Abstract
Cell migration requires the coordination of adhesion site assembly and turnover. Canonical models for nascent adhesion formation postulate that integrin binding to extracellular matrix (ECM) proteins results in the rapid recruitment of cytoskeletal proteins such as talin and paxillin to integrin cytoplasmic domains. It is thought that integrin-talin clusters recruit and activate tyrosine kinases such as focal adhesion kinase (FAK). However, the molecular connections of this linkage remain unresolved. Our recent findings support an alternative model whereby FAK recruits talin to new sites of β1 integrin-mediated adhesion in mouse embryonic fibroblasts and human ovarian carcinoma cells. This is dependent on a direct binding interaction between FAK and talin and occurs independently of direct talin binding to β1 integrin. Herein, we discuss differences between nascent and mature adhesions, interactions between FAK, talin and paxillin, possible mechanisms of FAK activation and how this FAK-talin complex may function to promote cell motility through increased adhesion turnover.
Keywords: adhesion assembly, focal adhesion kinase, focal adhesion turnover, motility, nascent adhesion, recruitment, talin, β1 integrin
Introduction
Integrin-based adhesions support cell movement via indirect linkages to the actin cytoskeleton.1 Integrins are transmembrane α and β subunit comprised receptors for distinct extracellular matrix (ECM) proteins. Sites of integrin clustering form distinct types of adhesions: nascent adhesions, focal complexes, focal adhesions, and fibrillar adhesions.2 These adhesions form in cultured cells plated on two-dimensional surfaces and within three-dimensional matrices.3 It is thought that different types of adhesions sequentially form as a function of cell tension generation and maturation.4 Despite knowing much about the protein composition of adhesions,5 the sequence of events controlling adhesion assembly and disassembly remain unresolved. Here, we will discuss recent discoveries regarding the recruitment of the cytoplasmic focal adhesions kinase (FAK) and talin to β1-integrin-containing adhesions. Moreover, the direct binding of FAK and talin within adhesions is also of key importance in promoting adhesion turnover needed for normal fibroblast and tumor cell movement.
Cell Adhesions
Efficient cell movement requires a regulated cycle of adhesion formation and disassembly. Stabilization of a protruding cell edge is initiated by nascent adhesions that are small and transient structures that either disassemble rapidly or mature in focal complexes. Actin-myosin tension generation within cells facilitates the maturation of focal complexes into focal adhesions.6,7 The “adhesome” can contain over 900 different receptors, signaling and cytoskeletal or adaptor proteins.5,6 Fibrillar adhesions are elongated structures that contribute to ECM remodeling.8 Importantly, initial protein recruitment, maturation and elongation of adhesions are balanced by protein dissociation and disassembly as a cycle of changes regulated in time and space within migrating cells.
Canonical models for the sequence of events associated with adhesion formation postulate that ECM binding by integrins triggers the rapid recruitment of cytoskeletal proteins such as talin and paxillin to integrin cytoplasmic domains. Talin is a large cytoskeletal protein comprised of a N-terminal head or FERM (band 4.1, ezrin, radixin, moesin homology) domain that binds to β1- and β3-integrin cytoplasmic tails, PIP5K1C and FAK. The C-terminal talin rod domain binds vinculin, actin, and contains a second integrin-binding site.9 Structural studies have provided insights on the molecular mechanisms through which talin promotes inside to out activation of platelet integrin αIIbβ3.10 However, it remains undetermined whether a similar sequence of events occur during outside to in integrin signaling upon binding of mesenchymal or tumor cells to ECM.
