Abstract
The L1 cell adhesion molecule (L1CAM) plays a major role in the development of the nervous system and in the malignancy of human tumors. In terms of biological function, L1CAM comes along in two different flavors: (1) a static function as a cell adhesion molecule that acts as a glue between cells; (2) a motility promoting function that drives cell migration during neural development and supports metastasis of human cancers. Important factors that contribute to the switch in the functional mode of L1CAM are: (1) the cleavage from the cell surface by membrane proximal proteolysis and (2) the ability to change binding partners and engage in L1CAM-integrin binding. Recent studies have shown that the cleavage of L1CAM by metalloproteinases and the binding of L1CAM to integrins via its RGD-motif in the sixth Ig-domain activate signaling pathways distinct from the ones elicited by homophilic binding. Here we highlight important features of L1CAM proteolysis and the signaling of L1CAM via integrin engagement. The novel insights into L1CAM downstream signaling and its regulation during tumor progression and epithelial-mesenchymal transition (EMT) will lead to a better understanding of the dualistic role of L1CAM as a cell adhesion and/or motility promoting cell surface molecule.
Keywords: ADAM10, ADAM17, EMT, ERM proteins, NFκB, cell adhesion, integrins, regulated intramembrane proteolysis, signaling
L1CAM in Neural and Tumor Biology
The L1 cell adhesion molecule (L1CAM) plays an essential role in the development of the nervous system but it is also highly relevant for the progression of human tumors.1 L1CAM is the prototype member of the L1-family of closely related neural adhesion molecules. This family comprises four different members in vertebrates: L1CAM,2 Close Homolog of L1 (CHL1), NrCAM and Neurofascin.3 The L1CAM molecule was initially identified in mice4 and homologous proteins have been detected in human (L1CAM),5 chicken (NgCAM),6 rat (NILE)7 and Drosophila (Neuroglian).8
L1CAM is a 200–220 kDa transmembrane glycoprotein of the immunoglobulin (Ig) superfamily composed of six Ig-like domains and five fibronectin type III repeats followed by a transmembrane region and a highly conserved cytoplasmic tail.2 Work over the past 25 y has shown that L1CAM can interact with various binding partners in cis (within the same plasma membrane) or trans (on an adjacent cell) (for review see refs. 9–11). L1CAM can bind to itself (homophilic) or heterophilically to other neural cell adhesion molecules, integrins, CD24, neurocan and neuropilin-1.10 The cytoplasmic tail of L1CAM can interact with the cytoskeletal proteins ankyrin, actin, spectrin and ERM proteins.12,13
L1CAM was discovered as a cell adhesion molecule in the nervous system.4 Thus, a large part of the knowledge about its function comes from studies in the field of neurobiology. During brain development, L1CAM is involved in neurite outgrowth14 and fasciculation,15 as well as adhesion16 and migration.17 In addition, L1CAM participates in myelination processes by mediating the elongation of Schwann cells along the axon18 and promotes neural cell survival.1,9,10,19 The important role of L1CAM in the human brain is underlined by the clinical symptoms associated with mutations or polymorphisms of the L1CAM gene located at the q28 locus of the X-chromosome.20 These mutations cause severe recessive cerebral disorders like X-linked mental retardation and schizophrenia and frequently lead to neonatal death.21 Clinical manifestations of the so-called “L1 syndrome” are summarized by the abbreviation MASA (mental retardation, aphasia, shuffling gait, adducted thumbs) or CRASH (corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraplegia and hydrocephalus). Most disease-associated mutations interfere with ligand binding, cell surface expression or intracellular transport of L1CAM.22
Structural studies have suggested that during homophilic binding the L1CAM Ig-domains 1–4 form paired zipper like structures along the intercellular boundaries supporting static cell adhesion.23,24 However, the ability of L1CAM to promote motility of neuronal cells is essential for the development or remodelling of the nervous system.3 These observations point to a switch from a static to a dynamic L1CAM function.
Subsequent work in tumor biology has significantly extended our view on the importance of L1CAM in human disease. L1CAM is overexpressed in many human cancers, such as ovarian and endometrial carcinoma (EC), pancreatic ductal adenocarcinoma (PDAC), melanoma and glioblastoma. In this context, L1CAM expression is generally associated with poor prognosis, an aggressive phenotype, and advanced tumor stages (for review see refs. 1 and 25). Unlike in neural cells, L1CAM in the tumor setting rarely promotes static cell-cell adhesion that keeps tumor cells together, but induces a motile and invasive phenotype, supporting an aggressive tumor growth, metastasis and chemoresistance (reviewed in ref. 11). Mechanistically, these fundamental differences and dualistic mode of action of L1CAM are not well understood. Would it be feasible to block the pro-metastatic function of L1CAM in tumors by reverting its function to a static adhesion molecule?
Work over the last years has shown that two important factors may contribute to the switch in the functional mode of L1CAM: (1) the cleavage from the cell surface by membrane proximal proteolysis and (2) the ability to change binding partners and engage in L1CAM-integrin interactions. Recent studies have shown that cleavage of L1CAM via metalloproteinases and the binding of L1CAM to integrins via its RGD-motif in the sixth Ig-domain activate signaling pathways that are distinct from the ones triggered by homophilic binding. Herein, we highlight important features of L1CAM proteolysis and the signaling of L1CAM via integrins.
L1CAM-Proteolysis and Ectodomain Cleavage
L1CAM can be proteolytically cleaved within the third FN repeat of the ectodomain by the serine proteinases plasmin26 and trypsin27 or the pro-protein convertase PC5A.28 This particular cleavage site consists of the consensus sequence 840RKHSKR845 which resembles the recognition motif for pro-protein convertases, (R/K)X0,2,4,6(K/R).28 Similar to other pro-protein convertase substrates, proteolysis of L1CAM does not necessarily lead to the release of the N-terminal cleavage fragment. Biochemical evidence suggests that it is retained in the membrane as it can be detected by cell surface iodination.29,30
The membrane proximal cleavage of the L1CAM ectodomain is mediated by a disintegrin and metalloproteinases (ADAMs) and occurs in tumor cell lines as well as in the developing mouse brain30-32 (see Fig. 1A). This process leads to the release of the whole ectodomain (termed L1-200), which as a consequence becomes water-soluble.30 Ectodomain shedding of L1CAM occurs at the cell surface as well as in tumor cell derived vesicles called exosomes.33,34 L1CAM shedding from exosomes or the constitutive release from the cell surface is ADAM10 dependent, while the induced cleavage in response to the phorbolester PMA (a common shedding inducer) is mediated by ADAM17.32,34,35 As ADAMs do not have consensus cleavage motifs, the exact cleavage site in L1CAM is not known but is likely to be close to the plasma membrane (see Fig. 1B).
