Abstract
In the past year, three papers have been published exploring the role of the matricellular protein periostin in excisional skin repair. These papers all show a delay in wound closure and the kinetics of this delay are strikingly similar across the three reports. The similarities between these papers end, however, when each investigates the mechanism through which periostin influences skin repair. Three proposed mechanisms have been identified: (1) myofibroblast differentiation, (2) keratinocyte proliferation and (3) fibroblast proliferation and migration. The aim of this commentary is to compare and contrast the three studies performed to date in an attempt to decipher the role of periostin in the repair of full-thickness skin wounds.
Keywords: periostin, skin repair, proliferation, migration, myofibroblast
Introduction
Since its discovery in 1993, the matricellular protein periostin, has garnered much attention, with primary focuses on the role of periostin in cardiac development and the progression of pathologies such as cancer and fibrosis.1-3 The physiological role of periostin in the healthy adult animal is clearly linked to biomechanically active tissues2 as well as in tissue repair and remodeling. Following acute injury periostin has been shown to be potently upregulated in bone,4 heart,5,6 vasculature7,8 and muscle.9 Beginning with Lindner and colleagues in 2005,8 and more recently from our lab,10,11 periostin has also been implicated in skin development and repair. In all described remodeling situations, periostin upregulation follows a highly conserved pattern (beginning at day 3, peaking around day 7 and eventually returning to baseline by 4 weeks), suggesting a consistent role for periostin in tissue repair process.
We first described the expression pattern of periostin in excisional wound healing in 200910 and in incisional healing in 2010.11 In the last year, three papers have now been published describing potential roles for periostin in dermal wound repair from experiments performed using different derivations of periostin knockout mice (Postn−/−). Beginning with Nishiyama and colleagues,12 followed by our report13 and finally Ontsuka et al.,14 each report shows a significant delay in the closure of excisional wounds in the absence of periostin. From these publications, it is clear that the wound closure results are reproducible even in different derivations of the knockout mouse. However, there are interesting and distinct differences in the mechanisms proposed by each paper as the underlying cause for the delayed wound closure in Postn−/− mice.
In this commentary we will discuss the similarities and the differences between these reports in an attempt to define more clearly the exact role(s) of periostin in adult excisional skin repair. We will conclude by highlighting where we think the field should focus its efforts moving forward.
Excisional Repair in the Postn−/− Mouse
Periostin promotes keratinocyte proliferation
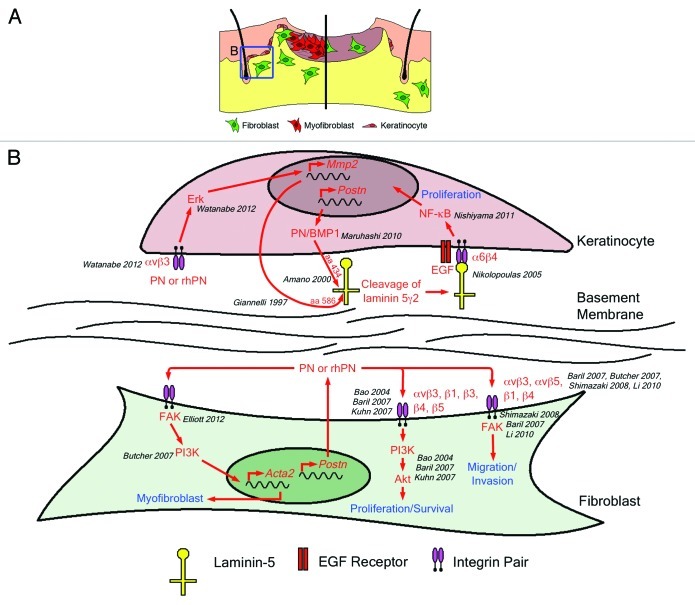
Nishiyama and colleagues were the first to report a delay in dermal wound repair due to the absence of periostin.12 This delay was observed at days 3, 5 and 8 following 3 mm full-thickness excisional wounding. The authors attributed this delay in wound closure to a defect in re-epithelialization, brought on by reduced keratinocyte proliferation in the hair follicles (Table 1 and Fig. 1). No direct in vitro test of the loss of periostin on keratinocyte proliferation was reported and overexpression of murine periostin in a human keratinocyte cell line (HaCaT) resulted in no initial increase in proliferation. However, following one week of culture at confluence, periostin overexpressing HaCaT cells showed increased BrdU uptake (~5.5% positive vs. ~3% positive) and phospho-NFκB positive cells. The authors proposed a mechanism where periostin acts as a scaffold to facilitate processing of laminin5γ2 by BMP-1. In support of this mechanism, previous reports indicate that keratinocyte adhesion to laminin5 is an important step in NFκB induced keratinocyte migration and proliferation.15,16 The overall results presented in the paper, however, do not test this mechanism.