FAK is a non-receptor tyrosine kinase recruited to clustered integrins.11 Integrins activate FAK where it forms a complex with paxillin at nascent adhesions,12 but FAK localization to adhesions does not require FAK kinase activity.13 Three-dimensional nanoscale fluorescent microscopy has co-localized FAK, paxillin, integrin tails and talin FERM (head domain) to a proximal signaling layer at adhesions followed by the C-terminal part of talin (rod domain) and vinculin localized to a more distal layer of adhesions.14 FAK binds to talin and canonical models postulate that talin promotes FAK localization to adhesions. To test this hypothesis, we evaluated FAK, paxillin and talin localization upon fibroblast and ovarian carcinoma cell adhesion to fibronectin. Analyses were performed on cells with new or nascent (15 min on fibronectin) and mature (60 min on fibronectin) β1 integrin-containing adhesions. Surprisingly, loss of FAK expression in FAK-null fibroblasts or after FAK-knockdown in ovarian carcinoma cells prevented talin recruitment to nascent but not mature adhesions.15 Importantly, FAK loss did not alter adhesion formation or paxillin recruitment to these sites. As talin was present at mature adhesions in FAK-null cells, other mechanisms such as direct talin binding to integrins can also serve to facilitate adhesion recruitment. Moreover, nascent vs. mature adhesions likely represent distinct structures with potential differences in adhesome protein content. Future comparisons using replating of cells onto ECM to synchronize adhesion formation will be useful in identifying these differences.
It is known that initial fibroblast adhesion and spreading is not dependent on talin expression,16 but that talin may facilitate adhesion site maturation.17 Accordingly, talin knockdown in human umbilical vein endothelial cells that express primarily one of the talin isoforms, did not alter adhesion formation or paxillin-FAK recruitment to these sites. Together, these results support an alternate integrin signaling linkage model whereby FAK functions to promote talin localization to nascent adhesions (Fig. 1). Additionally, normal and FAK-null fibroblasts showed equal staining of a ligand-induced binding site antibody (9EG7) to β1 integrin at peripheral adhesions upon talin knockdown.15 Similar results were observed upon talin knockdown in mammary epithelial cells18 and suggest that talin may not be essential for β1 integrin activation in all cells. There are other proteins that bind to integrin cytoplasmic domains and can regulate integrin activation such as the kindlins.19 As alterations in the amount of adhesion-localized phosphatidylinositol 4,5-biphosphate (PtdIns 4,5 P2) disrupted talin and vinculin but not kindlin recruitment to new adhesions,20 evidence is accumulating that both FAK and kindlin recruitment to adhesions occur independently of talin.
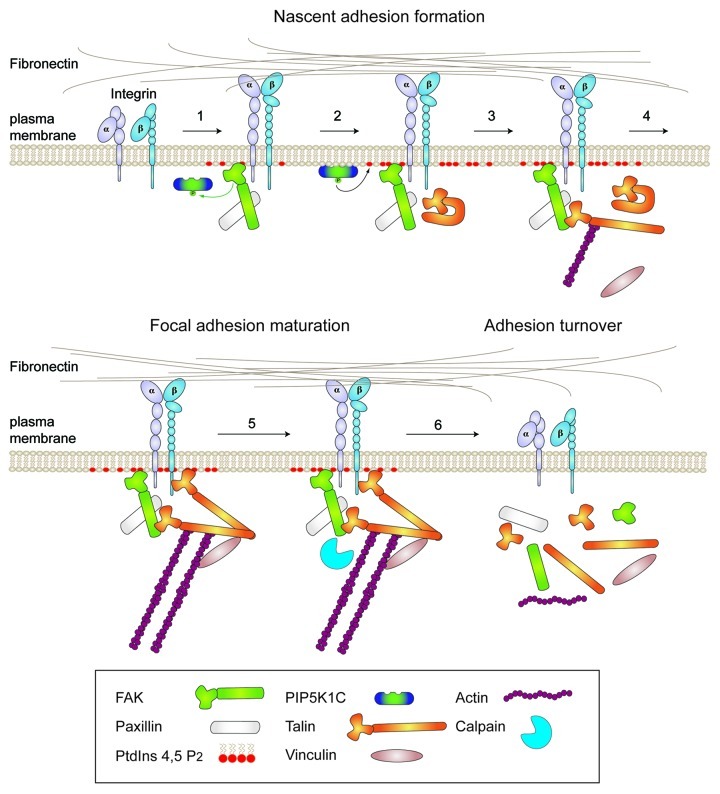
Figure 1. Model of a hierarchical linkage between integrins, FAK, talin and the control of cell motility. Simplified steps in adhesion assembly and turnover on fibronectin matrix. (1) Integrin engagement results in phosphatidylinositol-4,5-bisphosphate (PtdIns 4,5 P2) generation and the recruitment of FAK and paxillin to nascent adhesions within 15 min through undefined mechanisms. (2) FAK activation at nascent adhesions increases PtdIns 4,5 P2 generation by phosphorylating PIP5K1C. PtdIns 4,5 P2 can bind to the talin FERM domain and enable a conformational change in talin that would promote talin FERM binding to FAK and the recruitment of talin to nascent adhesions. (3) Talin dimerization allows for direct talin FERM binding to FAK and to β1-integrins for sustained adhesion signaling. (4) Myosin II-dependent tension generation and actomyosin contractility act in part through talin to enhance signaling and adhesion maturation into focal adhesions. (5) The binding of a protease such as calpain to FAK facilitates a multi-protein complex at adhesions. (6) FAK-enhanced and calpain-mediated cleavage of talin into head and rod domain fragments facilitates adhesion turnover required for efficient cell movement.