Figure 1. L1CAM structure and cleavage. (A) L1CAM is a type I transmembrane molecule of the immunoglobulin superfamily. It is composed of sixth immunoglobulin domains, five fibronectin-type III repeats and a conserved cytoplasmic domain. L1CAM can bind homophilically to other L1CAM molecules or heterophilically to various ligands. The RGD-site in the sixth Ig-domain supports binding to integrins such as α5β1 αvβ3 or αvβ5. L1CAM can be cleaved proximal to the plasma membrane by the metalloproteinases ADAM10 and ADAM17. This ectodomain shedding results in the release of the 200 kDa soluble ectodomain whereas the 32 kDa transmembrane stub is retained in the plasma membrane (see illustration). The RGD motif and the sites of proteolytic cleavage are specified. (B) The membrane proximal cleavage site. The amino acid sequence of mouse and human L1CAM is shown and the putative site of ADAM-mediated cleavage is indicated. It should be noted that the L1-32 cleavage fragment is insensitive to Endoglycosidase F treatment suggesting that it is devoid of N-linked glycans.
The process of ectodomain shedding has been demonstrated for other type I and type II trans-membrane proteins including a wide variety of molecules such as Ig-CAMs, selectins, growth factor receptor, growth factors and cadherins.36 It has emerged as a key mechanism for regulating the function of cell surface proteins.37 Cancer cells can cut homophilic adhesion molecules and enable cells to become mobile.38 The proteolytic cleavage leads to the downregulation of cell surface expression and not only reduces cell adhesion but also activates intracellular signaling processes (see below). Shedding of RPTPs in cancer cells may switch signals from stabilizing cell-cell adhesion to driving cell migration.39 There is an emerging role of RPTPs in cell-cell interaction as described in an accompanying article by Bouyain et al.40
Ectodomain Cleavage and Nuclear Signaling
Ectodomain shedding of L1CAM yields a C-terminal stub (L1-32), which is retained in the plasma membrane (see Fig. 1A). Interestingly, according to SDS-PAGE analysis this fragment has an apparent size of 32 kDa, which is more than double the calculated numerical size of approximately 13 kDa for the L1CAM intracellular domain (L1-ICD). The reasons for this discrepancy are presently unknown but could be a consequence of dimerization of the ICD or posttranslational modifications. Lutz et al. recently reported on a sumoylated transmembrane fragment, which is generated in mouse cerebellar neurons following treatment with L1CAM mAb or L1CAM-Fc and can translocate into the nucleus.41 There is further evidence that L1CAM is ubiquitinylated in its C-terminal part42 (Riedle, unpublished data). Whether these post-translational modifications play a role in L1CAM stability or affect L1CAM processing remains to be elucidated.
Numerous studies have shown that residual transmembrane remnants resulting from ectodomain shedding are substrates for RIP (regulated intramembrane proteolysis). These include APP,43 Notch,44 v-erb-a erythroblastic leukemia viral oncogene homolog (ErbB4),45,46 E-cadherin47 and CD44.48
Treatment of the ovarian carcinoma cell line OVMz with the γ-secretase inhibitor DAPT leads to an accumulation of L1-32 without the concomitant increase of the soluble ectodomain, providing evidence that L1-32 is a γ-secretase substrate. Processing of the L1-32 cleavage fragment by γ-secretase results in the release of a soluble L1CAM intracellular domain (L1-ICD) into the cytoplasm35,49 (Fig. 2A). This fragment can be detected in the nucleus49 (Fig. 2B) and subsequent studies indicate that the amount of the C-terminal fragment in the nucleus increases after treatment of cells with the shedding inducer PMA.50 L1CAM cleavage appears to be a prerequisite for nuclear translocation and L1CAM-mediated gene regulation, as both are abrogated by the inhibition of either processing step.49 Furthermore, regulation of several cancer-related genes such as the transcription factors homeobox A9 (HOX-A9), the activating enhancer-binding protein 2 α (AP2α), the tumor suppressor CRABPII, the regulator of apoptosis IER3, cathepsin and β3-integrin were identified in HEK293-L1CAM cells or OVMz ovarian carcinoma cells. The regulation of these genes was shown to depend on the L1CAM cytoplasmic part and L1CAM cleavage.49,51

Figure 2. Proteolytic cleavage and nuclear translocation of L1CAM-ICD. Following ADAM-mediated ectodomain shedding, the membraneous 32 kDa stub of L1CAM functions as a substrate for the intramembraneous cleavage by γ-secretase/presenilin. The resulting fragment represents the intracellular domain of L1CAM (L1-ICD). L1-ICD can translocate into the nucleus by an unknown mechanism and participate in the transcriptional regulation of genes including Cathepsin B, CRABPII, β3-integrin and MDK.11
L1CAM also plays an important role in the invasion, motility and survival of glioblastoma stem cells. A recent study investigated the protective role of L1CAM from radiation-induced damage by regulating DNA damage checkpoint responses through NBS1, a critical component of the MRE11-RAD50-NBS1 (MRN) complex that activates ataxia telangiectasia mutated (ATM) kinase.52 It was found that the L1CAM-mediated regulation of NBS1 depends on the translocation of the C-terminus to the nucleus.52 These findings indicate that cleavage-dependent L1CAM nuclear signaling can induce a pro-tumorigenic and an anti-apoptotic gene expression profile.
Function of the L1CAM Ectodomain
The release of the L1CAM ectodomain into the medium was observed in various cultured cell lines originating from different tumor types.28,30,31,35,53,54 Since the soluble ectodomain contains all binding elements for putative ligands it can in principle bind to cells in an autocrine or paracrine fashion. Importantly, soluble L1CAM is found in serum and ascites fluid of ovarian cancer patients,33,55,56 sera of patients with gastrointestinal stromal tumors57 and in cerebrospinal fluid of Alzheimer patients.58 These findings suggest a functional role of ADAM10-mediated cleavage of L1CAM in vivo. In many tumor tissues L1CAM is found to co-localize with ADAM10 at the invasive front53 and L1CAM/ADAM10 co-expression was found in ovarian and uterine carcinomas as well as in clear cell renal cancer.55,59
The soluble ectodomain of L1CAM is functionally active and promotes cell migration,32,60 protects cells from apoptosis,61,62 stimulates cell survival63,64 and acts as a pro-angiogenic factor.65,66 The L1CAM-RGD site in the sixth Ig-domain appears to be essential for many of the described functions.