Table 1. Major findings from reports on skin repair in Postn−/− mice.
| Elliott et al., 2012 | Nishiyama et al., 2011 | Ontsuka et al., 2012 | |
|---|---|---|---|
| Wound model |
6 mm punch biopsy |
3 mm punch biopsy |
8 mm or 10 mm punch biopsy |
| Main effect |
KO delay at D5 and D7 |
KO delay at D3, D5 and D7 |
KO delay at D3, D5, D7 and D11 |
| Proposed in vivo cause |
Myofibroblast differentiation |
Re-epithelialization via keratinocyte proliferation |
Fibroblast proliferation and migration |
| In vivo evidence |
Reduced α-smooth muscle actin gene expression and immunoreactivity in KO |
Measurements from H&E stained sections Reduced Ki-67 immunoreactivity around KO hair follicles |
No data |
| In vitro support |
Adult KO fibroblasts showed reduced: Force generation, Collagen gel contraction, α-smooth muscle actin immunofluorescence, α-smooth muscle actin protein |
No data |
Newborn KO fibroblasts show reduced proliferation KO MEFs show reduced migration |
| Rescue tool(s) (in vitro) |
Recombinant full-length human periostin (R&D Systems) produced in a mouse myeloma cell line (NS0) |
Expression vector for mouse periostin |
Recombinant full-length mouse periostin (R&D Systems) produced in an insect ovarian cell line (Sf21) Expression vector for full-length mouse periostin |
| Rescue? (in vitro) |
Adult KO fibroblasts showed restored: Collagen gel contraction, α-smooth muscle actin staining, α-smooth muscle actin protein. (Force generation not tested for rescue) |
Conflicting results: Overexpression of Ms Postn in human keratinocyte cell line (HaCaT) resulted in no difference in cell number when cultured for 96 h. However, the same cells cultured for one week beyond confluence showed an increase in BrdU labeling. |
Proliferation of newborn mouse fibroblasts (vector and recombinant) Proliferation of normal human dermal fibroblasts (recombinant) |
| Rescue tool (in vivo) |
Recombinant full-length human periostin (R&D Systems) incorporated into an electrospun collagen scaffold |
No in vivo rescue |
Recombinant full-length mouse periostin (R&D Systems) added directly onto wounds |
| Rescue? (in vivo) |
Increased α-smooth muscle actin immunoreactivity at D7 No wound closure kinetics |
No in vivo rescue |
Restored wound closure kinetics No evidence for mechanism |
| Additional findings | No difference in fibroblast migration No difference in re-epithelialization |
Similar Ki-67 numbers in granulation tissue and migrating keratinocytes |
Three recent studies on the role of periostin in skin repair have similarities in in vivo results. Yet, considerable differences in in vitro results and methodologies make it difficult to clearly define the mechanism through which periostin influences skin repair.
Figure 1. (A) Skin repair in wild-type mice (left) is a multistep process requiring contributions from the dermis and epidermis. Keratinocytes (pink) in the hair follicles proliferate and migrate to cover the defect. Simultaneously, fibroblasts (green) proliferate and migrate into the wound where they differentiate into myofibroblasts (red). Fibroblasts and myofibroblasts produce, secrete and organize new extracellular matrix while myofibroblasts contract the newly formed granulation tissue to draw the wound edges together. Recent reports suggest that delayed wound closure in the Postn−/− mouse (right) is due to decreased keratinocyte proliferation (leading to slowed re-epithelialization), decreased fibroblast proliferation and migration into the wound and failure of wound fibroblasts to differentiate into myofibroblasts (resulting in failed wound contraction). (B) There is considerable evidence in the literature to support a role for periostin in all of these cellular mechanisms. Predominantly, periostin has been shown to mediate its effects through integrins (such as αvβ3) and adhesive signaling.29-32 Although Nishiyama’s proposal requires intracellular interactions between periostin and BMP-1, the ability of rhPN to increase MMP2 expression, thereby facilitating laminin5γ2 cleavage, may represent an alternative mechanism for keratinocyte proliferation based on αvβ3 ligation and adhesion signaling.