The notion that FAK and talin localization to nascent β1 integrin adhesions could occur independently of direct talin binding to the integrin cytoplasmic tail was supported by experiments using Chinese hamster ovary (CHO) cells expressing chimeric β3/β1 integrins.21 At 15 min on ECM, a complex between integrin β1, talin and FAK was formed and localized to new adhesion sites in β1A wildtype-expressing CHO cells.15 Notably, mutation of β1 integrin (β1A Y783A) that prevents direct talin binding disrupted integrin co-immunoprecipitation with talin, but did not alter FAK and talin co-localization to nascent adhesion sites upon binding of CHO cells to ECM. These results support the conclusion that integrin-talin binding is not essential for talin recruitment to nascent adhesions. Moreover, if FAK and talin co-localize with integrins in the absence of direct talin binding to integrins, there must be alternative mechanisms facilitating these interactions.
How Does FAK Localize to Adhesions?
FAK and the adaptor protein paxillin are rapidly recruited to nascent adhesions where they form a complex.12 Like FAK, it remains unclear how paxillin localizes to adhesions. FAK is not essential for paxillin adhesion recruitment as paxillin localizes to new adhesions in FAK-null cells.15 Similarly, FAK localizes to a subset of adhesions formed in paxillin-null cells and is still activated by integrin stimulation, albeit with a delay.22 Although it is possible that paxillin-related proteins such as Hic-5 may compensate for the loss of paxillin expression, it is known that mutations in FAK that disrupt paxillin association do not necessarily prevent FAK adhesion localization.23 Despite the fact that the C-terminal region of FAK is designated the focal adhesion targeting (FAT) region,11 binding of paxillin and talin to this region of FAK may not be essential for targeting full-length FAK to adhesions. Alternatively, it is possible that FAK FAT domain interaction with other proteins such as p190RhoGEF (Rgnef) may also contribute to adhesion localization.
Another domain that has been implicated in connecting FAK to integrins is the FAK FERM domain. This region can bind peptides derived from the β1 integrin cytoplasmic domain and the FAK FERM domain can regulate FAK catalytic activity.24 The FAK FERM domain can also bind Arp3 of the Arp2/3 actin filament promoting complex25 and the RACK1 scaffolding protein.26 However, mutations in FAK FERM revealed that FAK was functioning to recruit Arp2/3 and RACK1 to nascent adhesions and not the other way around. Moreover, as exogenous expression of FAK FERM does not localize to nascent adhesions, it is unlikely that FAK FERM directly mediates recruitment to integrins. Instead, the FERM domain targets FAK to membranes, membrane-associated receptors, and to the nucleus.27 It is the potential PtdIns 4,5 P2 lipid binding activity of FAK FERM that may connect it to nascent adhesions and to the conformational regulation of FAK activity.28
To this end, PtdIns 4,5 P2 synthesis is rapidly triggered upon fibronectin replating of cells29 and occurs via β1 integrin clustering.30 Knockdown of PIP5KC1, involved in generating PtdIns 4,5 P2, results in decreased focal adhesion formation31 and this phenotype is also observed upon genetic deletion of the focal adhesion targeting region in PIP5KC1.20 FAK contributes to PtdIns 4,5 P2 generation via phosphorylation and activation of PIP5K1C.32 As PtdIns 4,5 P2 also regulates talin recruitment to adhesions20 by increasing its binding affinity to integrins,33 and loss of FAK also prevents talin localization to new adhesions,15 it is tempting to speculate that connections between FAK and talin may co-rely on the generation and binding of PtdIns 4,5 P2 at adhesions as previously reviewed34 (Fig. 1).
