac/Cdc42 and F-actin to sites of B. cenocepacia entry into macrophages. To assess the recruitment of active Rac/Cdc42 to sites of B. cenocepacia phagocytosis RAW 264.7 cells were transfected with a plasmid encoding the active Rac/Cdc42 reporter p21-binding domain of p21-activated kinase (PBD-PAK) fused to yellow fluorescent protein and were infected with live B. cenocepacia J2315 expressing mRFP as previously described.7 Cells were fixed with 4% (v/v) paraformaldehyde and were permeabilized with 0.1% (v/v) Triton X-100. Cells were then stained with phalloidin conjugated to Alexafluor-647 to evaluate the organization of F-actin prior to imaging. In (A) a representative image showing the localized accumulation of YFP-PBD-PAK at a site of B. cenocepacia phagocytosis is presented (top panels). The accumulation of F-actin at the same entry site is also shown [(A), bottom panels]. The hatched boxes demarcate the region of the cells depicted in the insets. In (B) the effect of pre-treating RAW 264.7 cells expressing YFP-PAK-PBD with 100 ng/ml C. difficile toxin B for 1 h prior to infection is presented. In the top panels of part B the distribution of YFP-PAK-PBD is shown and the organization of F-actin by phalloidin staining is also demonstrated (bottom panels). Note the absence of PAK-PBD or F-actin recruitment to the site where B. cenocepacia is bound to the RAW macrophage surface. The images in (A) and (B) reflect 5 min post-infection and were acquired by spinning disk confocal microscopy. Scale bars equal 12.7 μm.
The production of ROS by NOX2 is an important component of the armamentarium that is deployed by macrophages to combat infection. NOX2 is comprised of multiple subunits that are integral membrane proteins (gp91phox and p22phox, together called cytochrome b558) localized to the plasmalemma and the limiting membrane internal of vesicles, and soluble proteins that exist in a trimeric cytosolic complex (p67phox, p47phox and p40phox) (for a review see ref. 12). Upon the perception of activating signals cytochrome b558 interacts with p67phox/p47phox/p40phox at the forming phagosome, where association of GTP-bound Rac with p67phox enables the oxidase to become active.13 As suggested by Rosales-Reyes et al., the inactivation of Rac by B. cenocepacia could therefore prevent stimulation of the oxidase, affording protection to the invading pathogen.9 However, the timing of these events must be considered carefully. Using models of Fcγ receptor and integrin-dependent phagocytosis we and others can demonstrate that NOX2 starts generating ROS even prior to phagosomal sealing, indicating that the oxidase response is elicited without significant delay (data not shown and see ref. 13). Presumably this will also occur during the phagocytosis of viable B. cenocepacia unless the bacteria possess fast-acting mechanisms, other than Rac inactivation, to suppress NOX2 function. Exposure to an initial oxidative burst would clarify why B. cenocepacia requires a variety of ROS-detoxifying molecules—such as superoxide dismutase and a melanin-like pigment—for survival within macrophages.14,15 Presumably these ROS-scavenging mechanisms enable B. cenocepacia to survive an initial NOX2-dependent assault and provide the bacteria with sufficient time to express and deliver the GEF-inhibiting effector that will ultimately suppress Rac activity and consequently NOX2 function, as has been described.9 Detailed comparison of the time course of inactivation of Rac and of NOX2 by B. cenocepacia should clarify the relationship between these events.
In addition to impaired NOX2 function many other immune-related functions of the infected phagocyte are predicted to be perturbed by B. cenocepacia infection as a result of Rho-GTPase inactivation. A healthy phagocyte, such as a dendritic cell or macrophage, commonly combats infection in non-lymphoid tissue by internalizing prey (e.g., bacteria). After phagocytosis the cell harboring its prey will migrate to a draining lymph node, where it will present prey-derived antigens to lymphocytes and fulfill its role in initiating adaptive immunity.16 Cell migration is a sophisticated, actin-dependent process that requires coordinated activation of Rho-family GTPases including Rac and Cdc42; given that intracellular B. cenocepacia disrupt the activation of the GTPases and the associated actin polymerization, it is reasonable to expect that phagocyte migration will be severely compromised, preventing antigen presentation at the appropriate site. The assault on the process of antigen presentation will not be limited to phagocyte migration. In dendritic cells (and ostensibly macrophages) the delivery of major histocompatibility class II-positive compartments is also a Rho GTPase-dependent process that requires F-actin.17,18 Furthermore, the delivery of peptide-loaded MHCII to the plasmalemma requires membrane tubulation of MHCII-positive compartments.19,20 Interestingly, membrane tubulation requires active Rab7 and, as described previously, B. cenocepacia also disrupts Rab7 activation.5,18,21 Through the combined interference with Rac and Rab7 function Burkholderia will presumably ablate the ability of an infected phagocyte to present B. cenocepacia-derived antigens at the cell surface. At present we do not know if Rab7 inactivation is effected through T6SS or if the disruption of Rab7 and Rho GTPases is in any way related mechanistically, but it would be of considerable interest to evaluate the impact of B. cenocepacia infection on the cell biology of antigen presentation.
It has been appreciated for some time that B. cenocepacia survives within professional phagocytes, the very cells that are supposed to eradicate it from the body, but the mechanisms employed by the bacteria to accomplish this feat have remained elusive. The realization that Rho GTPase activation is impaired by B. cenocepacia is a significant step toward understanding at the molecular level how Burkholderia achieves intracellular survival and disrupts immune cell function. This mechanism, however is likely just the tip of a large iceberg, as B. cenocepacia is endowed with more than 8 million base pairs of coding sequence and is likely to possess many more effectors that disrupt host cell function in ways that we have yet to appreciate.