Periostin modulates myofibroblast differentiation
We have recently reported on the consequences of genetic deletion of periostin on dermal wound repair using a different derivation of the mouse.13 Following 6 mm full-thickness excisional wounding, Postn−/− mice17 exhibited a significant delay in wound closure at days 5 and 7. This delay corresponded with a significant decrease in α-smooth muscle actin (α-SMA) expression within the granulation tissue, although fibroblasts recruitment within the wound and collagen expression appeared unaltered. We therefore proposed that the delay in wound closure in Postn−/− mice was due to a defect in myofibroblast differentiation (Fig. 1), resulting in insufficient wound contraction (Table 1). In vitro support for this proposal was gathered by culturing wild-type (Postn+/+) and Postn−/− adult dermal fibroblasts from mouse skin biopsies. Postn−/− fibroblasts showed deficits in force generation and collagen gel contraction assays, consistent with a failure to develop the typical myofibroblast phenotype. Additionally, Postn−/− fibroblasts displayed reduced α-SMA protein in 3D and compliant 2D culture systems, compared with Postn+/+ fibroblasts. Of great significance, full-length recombinant human periostin (rhPN) was able to rescue the defects in collagen gel contraction and α-SMA protein expression evident in Postn−/− fibroblasts. The ability of rhPN to recover the myofibroblast phenotype was extended to in vivo punch wounds where delivery of exogenous rhPN via an electrospun periostin-collagen scaffold resulted in increased α-SMA immunoreactivity in the granulation tissue of day 7 Postn−/− wounds, compared with wounds receiving collagen scaffolds alone. Therefore, both our in vivo and in vitro results demonstrate that periostin modulates myofibroblast differentiation during excisional would repair and exogenous rhPN is sufficient to rescue this phenotype.
Periostin promotes fibroblast proliferation and migration
Very recently, Ontsuka and colleagues have reported on excisional wound repair in a Postn−/− mouse.14 As in the previous studies, a full-thickness excisional wound model was used, although it is unclear whether an 8 or 10 mm punch was used since both are stated in the methods (Table 1). Nevertheless, deletion of periostin resulted in a delay in wound closure, which was observed at days 3, 5, 7 and 11. The underlying cause for the delay was not established in vivo but the authors proposed that the loss of periostin resulted in defects in fibroblast proliferation and migration (Fig. 1), based on in vitro studies. Using dermal fibroblasts harvested from newborn Postn+/+ and Postn−/− mice, Ontsuka and colleagues detected a decreased proliferation rate when periostin was absent (approx 20% difference over 3 d). Postn−/− mouse embryonic fibroblasts (MEFs) exhibited a subtle but statistically significant delay in migration using an in vitro scratch wound assay. However, as the authors do not show in vivo evidence for a deficit in migration or proliferation, it is unclear whether these in vitro findings are of functional relevance. Addition of recombinant periostin directly to wounds did increase closure rates in Postn+/+ and Postn−/− mice, confirming that exogenous periostin sufficiently rescues Postn−/− wound closure. However, the mechanism for this rescue is not discussed.
Periostin is upregulated following excisional wounding
All three independent studies confirm that periostin is significantly upregulated following dermal wounding in mice. Moreover, all studies show a significant peak in periostin expression at day 7, confirming our initial finding.10 In normal skin periostin is localized at the dermal-epidermal junction (DEJ) and in hair follicles. Following excisional wounding in Postn+/+ mice, periostin is predominantly expressed in the granulation tissue and in neighboring hair follicles. The absence of periostin results in delayed wound closure, which is most pronounced between days 3 and 7. Even though these three studies each used different initial wounds sizes, the timing of the delay in wound closure and the peak of periostin expression were indeed very similar. The similarities in the studies begin to diverge, however, when we begin to look at the underlying mechanisms proposed by each of these reports (Table 1). Could it be that periostin modulates fibroblast proliferation, migration and differentiation into myofibroblasts, while simultaneously promoting keratinocyte proliferation and re-epithelialization? There is certainly evidence in the literature to support each claim individually (Table 2 and Fig. 1). In fact, rhPN has been shown to increase both proliferation and myofibroblastic behavior in palmar fascia fibroblasts.18 Upon closer inspection, however, it is clear that the evidence presented in the three reports on skin repair could arise as a result of differences in experimental methodologies.
Table 2. Evidence from the literature supports all three mechanisms.