Direct Binding of FAK and Talin
Both the talin and FAK FERM domains can bind PtdIns 4,5 P2 and this helps localize both proteins to membranes. The talin FERM also binds directly to the FAK C-terminal domain. Pull down assays revealed the minimal binding site as FAK residues 1,011–1,042 and point mutation analyses showed that E1015A disrupted full-length FAK binding to talin but not to paxillin.15 Re-expression of FAK E1015A in FAK-null fibroblasts co-localized with paxillin at nascent adhesions, but prevented talin localization to these sites. These results suggest that talin and paxillin bind independently to the FAK FAT domain. Importantly, results with E1015A FAK support the conclusion that FAK FAT binding functions to recruit talin to adhesions. Interestingly, mutational analyses also revealed that similar regions of the talin FERM domain bind FAK and β-integrins. It is possible that differential binding affinities may facilitate a sequential transition from FAK to β-integrin binding during adhesion site maturation. Alternatively, since talin may undergo dimerization,9 FAK and β-integrin may both potentially bind talin within the same dimer (Fig. 1).
Another notable finding with FAK E1015A was that this mutation did not disrupt adhesion-mediated FAK activation. This is not surprising since early studies using antibody-clustering of integrins in suspended cells revealed rapid increases in FAK tyrosine phosphorylation consistent with a model of intermolecular FAK autophosphorylation at Y397.35 However, this does not explain the potential lack of FAK Y397 phosphorylation detected in talin-depleted fibroblasts upon adhesion to ECM and postulated to be associated with the lack of tension generation.16 Alternatively, we did not detect significant differences in FAK Y397 phosphorylation upon fibroblast adhesion to ECM in the presence of blebbistatin, a pharmacological inhibitor of myosin II that prevents adhesion site maturation.15 Consistent with this, various cytoplasmic proteins including FAK and paxillin are recruited to integrin clustering sites in a force-independent manner.36 It is possible that data interpretation problems may occur with the use of phospho-specific antibodies to FAK Y397 to monitor FAK recruitment dynamics to adhesions. Despite this, the role of tension and RhoGTPase-mediated cell contractility is important in maintaining FAK activation downstream of integrins.37 A key distinction is that tensional changes are not likely essential for initial FAK activation at nascent adhesions. Moreover, talin binding is also not required for FAK activation, but a direct connection to talin is needed for efficient regulation of adhesion turnover and cell motility.
FAK and Talin in Adhesion Turnover
The adhesion “cycle” ends by protein dissociation and disassembly at both leading and trailing edges of moving cells.1 Loss of FAK expression prevents adhesion turnover with a subsequent inhibition of cell motility.38 Interestingly, reconstitution of FAK-null fibroblasts with E1015A FAK did not promote cell movement or efficient adhesion turnover even though this talin binding mutant of FAK exhibited normal adhesion-mediated kinase activation.15 The phenotype of E1015A FAK fibroblasts, with increased adhesion number and size, was very similar to fibroblasts derived from the kinase-dead FAK knockin mouse.13 Although the mechanism(s) associated with adhesion turnover defects in both FAK kinase-dead and E1015A fibroblasts remain unresolved, it is likely that FAK-talin complex formation is needed for the tyrosine phosphorylation of specific targets creating SH2 binding motifs or to facilitate the formation of a higher-order adhesome complex that have an intrinsic “off” switch.
