Abstract
Cell adhesion to DPP substrate is an integrin-mediated event and involves integrin binding, clustering, assembly of focal adhesion complexes and cytoskeletal organization. Cells perceive the DPP substrate through the integrin receptor αvβ1 and bind the actin cytoskeleton to the membrane via focal adhesion sites. The cells respond to this proteinaceous rigid substrate by activating the mechano-chemical signaling events leading to cell spreading and formation of focal adhesions. Focal adhesions, which are sites of integrin binding to the extracellular matrix, form in the leading edge during cell migration. These sites are dynamic and the supramolecular assemblies contain structural and signaling components regulating cell functions. In our study, we present a scenario that integrins utilize the actin network to permit activation of the mitogen-activated kinase modules to transduce signals through the cytoplasm to the nucleus in the presence of DPP. We specifically demonstrate that ERK-mediated transcriptional events impinge on activation of transcription factors leading to cell differentiation.
Keywords: DPP, integrin, FAK, extracellular matrix, adhesion, MAP kinase
Introduction
Mineral deposition is essential for the development of hard tissues like bone and teeth.1 In these tissues type I collagen defines the framework for mineral deposition and by itself is not sufficient to support nucleation of hydroxyapatite.2 However, in the presence of noncollagenous proteins, nucleation sites have been identified within the assembled collagen fibrils. Noncollagenous proteins constitute 5–10% of the total extracellular matrix proteins. Phosphophoryn (DPP), a major noncollagenous protein of dentin is a highly phosphorylated serine and aspartic acid rich protein.3,4 Because of its high phosphate content, net negative charge and avid binding of Ca2+ ions, DPP has been linked to dentin mineralization.4 DMP1 is another noncollagenous protein in dentin and bone matrices and has been implicated to play a regulatory role in mineralization.1
During tooth development, strong expression of DPP in undifferentiated mesenchymal cells was observed. Therefore, we proceeded to assess additional functional role of DPP in the extracellular matrix. In our published study, DPP in the extracellular matrix was simulated by coating glass slides with recombinant DPP and plating undifferentiated C3H10T1/2 mesenchymal cells and studying the downstream signaling effects during DPP mediated cell adhesion.5
Cell Attachment and Migration
The ability of mammalian cells to adhere and to migrate is an essential requirement for organogenesis. Both DPP and DMP1 contain an integrin binding RGD domain, which is well conserved across several species. When cells were plated on DPP substrate, adhesive interactions between cells and DPP were observed. It is well known that these interactions are facilitated by cell surface receptors called integrins.6 As integrin receptors are composed of α and β subunits, we identified that cell surface integrin receptor specific to bind DPP ligand was αvβ1. Cells were able to sense the DPP environment and attached within 4 h. Proteins like DPP in the ECM of the dentin matrix provide cellular microenvironments thereby regulating various cellular functions.
Cytoskeletal Organization
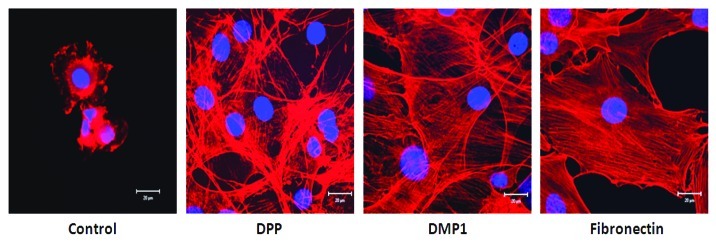
Environmental signals from the ECM and particularly growth factors are necessary to achieve tissue development. Interaction of transmembrane integrins to their target ligand leads to its activation via changes in conformation, triggering intracellular biochemical signals. Binding of integrin receptors to DPP ligand was seen to activate cytoskeletal organization. It is well established that binding of proteins such as actinin-α and talin to integrin cytoplasmic tails, and the subsequent recruitment of the actin-binding protein vinculin are important steps in linking adhesion complexes to the actin cytoskeleton.7-15 Cells on DPP coated substrate showed distinct cell responses such as cell spreading and adhesion as indicated by their F-actin cytoskeletal structure, formation of multiple filopodia and protrusions of the lamellipodia while cells on non-coated plates were round and showed no spreading (Fig. 1). Actin polymerization drives the extension of sheet-like (lamellipodia) and rod-like protrusions (filopodia) at the cell front.
Figure 1. Cell spreading and adhesion on different matrices. C3H10T1/2 cells were seeded on either DPP, DMP1 or fibronectin coated coverglass for 24 h. The cells were fixed and stained for actin. Confocal microscopy was used to assess cell adhesion and spreading. Representative confocal images show prominent F-actin cytoskeletal structure in cells coated on DPP and DMP1 substrates when compared with cells cultured on positive control fibronectin substrate. Scale bars, 20 µm.