| Myofibroblast | Proliferation | Migration |
|---|---|---|
| Shimazaki 2008: Postn−/− mice had an increased incidence of ventricular rupture following myocardial infarction due to reduced α-SMA positive cells and impaired collagen formation.6 |
Ben 2011: Transfection of pancreatic cancer cell lines (BxPC-3 and Panc-1) with a periostin expression vector (Ad5-PN) promoted anchorage-independent growth.33 |
Shimazaki 2008: Recombinant periostin (ΔbΔe splice variant) enhanced chemotaxis of cardiac fibroblasts from Postn−/− mice. This increase was attenuated by an anti-periostin antibody.6 |
| Erkan 2007: Addition of rhPN to pancreatic stellate cells resulted in increased expression of α-SMA, collagen 1, fibronectin, TGFβ1 and periostin. Silencing periostin decreased α-SMA expression.34 |
Erkan 2007: Under serum deprivation, rhPN stimulated growth of Panc1, SU86.86, and T3M3 (pancreatic cancer cell lines).34 |
Ben 2011: Cells (BxPC-3 or Panc-1) infected with Ad5-PN migrated and invaded faster than controls in transwell assays.33 |
| Vi 2009: Fibroblasts isolated from Dupuytren disease (DD) overexpressed periostin and had an increased ability to contract a collagen matrix, which was further enhanced by addition of rhPN. DD cells in 3D culture induced α-SMA in response to rhPN.18 |
Vi 2009: Growth on rhPN coated plates resulted in an increase in proliferation of palmar fascia fibroblasts.18 |
|
| Sidhu 2010: Transfection of bronchial epithelial cells (BEAS2B) with a rhPN expression vector resulted in increased α-SMA protein and mRNA.23 |
Liu 2011: Periostin-silenced gastric cancer cells exhibited reduced cell proliferation.35 |
Liu 2011: Periostin-silenced gastric cancer cells exhibited reduced invasion using a Boyden chamber invasion assay.35 |
| Bozyk 2012: Hyperoxia exposure increased α-SMA positive myofibroblasts in the lungs of Postn+/+, but not Postn−/−, mice.36 Bozyk 2012: Periostin treatment increased α-SMA expression in neonatal lung mesenchymal stromal cells.36 |
Bozyk 2012: Periostin induced human mesenchymal stromal cell DNA synthesis in the presence of TGF-β1.36 |
|
| Hakuno 2010: High fat diet-induced α-SMA in cardiac valve complexes is attenuated in Postn−/− mice.22 |
Kuhn 2007: rhPN induced proliferation of neonatal cardiomyocytes in a PI3K/Akt dependent manner. Injecting rhPN into the myocardium induced DNA synthesis and division of nearby differentiated cardiomyocytes.37 |
Hakuno 2010: Conditioned media from periostin transfected cells increased migration of human coronary artery endothelial cells.22 |
| Yoshida 2011: Silencing of periostin splice variant III attenuated TGF-β2 induced α-SMA production in primary human retinal pigment epithelial cells.28 |
Zhu 2011: Neutralizing monoclonal antibody to periostin inhibited anchorage-independent growth of the periostin-expressing ovarian cancer cell line A2780.38 |
Zhu 2011: Neutralizing monoclonal antibody to periostin inhibited periostin-induced cancer cell migration and invasion.38 |
| Jackson-Boeters 2009: Periostin expression coincides with 〈-SMA expression within the granulation tissue of excisional wounds of mice.10 |
Liu 2010: Inhibition of periostin expression via RNA interference suppressed proliferation of a human osteosarcoma cell line (U2OS).39 |
Liu 2010: Inhibition of periostin gene expression via RNA interference suppressed migration and invasion of U2OS cells in transwell assays.39 |
| Lindner 2005: Acquisition of a smooth muscle cell phenotype (α-SMA expression) correlated with acquisition of periostin expression both in vitro and in vivo.8 |
Yan 2006: Mice that received periostin-producing 293T cells at the mammalian fat pad tissue had significantly larger local tumors than did mice receiving control cells.40 |
Lindner 2005: Periostin overexpressing C3H10T1/2 cells had greater migratory response to serum, which was attenuated by a periostin-blocking antibody.8 |
| Hong 2010: Periostin overexpressing A549 cells expressed higher levels of vimentin mRNA.41 |
Hong 2010: Periostin overexpressing A549 cells displayed increased proliferation.41 |
Hong 2010: Periostin overexpressing A549 cells migrated and closed scratch wounds at an increased rate.41 |
| Kikuchi 2008: Close approximation of periostin immunoreactivity to α-SMA positive cells (periocryptal fibroblast) in normal colonic mucosa. Decreased periostin immunoreactivity preceded a decrease of α-SMA positive cells.42 | Kikuchi 2008: Ki-67-positive epithelial cells were significantly decreased in the colonic crypts of Postn−/− mice.42 | Yan 2006: Using transwell assays, significantly more periostin-expressing 293T cells migrated into the membrane relative to control cells.40 |
A brief summary of the literature surrounding periostin shows that there is support for each proposed mechanism. Cell-type and tissue specific effects of periostin certainly cloud the issue. To confidently say which mechanism(s) is most important in skin repair we must focus our attention on the in vivo evidence for context specific clues.