This raises the question whether the soluble ectodomain on its own is sufficient to contribute to the bad prognosis generally seen in L1CAM positive tumors. A recent study addressed this question in a cohort of 232 serous ovarian carcinoma patients.56 L1CAM expression levels were determined in tumor lysate and malignant ascites fluid by ELISA.56 L1CAM-expressing tumors showed a highly invasive phenotype associated with restricted tumor resectability at primary debulking surgery and increased lymphogenic spread. Soluble L1CAM in ascites proved to be a marker for poor progression-free survival and chemoresistance.56
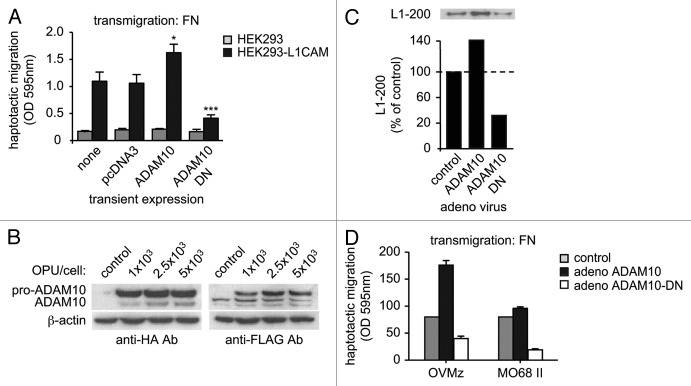
Earlier studies showed that the shed L1CAM ectodomain can be incorporated into the ECM where it augments haptotactic cell migration.31,67,68 Mechtersheimer et al. demonstrated that soluble L1CAM, as a result of ectodomain shedding, can bind to integrins in an autocrine/paracrine fashion and thereby promote cell migration.32 A similar effect is observed when HEK293 cells are treated with soluble L1CAM purified from human ascites, which acts as a potent trigger for cell migration.69 Furthermore, HEK293 or ovarian carcinoma cells stably transfected with L1CAM display integrin-dependent migration on fibronectin which can be blocked by an inactive dominant-negative form of ADAM10 (see Fig. 3) Thus, ADAM10 and L1CAM can cooperate to promote cancer cell motility.
Figure 3. L1CAM and its ADAM-mediated cleavage promote cell motility. (A) Stable overexpression of L1CAM in HEK293 cells promotes haptotactic cell migration in a Boyden chamber migration assay with fibronectin (FN) as substrate. Transient overexpression of ADAM10 augments, whereas a dominant-negative, proteolytically non-active form of ADAM10 (ADAM10-DN) blocks cell migration. (B) Adenovirus encoding ADAM10 (HA-tagged) or ADAM10-DN (FLAG tagged) were transduced in OVMz cells (L1CAM positive) and cell lysated were probed with the indicated mAbs to detect successful overexpression. (C) Detection of soluble L1CAM in the culture supernatant of transduced cells. The supernantant from equal number of cells was depleted from cellular debris by centrifugation and soluble proteins were precipitated by the addition of TCA (trichloroacetic acid). Soluble L1-200 was detected after SDS-PAGE and western blot analysis as described.31 (D) Haptotactic migration of ovarian carcinoma cell lines OVMz and MO68II (both L1CAM positive) after transduction with adenovirus.
Importantly, L1CAM-mediated enhancement of integrin-dependent migration may not depend on the presence of soluble L1CAM alone. Reports from Maness and colleagues have shown that overexpression of L1CAM (or the related molecule CL1) drives migration by augmenting L1CAM-β1 integrin interactions and β1 integrin internalization and recycling.70-72 These studies further highlight the importance of the RGD-binding site (or a related site DGEA in CHL1) but do not address the role of L1CAM shedding.
It is still unclear which biological effects of L1CAM are mediated by soluble L1CAM or by the membrane bound full-length form of L1CAM. To address this question, Kiefel et al. expressed various domains of L1CAM, i.e., the cytoplasmic tail (L1cyt), the ectodomain (L1ecto) or full-length L1CAM, in tumor cells and studied cell proliferation, motility and gene regulation.50 Full-length L1CAM augments cell motility to fibronectin and collagen type I, while cells expressing the secreted L1ecto show increased cell migration only on fibronectin (Kiefel et al., unpublished data). A different study by Shtutman et al. demonstrates that overexpression of L1CAM augments wound healing of a MCF7 breast cancer cell line monolayer.73 In contrast, a truncated version of L1CAM lacking the cytoplasmic domain, that over-produces soluble L1CAM when expressed in cells fails to enhance cell migration.73 Taken together, these data suggest that L1CAM-mediated migration involves different mechanisms depending on the substrate engaged. While soluble L1CAM might activate cell motility exclusively through the interaction with cell surface molecules, full-length L1CAM could induce additional signaling pathways either directly through its cytoplasmic domain or through the generation of signaling complexes and clusters in the plasma membrane.
A considerable amount of literature demonstrates that L1CAM expression induces an invasive and aggressive phenotype in cancer cells by augmenting cell migration, proliferation and tumor growth. A functional role of soluble L1CAM in cell motility via an autocrine/paracrine mechanism has been suggested. Other L1CAM-dependent functions require the presence of the full-length molecule, suggesting a signaling mechanism that involves both the extracellular and the cytoplasmic domain.
L1CAM Signaling via Integrins
The RGD-site in the sixth Ig-domain of L1CAM serves as a substrate for RGD-binding integrins αvβ5, α5β1, αvβ1, αvβ3 and αIIbβ3, thereby supporting cell adhesion and motility.67,74-79 But the functional significance of L1CAM-integrin binding in cell-cell communication had to be demonstrated first.
Previous work has identified PI3K, Rac-1 and ERK as downstream effectors of L1 signaling in B35 neuroblastoma cells.80 Recently, the expression of L1CAM was shown to induce constitutive NFκB activation in tumor cells.50,81 The Ben-Ze’ev lab showed that L1CAM confers metastasic potential to colorectal cancer cells (CRC) via NFκB and that overexpression of the NFκB p65 subunit is sufficient to increase cell proliferation, motility, and metastasis.81 Binding of the L1CAM cytoplasmic domain to ezrin (a cytoskeleton-crosslinking protein) was required for CRC metastasis and NFκB activation. Kiefel et al. showed that full-length L1CAM expressing cells upregulate IL-1β expression (a classical NFκB inducer) and show constitutive NFκB activity.82 Depletion of α5-integrin or expression of the L1CAM-RGE mutant, which is deficient in integrin binding, reverses NFκB activation and IL-1β production,50 suggesting that L1CAM-integrin binding is required for the induction of IL-1β and NFκB activation (for review see ref. 83). Recent data from our laboratory provide a possible link between L1CAM-integrin induced IL-1β production and ezrin dependent NFκB activation, as depletion of ezrin abrogated both IL-1β secretion and invasion in L1CAM expressing cells (Kiefel et al., unpublished data and ref. 82).