Acknowledgments
R.S.F. is a recipient of a RESTRACOMP fellowship from the Hospital for Sick Children and acknowledges Dr Sergio Grinstein for his helpful comments and mentorship. Original work in the laboratory of Dr S. Grinstein is supported by Cystic Fibrosis Canada.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20487
References
- 1.Flannagan RS, Jaumouillé V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 2.Saini LS, Galsworthy SB, John MA, Valvano MA. Intracellular survival of Burkholderia cepacia complex isolates in the presence of macrophage cell activation. Microbiology. 1999;145:3465–75. doi: 10.1099/00221287-145-12-3465. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald KL, Speert DP. Differential modulation of innate immune cell functions by the Burkholderia cepacia complex: Burkholderia cenocepacia but not Burkholderia multivorans disrupts maturation and induces necrosis in human dendritic cells. Cell Microbiol. 2008;10:2138–49. doi: 10.1111/j.1462-5822.2008.01197.x. [DOI] [PubMed] [Google Scholar]
- 4.Lamothe J, Huynh KK, Grinstein S, Valvano MA. Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell Microbiol. 2007;9:40–53. doi: 10.1111/j.1462-5822.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- 5.Huynh KK, Plumb JD, Downey GP, Valvano MA, Grinstein S. Inactivation of macrophage Rab7 by Burkholderia cenocepacia. J Innate Immun. 2010;2:522–33. doi: 10.1159/000319864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keith KE, Hynes DW, Sholdice JE, Valvano MA. Delayed association of the NADPH oxidase complex with macrophage vacuoles containing the opportunistic pathogen Burkholderia cenocepacia. Microbiology. 2009;155:1004–15. doi: 10.1099/mic.0.026781-0. [DOI] [PubMed] [Google Scholar]
- 7.Flannagan RS, Jaumouillé V, Huynh KK, Plumb JD, Downey GP, Valvano MA, et al. Burkholderia cenocepacia disrupts host cell actin cytoskeleton by inactivating Rac and Cdc42. Cell Microbiol. 2012;14:239–54. doi: 10.1111/j.1462-5822.2011.01715.x. [DOI] [PubMed] [Google Scholar]
- 8.Bingle LE, Bailey CM, Pallen MJ. Type VI secretion: a beginner’s guide. Curr Opin Microbiol. 2008;11:3–8. doi: 10.1016/j.mib.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Rosales-Reyes R, Skeldon AM, Aubert DF, Valvano MA. The Type VI secretion system of Burkholderia cenocepacia affects multiple Rho family GTPases disrupting the actin cytoskeleton and the assembly of NADPH oxidase complex in macrophages. Cell Microbiol. 2012;14:255–73. doi: 10.1111/j.1462-5822.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 10.Tse SML, Furuya W, Gold E, Schreiber AD, Sandvig K, Inman RD, et al. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J Biol Chem. 2003;278:3331–8. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- 11.Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–94. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol. 2010;32:415–30. doi: 10.1007/s00281-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KE, Chessa TAM, Davidson K, Henderson RB, Walker S, Tolmachova T, et al. PtdIns3P and Rac direct the assembly of the NADPH oxidase on a novel, pre-phagosomal compartment during FcR-mediated phagocytosis in primary mouse neutrophils. Blood. 2010;116:4978–89. doi: 10.1182/blood-2010-03-275602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keith KE, Killip L, He P, Moran GR, Valvano MA. Burkholderia cenocepacia C5424 produces a pigment with antioxidant properties using a homogentisate intermediate. J Bacteriol. 2007;189:9057–65. doi: 10.1128/JB.00436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keith KE, Valvano MA. Characterization of SodC, a periplasmic superoxide dismutase from Burkholderia cenocepacia. Infect Immun. 2007;75:2451–60. doi: 10.1128/IAI.01556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–61. doi: 10.1016/S1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 17.Ocana-Morgner C, Wahren C, Jessberger R. SWAP-70 regulates RhoA/RhoB-dependent MHCII surface localization in dendritic cells. Blood. 2009;113:1474–82. doi: 10.1182/blood-2008-04-152587. [DOI] [PubMed] [Google Scholar]
- 18.Neefjes J, Jongsma MLM, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–36. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 19.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–94. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 20.Vyas JM, Kim Y-M, Artavanis-Tsakonas K, Love JC, Van der Veen AG, Ploegh HL. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol. 2007;178:7199–210. doi: 10.4049/jimmunol.178.11.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–25. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y, Galán JE. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–7. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 23.Von Pawel-Rammingen U, Telepnev MV, Schmidt G, Aktories K, Wolf-Watz H, Rosqvist R. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol Microbiol. 2000;36:737–48. doi: 10.1046/j.1365-2958.2000.01898.x. [DOI] [PubMed] [Google Scholar]
- 24.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science. 2009;323:269–72. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- 25.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–3. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 26.Würtele M, Wolf E, Pederson KJ, Buchwald G, Ahmadian MR, Barbieri JT, et al. How the Pseudomonas aeruginosa ExoS toxin downregulates Rac. Nat Struct Biol. 2001;8:23–6. doi: 10.1038/83007. [DOI] [PubMed] [Google Scholar]
- 27.Stasia MJ, Jouan A, Bourmeyster N, Boquet P, Vignais PV. ADP-ribosylation of a small size GTP-binding protein in bovine neutrophils by the C3 exoenzyme of Clostridium botulinum and effect on the cell motility. Biochem Biophys Res Commun. 1991;180:615–22. doi: 10.1016/S0006-291X(05)81110-6. [DOI] [PubMed] [Google Scholar]