The Devil is in the Details
Migration
Fibroblasts
Does the evidence support the hypothesis that fibroblast migration is impaired in Postn−/− mice? Based on our in vivo studies, we hypothesized that a deficit in fibroblast recruitment and migration into the wounds may be present; the presence of α-SMA positive myofibroblasts at the wound edges, but not within the granulation tissue of day 7 Postn−/− wounds suggests impaired migration. However, we found no evidence for a reduction in fibroblast number in the granulation tissue of day 7 Postn−/− wounds compared with wild-type controls. Immuno-labeling of fibroblasts with fibroblast specific protein-1 and collagen detection via hydroxyproline demonstrated that the number of fibroblasts in the granulation tissue was equivalent in Postn+/+ and Postn−/− wounds. When we employed the scratch wound assay in vitro, our findings mirrored our in vivo results in that we found no difference in migratory ability of Postn+/+ and Postn−/− adult dermal fibroblasts.
In contrast, Ontsuka et al. demonstrated that wound closure kinetics was altered in Postn−/− mice, and suggested that fibroblast migration was impaired in Postn−/− wounds. Their rational for suspecting impaired migration was based on previous reports of a role for periostin in fibroblast proliferation and migration, but not on in vivo observations. They performed in vitro scratch wound assays and showed that MEFs derived from Postn−/− mice had a slightly, although significantly, lower migratory ability compared with MEFs derived from Postn+/+ mice. The key issue with this paper is that they provided no direct in vivo evidence that fibroblast migration or proliferation was actually impaired during healing in knockout animals, with their hypothesis in direct contrast to our findings. Although the addition of exogenous periostin or periostin overexpression may influence cell behavior in vitro, it does not necessarily prove that periostin functions to enhance fibroblast migration in the in vivo environment.
Still, why did we see no difference in fibroblast migration, yet Ontsuka reported a difference using the same scratch wound assay? One explanation is that embryonic fibroblasts respond differently to periostin than the adult dermal fibroblasts. We have previously reported that periostin expression/localization is very different in developing and newborn mouse skin when compared with that of skin in the adult animal.11 From embryonic day 13.5 forward, periostin is expressed in the developing skin. At 2 and 9 d old, periostin is heavily expressed at the DEJ and in the dermis of the newborn skin. This pattern changes dramatically by day 19 where periostin expression in the dermis is largely absent. By day 60, periostin is restricted to hair follicles and DEJ, although at a reduced level. Perhaps the role of periostin in development, and thus MEFs, is vastly different from its role in adult skin and the residing dermal fibroblast. If this is true then we must be careful when choosing our in vitro tools to confirm in vivo findings. To emphasize this, we have shown that in 2D culture knockout fibroblasts do differentiate into myofibroblasts and it is only when the substrate rigidity is reduced or 3D culture employed, that the periostin deficiency and loss of the myolfibroblast phenotype manifests in knockout cells.
Keratinocytes
Nishiyama and colleagues attributed the delay in closure of Postn−/− wounds to reduced re-epithelialization. In support of this, they present evidence that the migration of keratinocytes across the wound surface is impaired; however, we were unable to duplicate this result (discussed below and in our report13). Nishiyama proposed that the deficit in keratinocyte migration in vivo was due to decreased proliferation in the hair follicles and not cellular migration. Using the same scratch wound assay employed by Ontsuka and our group, Nishiyama showed no difference in the migration rate of HaCaT cells vs. those overexpressing mouse periostin. Nishiyama et al. did not use Postn+/+ and Postn−/− keratinocytes and we did not assess the migratory ability of murine keratinocytes. However, in light of our observations with fibroblasts, we can suggest that a specific in vivo migratory role for periostin in wound repair remains to be determined.
Proliferation
Keratinocytes
Does the evidence support the hypothesis that the role of periostin, and therefore the cause for delayed Postn−/− wound closure, is cell proliferation? Nishiyama shows in vivo evidence for reduced keratinocyte proliferation in the hair follicles of wounded Postn−/− mice. Using the HaCaT cell line in vitro, they showed a positive influence of periostin overexpression on keratinocyte proliferation. However, they did not isolate keratinocytes from periostin knockout animals and compare them to wild types to directly measure whether loss of periostin influences proliferation.
Moreover, we did not see the delay in Postn−/− wound re-epithelialization, described by Nishiyama to be the direct result of reduced keratinocyte proliferation. We measured the epithelial migration distance of day 7 Postn+/+ and Postn−/− wounds and found no significant difference. We attribute these conflicting results to differences in the methods used to measure wound re-epithelialization. Nishiyama and colleagues presented their results as percent re-epithelialization, which is sensitive to differences in wound size, where as we reported epithelial migration distance. Postn−/− wounds are larger than Postn+/+ wounds at later time points, therefore the calculated percent re-epithelialization is inappropriately reduced for Postn−/− wounds. Epithelial migration distance, however, is not influenced by the size of the wound. Using this more appropriate measure we could not confirm Nishiyama’s claim that re-epithelialization is the principal defect in Postn−/− wound repair.