Meanwhile, the downstream signaling of L1CAM-integrin binding was investigated in more detail.82 It is quite known that one of the first kinases activated upon integrin ligation is the focal adhesion kinase (FAK), which binds to the cytoplasmic tails of β1-, β2- and β3-integrin subunits. During activation, FAK undergoes a conformational change, which enables its autophosphorylation.84 This allows binding of Src, thereby bringing Src into an active conformation, leading to its autophosphorylation and concomitant phosphorylation of FAK at tyrosine 925.85 The resulting active FAK/Src complex can then promote the activation of various downstream signaling pathways including PI3K/Akt and mitogen-activated protein kinases (MAPK).86
It was found that L1CAM expression enhances the phosphorylation of FAK at Y397 and Y925 and Src at Y416 and correlates with a change in PI3K phosphorylation and Akt activation.82 It has been shown before that PI3K and Akt induce NFκB activation via binding to IκBκ,87 activation of IκB kinase β and MAPKp38.88 In support of the hypothesis that L1CAM-dependent NFκB activation was ultimately mediated via integrin signaling, depletion of α5- and β1-integrin, ILK, FAK and p110β reversed NFκB activation and IL-1β expression in breast cancer and PDACs cell lines.48,82
These findings suggest that in tumor cells L1CAM-integrin binding initiates the classical signaling pathway leading to the expression of various NFκB target genes. Thus, in this setting L1CAM acts primary as a ligand for integrins rather than as a homophilic ligand.
Different Binding Partners—Different Signals?
A major obstacle in working with L1CAM is that the cellular models used for analysis express L1CAM, integrins and other heterophilic L1CAM binding partners at the same time. Thus, expression or depletion of L1CAM does not only alter L1CAM signaling but also interferes with the signaling network triggered by its ligands.
Previous work has focused on the role of L1CAM-dependent activation of the MAP kinase pathway in L1CAM signaling.53,61,89-92 Direct engagement of L1CAM, i.e., by clustering with antibodies or L1CAM protein induces the phosphorylation/activation of the MAPKs extracellular signal-regulated kinases 1 and 2 (Erk1/2)89,90 (see Fig. 4A). Sustained activation results in the nuclear translocation of ERK, where it regulates gene transcription.93 The recently discovered integrin-binding pathway adds a new dimension to L1CAM-signaling in general.
Figure 4. L1CAM can trigger different signaling pathways. Schematic illustration of distinct signaling pathways triggered by L1CAM due to the interacting with different binding partners. (A) L1CAM homophilic interactions promote static cell-cell binding and trigger predominantly the MAPK pathway. This signaling can be modulated by interactions with growth factor receptors (GFR). (B) The binding of L1CAM to integrins triggers NFκB activation and renders cells more motile and invasive. Cleavage of L1CAM from the membrane generates soluble L1CAM. The intracellular fragment L1-ICD can translocate into the nucleus and activate gene transcription. (C) Upregulation of L1CAM by TGF-β allows L1CAM-integrin downstream signaling via FAK-Src. This pathway induces production and release of IL-1β that in turn activates NFκB via binding to the IL-1 receptor (IL-1RI).
We suggest that L1CAM-homophilic and L1CAM-integrin interactions elicit distinct signaling pathways, although these may partially overlap. On the one hand, L1CAM homophilic binding may be confined to a more static function of L1CAM and could activate predominantly the MAPK pathway (Fig. 4A). This signaling pathway can be modulated by the coupling to various growth factor receptors that are known to interact with L1CAM or other neural cell adhesion molecules94 (see Fig. 4A). On the other hand, L1CAM-integrin binding could trigger NFκB activation that is instrumental in driving cell motility and invasiveness (see Fig. 4B). These different modes of signaling could be linked to the different functions of L1CAM either as a static adhesion or a motility-promoting molecule. The different signaling mechanisms could translate into the activation of different kinases that phosphorylate L1CAM and thereby regulate its interaction with cytoskeletal proteins such as ankyrin or ERM proteins. Previous studies have shown that the binding of L1CAM to ankyrin mediates stationary behavior by inhibiting the actin-dependent retrograde movement of L1CAM.95 The association to ERM proteins was found to be necessary for L1CAM-mediated neurite branching96 and the NFκB driven formation of tumor metastasis.81 Interestingly, Silletti et al. have proposed a model of an inside-out regulation of L1CAM. According to this, differential phosphorylation can affect L1CAM conformation, accessibility to different ligands and shedding proteinases.97 Further studies are needed to investigate whether L1CAM can indeed adopt distinct conformations and how these affect ligand binding and the activation of downstream signaling events.
L1CAM Regulation during EMT and a Possible Leukocyte Binding Partner
The epithelial to mesenchymal transition (EMT) is a key process during development. EMT is first identified by changes in cell morphology, when epithelial cells lose their baso-apical polarization and acquire a fibroblast-like shape with a front-rear polarization. Mesenchymal cells show reduced intercellular interactions mainly as a result of the destabilization of adherens junctions due to the loss of the cell adhesion molecule E-cadherin and its replacement by N-cadherin. EMT also leads to the acquisition of mesenchymal markers such as vimentin and the upregulation of integrins, thereby promoting cell-matrix interactions and an increased migratory and invasive cell phenotype (reviewed in ref. 98) (see Fig. 5).
Figure 5. L1CAM upregulation during EMT. Carcinoma cells at the primary tumor site can lose their epithelial phenotype (charcterized by expression of E-cadherin and keratins) and undergo EMT-like phenotypic changes under the influence of TGF-β produced by fibroblasts in the tumor stroma. Tumor cells possess a more mesenchymal phenotype charcterized by upregulation of vimentin, SLUG and L1CAM. L1CAM at the tumor invasive front is eventually cleaved by ADAMs producing soluble L1CAM. By interacting with integrins on neighboring tumor cells, stroma cells or invaded leukocytes, L1CAM induces NFkB activity via IL-1β or other factors. L1CAM expression thereby promotes cell motility and invasion.
In the past years the role of EMT in cancer metastasis has been widely accepted. In the metastatic process a small number of cells at the invasive front detaches from the tumor mass and invades the surrounding tissue, a process that requires attachment to the ECM, ECM remodeling and an increased cell motility. Metastatic tumor cells often show a mesenchymal phenotype and an EMT gene expression signature with loss of epithelial markers such as E-cadherin and cytokeratins and the activation of the EMT inducing transcription factors Snail, Slug and Twist99,100 (Fig. 5). As shown in the accompanying review article by LeBras et al., there are additional mechanisms influencing E-cadherin expression and EMT.101
In 2006 Shtutman et al. suggested for the first time a connection between L1CAM and EMT. Their study shows that L1CAM disrupts E-cadherin-containing adherens junctions in the breast cancer cell line MCF-7 resulting in increased cell motility.73 Notably, expression of L1CAM is often found at the invasive front of tumors and recent immunohistochemical data from EC and lung tissue sections demonstrate that L1CAM localizes in areas with absent E-cadherin and high vimentin expression. In vitro, treatment of EC or PDAC cell lines with the EMT inducer TGF-β1 leads to a Slug-dependent upregulation of L1CAM, which is accompanied by an increase in cell migration and invasion.82,102-104 These events have also been implicated in the early stages of tumor development in PDACs and CRCs.105,106 In colonic biopsies from inflammatory bowel disease (IBD) patients, L1CAM expression was found in inflamed intestinal epithelia and increased in the course of inflammation. High L1CAM expression was associated with the presence of macrophages in the tissue. Co-culture experiments of anti-inflammatory macrophages (secreting TGF-β1) with the intestinal epithelial cell line NCM460 led to an increased Slug and L1CAM expression.106 Inhibition of TGF-β1 signaling abrogated these effects and likewise inhibited TGF-β1-induced cell migration. Taken together these data suggest a relationship between immune cell activation, tumor microenvironment and the invasive and migratory phenotypes associated with L1CAM expression.106
However, L1CAM expression alone is sufficient to promote migration of CRC and breast carcinoma cell lines and does not affect E-cadherin expression in CRC cells lines.82,107 Thus, although not an EMT-mediator itself, L1CAM appears to be regulated by environmental factors, such as TGF-β1, in an EMT-like fashion. Once expressed L1CAM drives carcinogenesis, cancer cell motility, invasion and metastasis.