Activation of Focal Adhesion Kinase
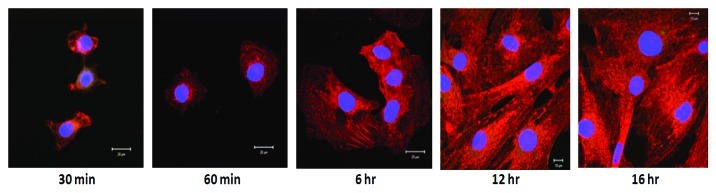
In order for a cell to correctly process the environmental motility promoting stimuli correctly, there must be intracellular signaling proteins that function as “integrators.” Focal contacts are formed at the ECM-integrin junctions that bring together cytoskeletal and signaling proteins at the sites of contact. These focal adhesions represent centers for cellular signaling mediated through integrin aggregation by ligand binding.16-18 C3H10T1/2 cells form focal adhesions when plated on DPP extracellular matrix. An important focal adhesion signaling protein is focal adhesion kinase (FAK) which is a tyrosine kinase. At the initial stages of attachment (30 min and 60 min) on a DPP coated substrate, the cells exhibit a round morphology with FAK localizing at the sites of transmembrane integrin receptor clustering. With progression in time from 6 to 24 h, FAK is seen at the edge of the cells forming lamellipodia that protrudes into the surrounding matrix (Fig. 2). Another interesting observation in this study is the interaction of FAK with microtubules (Fig. 2). At 12 and 16 h, localization of FAK was seen on microtubules in cells grown on DPP substrate. Thus, it can be envisaged that clustering of integrins by DPP leading to activation of FAK might be necessary for localized stabilization of microtubules.19 Palazzo et al. have suggested that integrin-FAK signaling pathway might enable Rho to stabilize microtubules in the leading edge of migrating cells.20
Figure 2. Localization of focal adhesion kinase during cell adhesion on DPP coated substrate. C3H10T1/2 cells were seeded on DPP coated cover glass for various time points as indicated. The cells were fixed and immunohistochemical analysis was performed with anti-FAK antibody. Representative confocal images show the successful spreading of the cells with prominent localization of FAK on the microtubules. Localization at the perinuclear region was also observed. Scale bars, 20 µm.
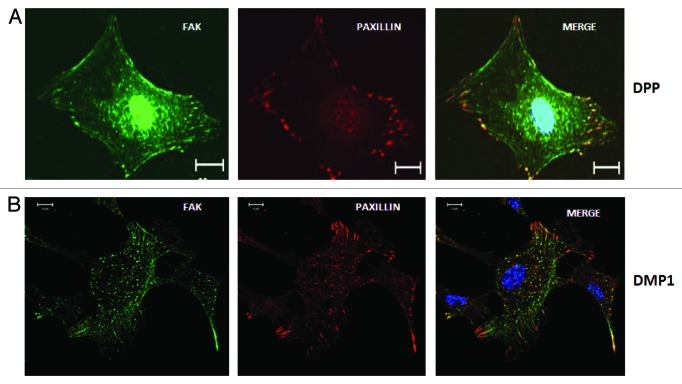
Autophosphorylation of FAK at Tyr397 creates a high affinity binding site for the Src homology 2 domains of Src family kinases.21 The interaction between Tyr 397 phosphorylated FAK and Src leads to a cascade of tyrosine phosphorylation of multiple sites in FAK as well as in other signaling molecules such as paxillin. Paxillin is known to be a substrate for FAK-Src complex and functions as an adaptor molecule for various signaling and structural proteins in adhesions.22,23 Phosphorylation of paxillin is reported to promote migration and thus modulate adhesion dynamics.24 Immunoprecipitation studies showed that DPP was able to induce association of FAK and paxillin. Although FAK was named according to its localization at focal adhesions, in this work we provide evidence that FAK is not only localized at the cytoplasmic regions where many filopodia are formed but could be clearly observed in the nucleus of the undifferentiated mesenchymal cells (Fig. 3). However, no nuclear localization of FAK was observed in cells grown on DMP1 coated substrate. Lim et al. have shown that nuclear FAK might regulate p53 activity.25 When cells are stressed, p53 accumulates and induces the expression of the gene encoding p21, which triggers cell cycle arrest. Nuclear FAK can mediate the ubiquitination and degradation of p5326 resulting in increased cell survival. Lim et al. concluded a kinase independent role for FAK as a nuclear scaffold protein that targets p53 for degradation and enhances cell-proliferation.27 Recently, nuclear FAK has been shown to regulate chromatin remodeling by its interaction with MBD2 (methyl CpG-binding protein 2).28 The nuclear FAK-MBD2 complexes alter heterochromatin reorganization and decrease MBD2 association with HDAC1 (histone deacetylase complex 1) and methyl CpG site in the myogenin promoter, thus inducing myogenin expression. Both nuclear localization signal (NLS) and nuclear export signal (NES) have been identified in FAK and this facilitates shuttling between the nuclear and cytoplasmic compartments.29
Figure 3. Localization of FAK and Paxillin in cells adherent on DPP and DMP1 coated substrate. C3H10T1/2 cells were seeded on DPP or DMP1 coated cover glass for 24 h and immunostained with anti-FAK (green) and anti-Paxillin (red) antibodies. Confocal microscopic images show successful spreading and formation of focal adhesions. Interestingly, FAK is localized in the nucleus on DPP coated substrate while it is present only at focal adhesions on DMP1 coated substrate. Paxillin is predominantly localized at the focal adhesions in cells cultured on both DPP and DMP1 substrate. Scale bars, 20 µm.