Fibroblasts
With respect to fibroblast proliferation, Ontsuka makes a very compelling argument for a significant role of periostin in regulating this cellular process. They demonstrated that the loss of periostin in newborn dermal fibroblasts results in a ~20% decrease in proliferation rate. The addition of recombinant mouse periostin, as well as overexpression of mouse periostin, increased the proliferation rate of both Postn+/+ and Postn−/− newborn dermal fibroblasts. Additionally, they showed that normal human dermal fibroblasts increased proliferation in a dose-dependent manner in the presence of recombinant periostin. However, there is no direct evidence that fibroblast proliferation is altered in vivo following wounding in the absence of periostin. In fact, Nishiyama describes the granulation tissue of day 3 Postn+/+ and Postn−/− wounds as having similar numbers of Ki-67 positive cells, suggesting no difference in fibroblast proliferation. We have shown that adult Postn+/+ and Postn−/− dermal fibroblasts exhibit no differences in proliferation in vitro (Fig. 2), which confirms the findings of Nishiyama et al. In fact, Postn−/− fibroblast proliferation was consistently higher, although not statistically significant, after 10 d of culture. The in vitro evidence presented by Ontsuka is quite convincing, yet the in vivo evidence suggests that fibroblast proliferation is not the principal defect in the Postn−/− wound repair process. As with migration, we suspect that newborn dermal fibroblasts respond differently to periostin than adult dermal fibroblasts, potentially explaining these conflicting results.
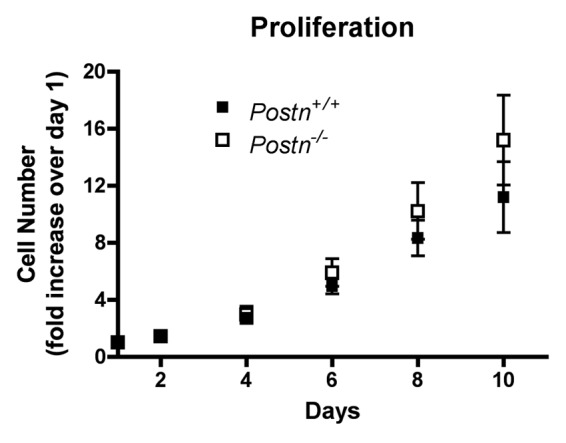
Figure 2. Loss of periostin does not negatively affect proliferation of adult murine dermal fibroblasts. Primary murine dermal fibroblasts were seeded at 2,000 cells/well in 24-well plates in 10% FBS supplemented media. Media was changed every 48 h throughout the course of the experiments. At the desired time-points, media was completely aspirated and the plate was frozen at -80°C. Once all time-points were captured, the CyQUANT cell proliferation assay kit (Invitrogen, C7026) was used to determine cell number as per the manufacturer’s protocol. A standard curve was used to obtain cell number. Error bars indicate standard error of the mean from four experiments, using four independent cell isolations.
Hints from Rescue Experiments
Knowing that the loss of periostin results in delayed skin repair, can the addition of periostin to a wound result in accelerated repair? We have shown that the addition of rhPN to Postn−/− wounds results in increased α-SMA protein at day 7, demonstrating that rhPN can modulate fibroblast to myofibroblast transition during wound healing. Whether this increase in α-SMA translates into accelerated wound closure kinetics was reserved for future publication. Ontsuka also recognized the importance of in vivo rescue experiments using recombinant periostin. In their experiments, they documented the closure kinetics of Postn+/+ and Postn−/− wounds, with and without addition of recombinant periostin. Their findings were very encouraging; showing a complete restoration of normal closure kinetics in Postn−/− wounds and accelerated closure in Postn+/+ wounds. Together these two outcomes paint a very promising picture for periostin as a therapeutic.
Interestingly, in both rescue attempts periostin was added to the wounds earlier than the endogenous peak (day 0 in our report, day 1 and every second day to day 9 for Ontsuka). The wound closure kinetics provided by Ontsuka show an immediate response to exogenous periostin, statistically significant at day 3. We show that exogenous periostin increases α-SMA by day 7, but can myofibroblast differentiation be a major contributor to wound closure at day 3? The day 3 wound is largely a pool of inflammatory cells and relatively few fibroblasts. Periostin may be stimulating chemokine releases by neutrophils and macrophages,19 but inflammatory cells do not close wounds. At such an early time point fibroblast migration into the wound is a key event. There is support for the hypothesis that tractional forces of fibroblast migration alone, as opposed to myofibroblast based contraction, can generate sufficient force to initiate wound contraction.20,21 As resistance in the newly formed matrix increases fibroblasts differentiate into myofibroblasts to complete closure. It is possible that the presence of exogenous periostin influences fibroblast migration at earlier time points. However, there is no in vivo evidence to support this and our data argues against a migratory role for periostin in vivo.