TGF-β1 is generally regarded as an anti-inflammatory cytokine that is involved in the control of wound healing processes. In early stages of tumor development it acts as a tumor suppressor, but promotes tumor progression at later stages by augmenting tumor cell invasion and metastasis.100
TGF-β1-mediated EMT involves a cross talk between many different pathways including Smads, β-catenin, Rho-family GTPases, integrins and NFκB.100 Interestingly, Huber et al. could show that TGF-β1-dependent EMT induction in breast cancer cell lines required NFκB activity.108 In MDA-MB231 breast cancer cells TGF-β1 treatment does not only result in the upregulation of L1CAM but it also induces IL-1β expression and concomitant NFκB activation (Fig. 4C). Both NFκB activation and L1CAM expression were necessary for TGF-β1-induced cell invasion. Interestingly, L1CAM-mediated NFκB activity requires the activation of the integrin-FAK-Src-Akt signaling pathway, which in turn depends on an intact L1CAM-integrin binding.82 An extensive cross talk has been also described for the TGF-β1 and integrin signaling. Integrin-binding can cooperate with TGF-β1-induced signal transduction leading to the activation of downstream signaling molecules such as Erk1/2 or p38 MAPKs (reviewed in ref. 109). In mammary epithelial cells TGF-β1 induces clustering of integrins with the growth factor receptor HER2, which mediates PI3K signaling through the activation of the c-Src-FAK complex.110
Summarizing this variety of observations, we hypothesize that TGF-β1-mediated upregulation of L1CAM triggers binding of integrins, resulting in an amplification of the overlapping TGF-β1-integrin signaling which finally leads to NFκB activation. As many immune cells express integrins and L1CAM-integrin interactions can occur in trans (between different cells), it is feasible that L1CAM expression can induce IL-1β secretion and NFκB activation not only in tumor cells but also in immune cells. This could promote a pro-tumorigenic environment and EMT induction, leading to aggressive and invasive tumor growth (Fig. 5). Further studies are needed to investigate the relationship between L1CAM signaling and the immune microenvironment of cancers.
Thus, clarification of the detailed signaling sequences in the L1CAM-TGF-β1 integrin crosstalk would not only increase the understanding of L1CAM signaling in cancer but also provide important insights for new treatment strategies including a L1CAM mAb therapy.
Concluding Remarks
Here we have addressed the different types of function of L1CAM as a static adhesion molecule or motility-promoting molecule from a signaling point of view. The new data on L1CAM-integrin signaling suggest that different simultaneously occurring events including L1CAM proteolytic processing, autocrine stimulation by soluble L1CAM, nuclear signaling and homo- and hetero-philic interactions by full-length L1CAM drive various functions. These diverse signaling events built the basis for the dualistic roles of L1CAM in both health and disease. We have learned a lot in recent years and there is great hope that this knowledge can be translated into novel therapeutic measures to fight human cancer.
Acknowledgments
This work was supported by grants to P.A. from Deutsche Krebshilfe (108739) and Wilhelm-Sander Stiftung, Munich (2010.094.1).
Glossary
Abbreviations:
- ADAM
a disintegrin and metalloproteinase
- CAM
cell adhesion molecule
- CRABPII
cellular retinoic acid binding protein
- CRC
colorectal cancer
- EC
endometrial carcinoma
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- ERM
ezrin-radixin-moesin
- FAK
focal adhesion kinase
- ICD
intracellular domain
- IL
interleukin
- ILK
integrin-linked kinase
- L1CAM
L1 cell adhesion molecule
- MAPK
mitogen-activated protein kinase
- NBS1
Nijmegen breakage syndrome 1
- NFκB
nuclear factor κB
- PDAC
pancreatic ductal adenocarcinoma
- PI3-K
phosphoinositide 3-kinase
- PMA
phorbol myristate acetate
- RIP
regulated intramembrane proteolysis
- RGD
arginine-glycine-aspartic acid
- RPTP
receptor protein tyrosine phosphatase
- Src
Rous sarcoma oncogne cellular homolog
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20832
References
- 1.Arlt A, Vorndamm J, Müerköster S, Yu H, Schmidt WE, Fölsch UR, et al. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res. 2002;62:910–6. [PubMed] [Google Scholar]
- 2.Moos M, Tacke R, Scherer H, Teplow D, Früh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–3. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- 3.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 4.Rathjen FG, Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984;3:1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff JM, Frank R, Mujoo K, Spiro RC, Reisfeld RA, Rathjen FG. A human brain glycoprotein related to the mouse cell adhesion molecule L1. J Biol Chem. 1988;263:11943–7. [PubMed] [Google Scholar]
- 6.Grumet M, Edelman GM. Heterotypic binding between neuronal membrane vesicles and glial cells is mediated by a specific cell adhesion molecule. J Cell Biol. 1984;98:1746–56. doi: 10.1083/jcb.98.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee VM, Greene L, Shelanski ML. Identification of neural and adrenal medullary surface membrane glycoproteins recognized by antisera to cultured rat sympathetic neurons and PC12 pheochromocytoma cells. Neuroscience. 1981;6:2773–86. doi: 10.1016/0306-4522(81)90119-6. [DOI] [PubMed] [Google Scholar]
- 8.Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, et al. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–60. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 9.Schachner M. Neural recognition molecules and synaptic plasticity. Curr Opin Cell Biol. 1997;9:627–34. doi: 10.1016/S0955-0674(97)80115-9. [DOI] [PubMed] [Google Scholar]
- 10.Brümmendorf T, Kenwrick S, Rathjen FG. Neural cell recognition molecule L1: from cell biology to human hereditary brain malformations. Curr Opin Neurobiol. 1998;8:87–97. doi: 10.1016/S0959-4388(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 11.Schäfer MKE, Altevogt P. L1CAM malfunction in the nervous system and human carcinomas. Cell Mol Life Sci. 2010;67:2425–37. doi: 10.1007/s00018-010-0339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loers G, Schachner M. Recognition molecules and neural repair. J Neurochem. 2007;101:865–82. doi: 10.1111/j.1471-4159.2006.04409.x. [DOI] [PubMed] [Google Scholar]