Thus, FAK, which is an important component of focal adhesions, promotes both scaffolding and signaling function. In a recent study an unconventional function of focal adhesions was identified, namely the regulated degradation of the surrounding matrix.30 In this study it was determined that MT1-MMP (membrane type MMPs) is targeted to focal adhesions through a novel interaction with a FAK-p130Cas complex.
Focal Adhesions Mediated by DPP Activate MAP Kinase Signaling Pathway
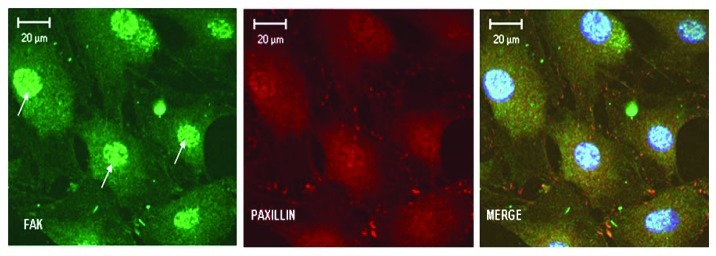
Focal adhesions are not only structural links between the cytoskeleton and the extracellular matrix to mediate cell adhesion but can transduce signals initiated in the extracellular matrix to the nucleus.6 Activated paxillin can recruit and activate Pak a downstream effector of Rac1. Pak can phosphorylate the MAP-kinase kinase MEK leading to possible convergence between integrin mediated activated focal adhesions, Rac1 and mitogen-activated protein kinase (MAPK) signaling.31 MAP kinase cascades play a key role in transducing extracellular signals to cellular responses resulting in cell growth and differentiation. Several studies have shown that cell adhesion can regulate ERK signaling and regulate diverse cellular processes via the phosphorylation of multiple substrates.32 In this study, the integrins present on the cell surface could utilize the polymerized actin network as a scaffold to enhance DPP mediated activation of the ERK pathway. Phosphorylated ERK can translocate to the nucleus and phosphorylate multiple substrates, including Elk-1, Sap-1a, Etd1, c-Myc, c-fos, etc. DPP mediated adhesion permits phosphorylation of ERK and its translocation to the nucleus where it phosphorylates a key transcriptional factor Elk-1. Interestingly, cells treated with the ERK1/2 inhibitor PD98059, did not have any effect on the activation of FAK and paxillin (Fig. 4). This suggests that ERK signaling is a downstream event and is activated by binding of cell surface integrin receptors to DPP ligand.
Figure 4. Blocking ERK1/2 activation does not abrogate nuclear translocation of FAK and formation of focal adhesions. C3H10T1/2 cells were incubated with 30 µM of PD98059 (ERK1/2 inhibitor) for 2 h and then plated on DPP coated plates and stained with anti-FAK (green) and anti-Paxillin (red) antibody after 48 h. Confocal images show that ERK activation is not required for cell adhesion and formation of focal adhesions. Activation of FAK and paxillin is upstream of ERK signaling. Arrows point to FAK in the nucleus. Scale bars, 20 µm.
Activation and translocation of ERK to the nucleus provided an important clue to explain differentiation of undifferentiated mesenchymal cells to odontoblast-lineage. Our results demonstrate for the first time that C3H10T1/2 cells on DPP substrate express differentiation markers such as DSP (dentin sialoprotein), DMP1 (dentin matrix protein 1) and OCN (osteocalcin). Blocking integrin activation by RGD peptide abrogated this effect.
In summary, we suggest that integrin-dependent adhesion to DPP promotes cell migration and differentiation by aggregation of adhesion molecules which consequently form adhesion complexes. This supramolecular adhesion complex contains FAK, paxillin and integrins. A central signaling component of focal adhesions is activation of focal adhesion kinase. Disruption of the integrin-mediated adhesions demonstrated the importance of FAK in downstream signaling events. An interesting component of this study is the adhesion dependent activation of MAP kinase signaling pathway leading to cell differentiation. Some of the genes upregulated by adhesion to DPP have been shown to be relevant to odontoblast differentiation.