One of these off switches involves the regulated proteolysis of both FAK and talin linked to adhesion turnover. In tumor cells, caspase-8 promotes cell migration via formation of a complex between FAK and calpain 2 leading to the enhanced cleavage of adhesome-associated proteins such as talin.39 Talin cleavage into head and rod domain fragments is associated with increased adhesion turnover dynamics.40 Although the talin head domain can activate integrins, it can also be rapidly degraded through Smurf-1-mediated ubiquitinylation correlated with increased adhesion turnover.41 In ovarian carcinoma cells, FAK knockdown prevents talin head domain generation and this is restored by re-expression of wildtype but not E1015A FAK.15 Thus, our results support a model whereby the presence of a FAK-talin complex within adhesions regulates a cycle of talin proteolysis and adhesion turnover enabling efficient cell movement (Fig. 1). The mechanism(s) and proteases involved in these events remain to be identified.
Conclusions
Our recent studies highlight a new linkage between integrins, FAK, talin and the control of cell motility. We propose an alternative model whereby FAK may be upstream of talin and functions to recruit talin to new sites of β1 integrin-mediated adhesion (Fig. 1). Additionally, regulation of the FAK and talin complex facilitates adhesion turnover during cell movement. Although FAK and talin are present in both nascent and mature adhesions, these structures are distinct and functional roles for these proteins within these structures may be different. Further efforts will be needed to answer such questions as: how does FAK get recruited and activated to nascent adhesions? Are there cell-type or integrin-associated differences in the process of adhesion assembly and turnover? What are the mechanisms of how the FAK-talin complex is regulated and what signals trigger talin proteolysis associated with adhesion turnover and cell motility? When answering these questions, the timing of these analyses, either focusing on nascent or mature adhesions, will be of key importance. After all, timing is everything.
Acknowledgments
We thank the members of the Schlaepfer lab for useful discussions and critical insights. We also thank Ssang-Taek Lim PhD (University of Alabama, Mobile, AL) for his helpful comments and suggestions. C.L. is supported by a Canadian Institutes of Health Research fellowship (200810MFE-193594-139144) and D.S. by grants from the NIH (GM087400, HL093156, and CA102310).
Glossary
Abbreviations:
- ARP3
actin-related protein 3
- CHO
Chinese hamster ovary
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- FAT
focal adhesion targeting
- FERM
band 4.1, ezrin, radixin, moesin homology
- FN
fibronectin
- PIP5K1C
phosphatidylinositol-4-phosphate 5-kinase, type I, gamma
- PtdIns 4,5 P2
phosphatidylinositol-4, 5-bisphosphate
- RACK1
receptor for activated kinase C 1
- p190RhoGEF (Rgnef)
190kDa Rho guanine nucleotide exchange factor
- Smurf-1
SMAD specific E3 ubiquitin protein ligase
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20488
References
- 1.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–43. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–33. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nat Cell Biol. 2011;13:3–5, author reply 5-7. doi: 10.1038/ncb0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci. 2010;123:1385–8. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–93. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–90. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilicá D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevicá N, Han Q, et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117:177–87. doi: 10.1242/jcs.00845. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler WH, Gingras AR, Critchley DR, Emsley J. Integrin connections to the cytoskeleton through talin and vinculin. Biochem Soc Trans. 2008;36:235–9. doi: 10.1042/BST0360235. [DOI] [PubMed] [Google Scholar]