- 13.Herron LR, Hill M, Davey F, Gunn-Moore FJ. The intracellular interactions of the L1 family of cell adhesion molecules. Biochem J. 2009;419:519–31. doi: 10.1042/BJ20082284. [DOI] [PubMed] [Google Scholar]
- 14.Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell surface glycoproteins. J Cell Biol. 1987;104:355–62. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer G, Künemund V, Schachner M. Neurite outgrowth patterns in cerebellar microexplant cultures are affected by antibodies to the cell surface glycoprotein L1. J Neurosci. 1986;6:605–12. doi: 10.1523/JNEUROSCI.06-02-00605.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keilhauer G, Faissner A, Schachner M. Differential inhibition of neurone-neurone, neurone-astrocyte and astrocyte-astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature. 1985;316:728–30. doi: 10.1038/316728a0. [DOI] [PubMed] [Google Scholar]
- 17.Lindner J, Rathjen FG, Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature. 1983;305:427–30. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- 18.Wood PM, Schachner M, Bunge RP. Inhibition of Schwann cell myelination in vitro by antibody to the L1 adhesion molecule. J Neurosci. 1990;10:3635–45. doi: 10.1523/JNEUROSCI.10-11-03635.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conacci-Sorrell M, Kaplan A, Raveh S, Gavert N, Sakurai T, Ben-Ze’ev A. The shed ectodomain of Nr-CAM stimulates cell proliferation and motility, and confers cell transformation. Cancer Res. 2005;65:11605–12. doi: 10.1158/0008-5472.CAN-05-2647. [DOI] [PubMed] [Google Scholar]
- 20.Djabali M, Mattei MG, Nguyen C, Roux D, Demengeot J, Denizot F, et al. The gene encoding L1, a neural adhesion molecule of the immunoglobulin family, is located on the X chromosome in mouse and man. Genomics. 1990;7:587–93. doi: 10.1016/0888-7543(90)90203-7. [DOI] [PubMed] [Google Scholar]
- 21.Jouet M, Strain L, Bonthron D, Kenwrick S. Discordant segregation of Xq28 markers and a mutation in the L1 gene in a family with X linked hydrocephalus. J Med Genet. 1996;33:248–50. doi: 10.1136/jmg.33.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Angelis E, Watkins A, Schäfer M, Brümmendorf T, Kenwrick S. Disease-associated mutations in L1 CAM interfere with ligand interactions and cell-surface expression. Hum Mol Genet. 2002;11:1–12. doi: 10.1093/hmg/11.1.1. [DOI] [PubMed] [Google Scholar]
- 23.He Y, Jensen GJ, Bjorkman PJ. Cryo-electron tomography of homophilic adhesion mediated by the neural cell adhesion molecule L1. Structure. 2009;17:460–71. doi: 10.1016/j.str.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouveia RM, Gomes CM, Sousa M, Alves PM, Costa J. Kinetic analysis of L1 homophilic interaction: role of the first four immunoglobulin domains and implications on binding mechanism. J Biol Chem. 2008;283:28038–47. doi: 10.1074/jbc.M804991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavert N, Ben-Shmuel A, Raveh S, Ben-Ze’ev A. L1-CAM in cancerous tissues. Expert Opin Biol Ther. 2008;8:1749–57. doi: 10.1517/14712598.8.11.1749. [DOI] [PubMed] [Google Scholar]
- 26.Nayeem N, Silletti S, Yang X, Lemmon VP, Reisfeld RA, Stallcup WB, et al. A potential role for the plasmin(ogen) system in the posttranslational cleavage of the neural cell adhesion molecule L1. J Cell Sci. 1999;112:4739–49. doi: 10.1242/jcs.112.24.4739. [DOI] [PubMed] [Google Scholar]
- 27.Sadoul K, Sadoul R, Faissner A, Schachner M. Biochemical characterization of different molecular forms of the neural cell adhesion molecule L1. J Neurochem. 1988;50:510–21. doi: 10.1111/j.1471-4159.1988.tb02941.x. [DOI] [PubMed] [Google Scholar]
- 28.Kalus I, Schnegelsberg B, Seidah NG, Kleene R, Schachner M. The proprotein convertase PC5A and a metalloprotease are involved in the proteolytic processing of the neural adhesion molecule L1. J Biol Chem. 2003;278:10381–8. doi: 10.1074/jbc.M208351200. [DOI] [PubMed] [Google Scholar]
- 29.Hubbe M, Kowitz A, Schirrmacher V, Schachner M, Altevogt P. L1 adhesion molecule on mouse leukocytes: regulation and involvement in endothelial cell binding. Eur J Immunol. 1993;23:2927–31. doi: 10.1002/eji.1830231130. [DOI] [PubMed] [Google Scholar]
- 30.Beer S, Oleszewski M, Gutwein P, Geiger C, Altevogt P. Metalloproteinase-mediated release of the ectodomain of L1 adhesion molecule. J Cell Sci. 1999;112:2667–75. doi: 10.1242/jcs.112.16.2667. [DOI] [PubMed] [Google Scholar]
- 31.Gutwein P, Oleszewski M, Mechtersheimer S, Agmon-Levin N, Krauss K, Altevogt P. Role of Src kinases in the ADAM-mediated release of L1 adhesion molecule from human tumor cells. J Biol Chem. 2000;275:15490–7. doi: 10.1074/jbc.275.20.15490. [DOI] [PubMed] [Google Scholar]
- 32.Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, et al. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol. 2001;155:661–73. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Gast D, Joumaa S, et al. ADAM10-mediated cleavage of L1 adhesion molecule at the cell surface and in released membrane vesicles. FASEB J. 2003;17:292–4. doi: 10.1096/fj.02-0430fje. [DOI] [PubMed] [Google Scholar]
- 34.Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F, et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J. 2006;393:609–18. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maretzky T, Schulte M, Ludwig A, Rose-John S, Blobel C, Hartmann D, et al. L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol Cell Biol. 2005;25:9040–53. doi: 10.1128/MCB.25.20.9040-9053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiss K, Ludwig A, Saftig P. Breaking up the tie: disintegrin-like metalloproteinases as regulators of cell migration in inflammation and invasion. Pharmacol Ther. 2006;111:985–1006. doi: 10.1016/j.pharmthera.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem J. 1997;321:265–79. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craig SE, Brady-Kalnay SM. Cancer cells cut homophilic cell adhesion molecules and run. Cancer Res. 2011;71:303–9. doi: 10.1158/0008-5472.CAN-10-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips-Mason PJ, Craig SE, Brady-Kalnay SM. Should I stay or should I go? Shedding of RPTPs in cancer cells switches signals from stabilizing cell-cell adhesion to driving cell migration. Cell Adh Migr. 2011;5:298–305. doi: 10.4161/cam.5.4.16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikolaienko RM, Agyekum B, Bouyain S. Receptor protein tyrosine phosphatases and cancer: New insights from structural biology. Cell Adhes Migr. 2012 doi: 10.4161/cam.21242. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutz D, Wolters-Eisfeld G, Joshi G, Djogo N, Jakovcevski I, Schachner M, et al. Generation and nuclear translocation of sumoylated transmembrane fragment of cell adhesion molecule l1. J Biol Chem. 2012;287:17161–75. doi: 10.1074/jbc.M112.346759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schäfer MK, Schmitz B, Diestel S. L1CAM ubiquitination facilitates its lysosomal degradation. FEBS Lett. 2010;584:4475–80. doi: 10.1016/j.febslet.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y, Pimplikar SW. The gamma -secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc Natl Acad Sci U S A. 2001;98:14979–84. doi: 10.1073/pnas.261463298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 45.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 46.Lee HJ, Jung KM, Huang YZ, Bennett LB, Lee JS, Mei L, et al. Presenilin-dependent gamma-secretase-like intramembrane cleavage of ErbB4. J Biol Chem. 2002;277:6318–23. doi: 10.1074/jbc.M110371200. [DOI] [PubMed] [Google Scholar]
- 47.Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, et al. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–45. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol. 2001;155:755–62. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riedle S, Kiefel H, Gast D, Bondong S, Wolterink S, Gutwein P, et al. Nuclear translocation and signalling of L1-CAM in human carcinoma cells requires ADAM10 and presenilin/gamma-secretase activity. Biochem J. 2009;420:391–402. doi: 10.1042/BJ20081625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiefel H, Bondong S, Erbe-Hoffmann N, Hazin J, Riedle S, Wolf J, et al. L1CAM-integrin interaction induces constitutive NF-kappaB activation in pancreatic adenocarcinoma cells by enhancing IL-1beta expression. Oncogene. 2010;29:4766–78. doi: 10.1038/onc.2010.230. [DOI] [PubMed] [Google Scholar]
- 51.Gast D, Riedle S, Issa Y, Pfeifer M, Beckhove P, Sanderson MP, et al. The cytoplasmic part of L1-CAM controls growth and gene expression in human tumors that is reversed by therapeutic antibodies. Oncogene. 2008;27:1281–9. doi: 10.1038/sj.onc.1210747. [DOI] [PubMed] [Google Scholar]
- 52.Cheng L, Wu Q, Huang Z, Guryanova OA, Huang Q, Shou W, et al. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J. 2011;30:800–13. doi: 10.1038/emboj.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168:633–42. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Galileo DS. Soluble L1CAM promotes breast cancer cell adhesion and migration in vitro, but not invasion. Cancer Cell Int. 2010;10:34. doi: 10.1186/1475-2867-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fogel M, Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Smirnov A, et al. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet. 2003;362:869–75. doi: 10.1016/S0140-6736(03)14342-5. [DOI] [PubMed] [Google Scholar]
- 56.Bondong S, Kiefel H, Hielscher T, Zeimet AG, Zeillinger R, Pils D, et al. Prognostic significance of L1CAM in ovarian cancer and its role in constitutive NF-κB activation. Ann Oncol. 2012;23:1795–802. doi: 10.1093/annonc/mdr568. [DOI] [PubMed] [Google Scholar]
- 57.Zander H, Rawnaq T, von Wedemeyer M, Tachezy M, Kunkel M, Wolters G, et al. Circulating levels of cell adhesion molecule L1 as a prognostic marker in gastrointestinal stromal tumor patients. BMC Cancer. 2011;11:189–, 1-7. doi: 10.1186/1471-2407-11-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strekalova H, Buhmann C, Kleene R, Eggers C, Saffell J, Hemperly J, et al. Elevated levels of neural recognition molecule L1 in the cerebrospinal fluid of patients with Alzheimer disease and other dementia syndromes. Neurobiol Aging. 2006;27:1–9. doi: 10.1016/j.neurobiolaging.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Doberstein K, Pfeilschifter J, Gutwein P. The transcription factor PAX2 regulates ADAM10 expression in renal cell carcinoma. Carcinogenesis. 2011;32:1713–23. doi: 10.1093/carcin/bgr195. [DOI] [PubMed] [Google Scholar]
- 60.Yang M, Adla S, Temburni MK, Patel VP, Lagow EL, Brady OA, et al. Stimulation of glioma cell motility by expression, proteolysis, and release of the L1 neural cell recognition molecule. Cancer Cell Int. 2009;9:27. doi: 10.1186/1475-2867-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoeck A, Gast D, Sanderson MP, Issa Y, Gutwein P, Altevogt P. L1-CAM in a membrane-bound or soluble form augments protection from apoptosis in ovarian carcinoma cells. Gynecol Oncol. 2007;104:461–9. doi: 10.1016/j.ygyno.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 62.Sebens Müerköster S, Werbing V, Sipos B, Debus MA, Witt M, Grossmann M, et al. Drug-induced expression of the cellular adhesion molecule L1CAM confers anti-apoptotic protection and chemoresistance in pancreatic ductal adenocarcinoma cells. Oncogene. 2007;26:2759–68. doi: 10.1038/sj.onc.1210076. [DOI] [PubMed] [Google Scholar]
- 63.Voura EB, Ramjeesingh RA, Montgomery AM, Siu CH. Involvement of integrin alpha(v)beta(3) and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol Biol Cell. 2001;12:2699–710. doi: 10.1091/mbc.12.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishimune H, Bernreuther C, Carroll P, Chen S, Schachner M, Henderson CE. Neural adhesion molecules L1 and CHL1 are survival factors for motoneurons. J Neurosci Res. 2005;80:593–9. doi: 10.1002/jnr.20517. [DOI] [PubMed] [Google Scholar]
- 65.Hall H, Hubbell JA. Matrix-bound sixth Ig-like domain of cell adhesion molecule L1 acts as an angiogenic factor by ligating alphavbeta3-integrin and activating VEGF-R2. Microvasc Res. 2004;68:169–78. doi: 10.1016/j.mvr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Friedli A, Fischer E, Novak-Hofer I, Cohrs S, Ballmer-Hofer K, Schubiger PA, et al. The soluble form of the cancer-associated L1 cell adhesion molecule is a pro-angiogenic factor. Int J Biochem Cell Biol. 2009;41:1572–80. doi: 10.1016/j.biocel.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Montgomery AM, Becker JC, Siu CH, Lemmon VP, Cheresh DA, Pancook JD, et al. Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin alpha v beta 3. J Cell Biol. 1996;132:475–85. doi: 10.1083/jcb.132.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oleszewski M, Gutwein P, von der Lieth W, Rauch U, Altevogt P. Characterization of the L1-neurocan-binding site. Implications for L1-L1 homophilic binding. J Biol Chem. 2000;275:34478–85. doi: 10.1074/jbc.M004147200. [DOI] [PubMed] [Google Scholar]