Acknowledgments
This work was supported by grants from the National Institute of Health DE 19633 and the Brodie Endowment Fund.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20627
References
- 1.George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 2008;108:4670–93. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veis A. Mineral-matrix interactions in bone and dentin. J Bone Miner Res. 1993;8(Suppl 2):S493–7. doi: 10.1002/jbmr.5650081312. [DOI] [PubMed] [Google Scholar]
- 3.Veis A. Phosphoproteins from teeth and bone. Ciba Found Symp. 1988;136:161–77. doi: 10.1002/9780470513637.ch11. [DOI] [PubMed] [Google Scholar]
- 4.Stetler-Stevenson WG, Veis A. Bovine dentin phosphophoryn: composition and molecular weight. Biochemistry. 1983;22:4326–35. doi: 10.1021/bi00287a025. [DOI] [PubMed] [Google Scholar]
- 5.Eapen AS, Ramachandran A, George A. Dentin phosphoprotein (DPP) activates integrin-mediated anchorage-dependent signals in undifferentiated mesenchymal cells. J Biol Chem. 2012;287:5211–24. doi: 10.1074/jbc.M111.290080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wehrle-Haller B. Structure and function of focal adhesions. Curr Opin Cell Biol. 2012;24:116–24. doi: 10.1016/j.ceb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Cabodi S, Di Stefano P, Leal MdelP, Tinnirello A, Bisaro B, Morello V, et al. Integrins and signal transduction. Adv Exp Med Biol. 2010;674:43–54. doi: 10.1007/978-1-4419-6066-5_5. [DOI] [PubMed] [Google Scholar]
- 8.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruthamuthu V, Aratyn-Schaus Y, Gardel ML. Conserved F-actin dynamics and force transmission at cell adhesions. Curr Opin Cell Biol. 2010;22:583–8. doi: 10.1016/j.ceb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A. 2011;17:713–24. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3:a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali O, Guillou H, Destaing O, Albigès-Rizo C, Block MR, Fourcade B. Cooperativity between integrin activation and mechanical stress leads to integrin clustering. Biophys J. 2011;100:2595–604. doi: 10.1016/j.bpj.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–8. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keely PJ. Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion. J Mammary Gland Biol Neoplasia. 2011;16:205–19. doi: 10.1007/s10911-011-9226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouaouina M, Harburger DS, Calderwood DA. Talin and signaling through integrins. Methods Mol Biol. 2012;757:325–47. doi: 10.1007/978-1-61779-166-6_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol. 2011;12:189–97. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- 18.Serrels B, Frame MC. FAK and talin: who is taking whom to the integrin engagement party? J Cell Biol. 2012;196:185–7. doi: 10.1083/jcb.201112128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol. 2007;176:667–80. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–9. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 21.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–19. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Ilicá D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevicá N, Han Q, et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117:177–87. doi: 10.1242/jcs.00845. [DOI] [PubMed] [Google Scholar]
- 23.Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am J Respir Cell Mol Biol. 2009;40:99–107. doi: 10.1165/rcmb.2008-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes CD, Petrie TA, García AJ. Mixed extracellular matrix ligands synergistically modulate integrin adhesion and signaling. J Cell Physiol. 2008;217:450–8. doi: 10.1002/jcp.21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golubovskaya VM, Cance WG. FAK and p53 Protein Interactions. Anticancer Agents Med Chem. 2011;11:617–9. doi: 10.2174/187152011796817619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim ST, Miller NL, Nam JO, Chen XL, Lim Y, Schlaepfer DD. Pyk2 inhibition of p53 as an adaptive and intrinsic mechanism facilitating cell proliferation and survival. J Biol Chem. 2010;285:1743–53. doi: 10.1074/jbc.M109.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei L, Xiong WC. FAK interaction with MBD2: A link from cell adhesion to nuclear chromatin remodeling? Cell Adh Migr. 2010;4:77–80. doi: 10.4161/cam.4.1.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ossovskaya V, Lim ST, Ota N, Schlaepfer DD, Ilic D. FAK nuclear export signal sequences. FEBS Lett. 2008;582:2402–6. doi: 10.1016/j.febslet.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–85. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livshits G, Kobielak A, Fuchs E. Governing epidermal homeostasis by coupling cell-cell adhesion to integrin and growth factor signaling, proliferation, and apoptosis. Proc Natl Acad Sci U S A. 2012;109:4886–91. doi: 10.1073/pnas.1202120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aplin AE, Stewart SA, Assoian RK, Juliano RL. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol. 2001;153:273–82. doi: 10.1083/jcb.153.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]