- 10.Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, McLean MA, et al. Recreation of the terminal events in physiological integrin activation. J Cell Biol. 2010;188:157–73. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–13. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 12.Choi CK, Zareno J, Digman MA, Gratton E, Horwitz AR. Cross-correlated fluctuation analysis reveals phosphorylation-regulated paxillin-FAK complexes in nascent adhesions. Biophys J. 2011;100:583–92. doi: 10.1016/j.bpj.2010.12.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim ST, Chen XL, Tomar A, Miller NL, Yoo J, Schlaepfer DD. Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem. 2010;285:21526–36. doi: 10.1074/jbc.M110.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–4. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, Schlaepfer DD. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol. 2012;196:223–32. doi: 10.1083/jcb.201108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10:1062–8. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopp PM, Bate N, Hansen TM, Brindle NP, Praekelt U, Debrand E, et al. Studies on the morphology and spreading of human endothelial cells define key inter- and intramolecular interactions for talin1. Eur J Cell Biol. 2010;89:661–73. doi: 10.1016/j.ejcb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P, Ballestrem C, Streuli CH. The C terminus of talin links integrins to cell cycle progression. J Cell Biol. 2011;195:499–513. doi: 10.1083/jcb.201104128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010;115:4011–7. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legate KR, Takahashi S, Bonakdar N, Fabry B, Boettiger D, Zent R, et al. Integrin adhesion and force coupling are independently regulated by localized PtdIns(4,5)2 synthesis. EMBO J. 2011;30:4539–53. doi: 10.1038/emboj.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieves B, Jones CW, Ward R, Ohta Y, Reverte CG, LaFlamme SE. The NPIY motif in the integrin beta1 tail dictates the requirement for talin-1 in outside-in signaling. J Cell Sci. 2010;123:1216–26. doi: 10.1242/jcs.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–15. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheswohl DM, Harrell JR, Rajfur Z, Gao G, Campbell SL, Schaller MD. Multiple paxillin binding sites regulate FAK function. J Mol Signal. 2008;3:1. doi: 10.1186/1750-2187-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010;11:802–14. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 25.Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, et al. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9:1046–56. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- 26.Serrels B, Sandilands E, Serrels A, Baillie G, Houslay MD, Brunton VG, et al. A complex between FAK, RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol. 2010;20:1086–92. doi: 10.1016/j.cub.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 27.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai X, Lietha D, Ceccarelli DF, Karginov AV, Rajfur Z, Jacobson K, et al. Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol Cell Biol. 2008;28:201–14. doi: 10.1128/MCB.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNamee HP, Ingber DE, Schwartz MA. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol. 1993;121:673–8. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNamee HP, Liley HG, Ingber DE. Integrin-dependent control of inositol lipid synthesis in vascular endothelial cells and smooth muscle cells. Exp Cell Res. 1996;224:116–22. doi: 10.1006/excr.1996.0118. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Li X, Sunkara M, Spearman H, Morris AJ, Huang C. PIPKIγ regulates focal adhesion dynamics and colon cancer cell invasion. PLoS One. 2011;6:e24775. doi: 10.1371/journal.pone.0024775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 33.Martel V, Racaud-Sultan C, Dupe S, Marie C, Paulhe F, Galmiche A, et al. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J Biol Chem. 2001;276:21217–27. doi: 10.1074/jbc.M102373200. [DOI] [PubMed] [Google Scholar]
- 34.Nayal A, Webb DJ, Horwitz AF. Talin: an emerging focal point of adhesion dynamics. Curr Opin Cell Biol. 2004;16:94–8. doi: 10.1016/j.ceb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Toutant M, Costa A, Studler JM, Kadaré G, Carnaud M, Girault JA. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol Cell Biol. 2002;22:7731–43. doi: 10.1128/MCB.22.22.7731-7743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu CH, Law JB, Suryana M, Low HY, Sheetz MP. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc Natl Acad Sci U S A. 2011;108:20585–90. doi: 10.1073/pnas.1109485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol. 2009;21:676–83. doi: 10.1016/j.ceb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 39.Barbero S, Mielgo A, Torres V, Teitz T, Shields DJ, Mikolon D, et al. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009;69:3755–63. doi: 10.1158/0008-5472.CAN-08-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–83. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 41.Huang C, Rajfur Z, Yousefi N, Chen Z, Jacobson K, Ginsberg MH. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol. 2009;11:624–30. doi: 10.1038/ncb1868. [DOI] [PMC free article] [PubMed] [Google Scholar]