It is also possible that exogenous periostin is having an immediate effect on keratinocyte proliferation within intact hair follicles and therefore accelerating re-epithelialization. Perhaps, even aiding in keratinocyte migration by increasing matrix metalloproteinase production.22,23 Again, there is no evidence currently available to confirm this. The proposition that periostin mediates laminin5γ2 cleavage by BMP-1, indirectly promoting keratinocyte proliferation, is based on a previously described interaction between periostin and BMP-1 inside the cell.24 If true, this mechanism would require intracellular periostin isoforms, not the exogenous periostin used in these studies. Interestingly, extracellular and recombinant periostin have been shown to increase MMP2 expression in several models.22,23,25 MMP2 is known to cleave laminin5γ2, albeit at a different cleavage site than BMP-1;26 resulting in increased epithelial cell migration.27 Although this is highly speculative, induction of MMP2 may offer an alternative mechanism for keratinocyte proliferation that accommodates extracellular periostin and adhesive signaling (Fig. 1). More work is needed to determine if the influence of periostin on keratinocyte proliferation is defined by intracellular interactions, or if its role can be explained by extracellular mechanisms.25
Based on the literature, an argument can be made for each of the three mechanisms proposed in these three reports (Table 2 and Fig. 1). With respect to in vivo rescue experiments, however, we must base our conclusions on the evidence at hand and the only mechanistic evidence available right now is that exogenous periostin results in increased α-SMA within day 7 Postn−/− wounds. It is possible that periostin is increasing myofibroblast differentiation even as early as day 3. Fibroblasts at the wound edges and those that have migrated into the granulation tissue are all in a position to differentiate and initiate wound contraction, but this information has yet to be published.
Concluding Remarks
The importance of in vivo mechanistic evidence and the use of appropriate in vitro tools cannot be overstated. We have shown impaired myofibroblast differentiation in vivo and we have reproduced this defect in vitro using fibroblasts taken directly from Postn+/+ and Postn−/− skin biopsies. The studies by Nishiyama and Ontsuka have used cell lines and/or primary cultures in vitro, to suggest a role for periostin in vivo. We propose that future work with periostin in skin repair should place special emphasis on the following items to avoid similarly conflicting reports:
(1) Wound closure kinetics
(2) In vivo evidence for a mechanism
(3) Cell types and their abundance within the wound
(4) Appropriate cell types for an in vitro study
Moving forward, there are still interesting questions surrounding periostin that, once answered, will have important implications for its application in skin repair. One major question is what role periostin’s C-terminus, and its many splice variants, has on its function(s). The work described in these three papers used full-length periostin, yet studies have shown that different isoforms may have differing effects.3,28 Another important question is how delivery of periostin, exogenous or overexpression, will impact its effectiveness.
Acknowledgments
This work was funded by the Canadian Foundation for Innovation Leaders Opportunity Fund (18742), the Canadian Institutes of Health Research operating grants (MOP115044) to D.W.H., the National Sciences and Engineering Research Council of Canada Canadian Graduate Scholarship program to C.G.E. and the Ontario Graduate Scholarship program to S.S.K.
Glossary
Abbreviations:
- α-SMA
alpha-smooth muscle actin
- BMP-1
bone morphogenetic protein 1
- EGF
epidermal growth factor
- Erk
extracellular signal-regulated kinase
- DEJ
dermal-epidermal junction
- FAK
focal adhesion kinase
- KO
knockout
- MEFs
mouse embryonic fibroblasts
- MMP2
matrix metalloproteinase 2
- PI3K
phosphatidylinositol 3-kinases
- PN
Postn, periostin
- rhPN
recombinant human periostin
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20879
References
- 1.Elliott CG, Hamilton DW. Deconstructing fibrosis research: do pro-fibrotic signals point the way for chronic dermal wound regeneration? J Cell Commun Signal. 2011;5:301–15. doi: 10.1007/s12079-011-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68:3201–7. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakazawa T, Nakajima A, Seki N, Okawa A, Kato M, Moriya H, et al. Gene expression of periostin in the early stage of fracture healing detected by cDNA microarray analysis. J Orthop Res. 2004;22:520–5. doi: 10.1016/j.orthres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–21. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Oparil S, Sanders JM, Zhang L, Dai M, Chen LB, et al. Phosphatidylinositol-3-kinase signaling mediates vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis. 2006;188:292–300. doi: 10.1016/j.atherosclerosis.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77–83. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- 9.Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics. 2003;14:261–71. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- 10.Jackson-Boeters L, Wen W, Hamilton DW. Periostin localizes to cells in normal skin, but is associated with the extracellular matrix during wound repair. J Cell Commun Signal. 2009;3:125–33. doi: 10.1007/s12079-009-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou HM, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal. 2010;4:99–107. doi: 10.1007/s12079-010-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, et al. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011;6:e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, et al. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci. 2012;125:121–32. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, et al. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012;21:331–6. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 15.Dellambra E, Vailly J, Pellegrini G, Bondanza S, Golisano O, Macchia C, et al. Corrective transduction of human epidermal stem cells in laminin-5-dependent junctional epidermolysis bullosa. Hum Gene Ther. 1998;9:1359–70. doi: 10.1089/hum.1998.9.9-1359. [DOI] [PubMed] [Google Scholar]
- 16.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Puri C, Tacchetti C, et al. Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol Cell Biol. 2005;25:6090–102. doi: 10.1128/MCB.25.14.6090-6102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, et al. periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–44. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vi L, Feng L, Zhu RD, Wu Y, Satish L, Gan BS, et al. Periostin differentially induces proliferation, contraction and apoptosis of primary Dupuytren’s disease and adjacent palmar fascia cells. Exp Cell Res. 2009;315:3574–86. doi: 10.1016/j.yexcr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46:677–86. doi: 10.1165/rcmb.2011-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au K, Ehrlich HP. When the Smad signaling pathway is impaired, fibroblasts advance open wound contraction. Exp Mol Pathol. 2010;89:236–40. doi: 10.1016/j.yexmp.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrlich HP, Keefer KA, Myers RL, Passaniti A. Vanadate and the absence of myofibroblasts in wound contraction. Arch Surg. 1999;134:494–501. doi: 10.1001/archsurg.134.5.494. [DOI] [PubMed] [Google Scholar]
- 22.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest. 2010;120:2292–306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010;107:14170–5. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285:13294–303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe T, Yasue A, Fujihara S, Tanaka E. PERIOSTIN regulates MMP-2 expression via the αvβ3 integrin/ERK pathway in human periodontal ligament cells. Arch Oral Biol. 2012;57:52–9. doi: 10.1016/j.archoralbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, et al. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem. 2000;275:22728–35. doi: 10.1074/jbc.M002345200. [DOI] [PubMed] [Google Scholar]
- 27.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida S, Ishikawa K, Asato R, Arima M, Sassa Y, Yoshida A, et al. Increased expression of periostin in vitreous and fibrovascular membranes obtained from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:5670–8. doi: 10.1167/iovs.10-6625. [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–39. doi: 10.1016/S1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 30.Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26:2082–94. doi: 10.1038/sj.onc.1210009. [DOI] [PubMed] [Google Scholar]
- 31.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256–66. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, et al. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208:358–65. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben QW, Jin XL, Liu J, Cai X, Yuan F, Yuan YZ. Periostin, a matrix specific protein, is associated with proliferation and invasion of pancreatic cancer. Oncol Rep. 2011;25:709–16. doi: 10.3892/or.2011.1140. [DOI] [PubMed] [Google Scholar]
- 34.Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–64. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Liu BA. Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J Gastroenterol. 2011;17:2674–80. doi: 10.3748/wjg.v17.i21.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozyk PD, Bentley JK, Popova AP, Anyanwu AC, Linn MD, Goldsmith AM, et al. Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS One. 2012;7:e31336. doi: 10.1371/journal.pone.0031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–9. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 38.Zhu M, Saxton RE, Ramos L, Chang DD, Karlan BY, Gasson JC, et al. Neutralizing monoclonal antibody to periostin inhibits ovarian tumor growth and metastasis. Mol Cancer Ther. 2011;10:1500–8. doi: 10.1158/1535-7163.MCT-11-0046. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Huang SJ, Qin ZL. Inhibition of periostin gene expression via RNA interference suppressed the proliferation, apoptosis and invasion in U2OS cells. Chin Med J (Engl) 2010;123:3677–83. [PubMed] [Google Scholar]
- 40.Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem. 2006;281:19700–8. doi: 10.1074/jbc.M601856200. [DOI] [PubMed] [Google Scholar]
- 41.Hong L, Sun H, Lv X, Yang D, Zhang J, Shi Y. Expression of periostin in the serum of NSCLC and its function on proliferation and migration of human lung adenocarcinoma cell line (A549) in vitro. Mol Biol Rep. 2010;37:2285–93. doi: 10.1007/s11033-009-9721-1. [DOI] [PubMed] [Google Scholar]
- 42.Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, Shimazaki M, et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem. 2008;56:753–64. doi: 10.1369/jhc.2008.951061. [DOI] [PMC free article] [PubMed] [Google Scholar]