- 69.Gutwein P, Stoeck A, Riedle S, Gast D, Runz S, Condon TP, et al. Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin Cancer Res. 2005;11:2492–501. doi: 10.1158/1078-0432.CCR-04-1688. [DOI] [PubMed] [Google Scholar]
- 70.Thelen K, Kedar V, Panicker AK, Schmid RS, Midkiff BR, Maness PF. The neural cell adhesion molecule L1 potentiates integrin-dependent cell migration to extracellular matrix proteins. J Neurosci. 2002;22:4918–31. doi: 10.1523/JNEUROSCI.22-12-04918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buhusi M, Midkiff BR, Gates AM, Richter M, Schachner M, Maness PF. Close homolog of L1 is an enhancer of integrin-mediated cell migration. J Biol Chem. 2003;278:25024–31. doi: 10.1074/jbc.M303084200. [DOI] [PubMed] [Google Scholar]
- 72.Panicker AK, Buhusi M, Erickson A, Maness PF. Endocytosis of beta1 integrins is an early event in migration promoted by the cell adhesion molecule L1. Exp Cell Res. 2006;312:299–307. doi: 10.1016/j.yexcr.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 73.Shtutman M, Levina E, Ohouo P, Baig M, Roninson IB. Cell adhesion molecule L1 disrupts E-cadherin-containing adherens junctions and increases scattering and motility of MCF7 breast carcinoma cells. Cancer Res. 2006;66:11370–80. doi: 10.1158/0008-5472.CAN-06-2106. [DOI] [PubMed] [Google Scholar]
- 74.Ruppert M, Aigner S, Hubbe M, Yagita H, Altevogt P. The L1 adhesion molecule is a cellular ligand for VLA-5. J Cell Biol. 1995;131:1881–91. doi: 10.1083/jcb.131.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebeling O, Duczmal A, Aigner S, Geiger C, Schöllhammer S, Kemshead JT, et al. L1 adhesion molecule on human lymphocytes and monocytes: expression and involvement in binding to alpha v beta 3 integrin. Eur J Immunol. 1996;26:2508–16. doi: 10.1002/eji.1830261035. [DOI] [PubMed] [Google Scholar]
- 76.Felding-Habermann B, Silletti S, Mei F, Siu CH, Yip PM, Brooks PC, et al. A single immunoglobulin-like domain of the human neural cell adhesion molecule L1 supports adhesion by multiple vascular and platelet integrins. J Cell Biol. 1997;139:1567–81. doi: 10.1083/jcb.139.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blaess S, Kammerer RA, Hall H. Structural analysis of the sixth immunoglobulin-like domain of mouse neural cell adhesion molecule L1 and its interactions with alpha(v)beta3, alpha(IIb)beta3, and alpha5beta1 integrins. J Neurochem. 1998;71:2615–25. doi: 10.1046/j.1471-4159.1998.71062615.x. [DOI] [PubMed] [Google Scholar]
- 78.Oleszewski M, Beer S, Katich S, Geiger C, Zeller Y, Rauch U, et al. Integrin and neurocan binding to L1 involves distinct Ig domains. J Biol Chem. 1999;274:24602–10. doi: 10.1074/jbc.274.35.24602. [DOI] [PubMed] [Google Scholar]
- 79.Duczmal A, Schöllhammer S, Katich S, Ebeling O, Schwartz-Albiez R, Altevogt P. The L1 adhesion molecule supports alpha v beta 3-mediated migration of human tumor cells and activated T lymphocytes. Biochem Biophys Res Commun. 1997;232:236–9. doi: 10.1006/bbrc.1997.6265. [DOI] [PubMed] [Google Scholar]
- 80.Schmid RS, Pruitt WM, Maness PF. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J Neurosci. 2000;20:4177–88. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gavert N, Ben-Shmuel A, Lemmon V, Brabletz T, Ben-Ze’ev A. Nuclear factor-kappaB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. J Cell Sci. 2010;123:2135–43. doi: 10.1242/jcs.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiefel H, Bondong S, Pfeifer M, Schirmer U, Erbe-Hoffmann N, Schäfer H, et al. EMT-associated up-regulation of L1CAM provides insights into L1CAM-mediated integrin signalling and NF-κB activation. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs220. In press. [DOI] [PubMed] [Google Scholar]
- 83.Kiefel H, Pfeifer M, Bondong S, Hazin J, Altevogt P. Linking L1CAM-mediated signaling to NF-κB activation. Trends Mol Med. 2011;17:178–87. doi: 10.1016/j.molmed.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113:3563–71. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 85.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 86.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 87.Béraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc Natl Acad Sci U S A. 1999;96:429–34. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–40. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 89.Schaefer AW, Kamiguchi H, Wong EV, Beach CM, Landreth G, Lemmon V. Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J Biol Chem. 1999;274:37965–73. doi: 10.1074/jbc.274.53.37965. [DOI] [PubMed] [Google Scholar]
- 90.Schmid RS, Pruitt WM, Maness PF. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J Neurosci. 2000;20:4177–88. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thelen K, Kedar V, Panicker AK, Schmid R-S, Midkiff BR, Maness PF. The neural cell adhesion molecule L1 potentiates integrin-dependent cell migration to extracellular matrix proteins. J Neurosci. 2002;22:4918–31. doi: 10.1523/JNEUROSCI.22-12-04918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silletti S, Yebra M, Perez B, Cirulli V, McMahon M, Montgomery AM. Extracellular signal-regulated kinase (ERK)-dependent gene expression contributes to L1 cell adhesion molecule-dependent motility and invasion. J Biol Chem. 2004;279:28880–8. doi: 10.1074/jbc.M404075200. [DOI] [PubMed] [Google Scholar]
- 93.Colucci-D’Amato L, Perrone-Capano C, di Porzio U. Chronic activation of ERK and neurodegenerative diseases. Bioessays. 2003;25:1085–95. doi: 10.1002/bies.10355. [DOI] [PubMed] [Google Scholar]
- 94.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 95.Gil OD, Sakurai T, Bradley AE, Fink MY, Cassella MR, Kuo JA, et al. Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface. J Cell Biol. 2003;162:719–30. doi: 10.1083/jcb.200211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng L, Itoh K, Lemmon V. L1-mediated branching is regulated by two ezrin-radixin-moesin (ERM)-binding sites, the RSLE region and a novel juxtamembrane ERM-binding region. J Neurosci. 2005;25:395–403. doi: 10.1523/JNEUROSCI.4097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen MM, Lee CY, Leland HA, Lin GY, Montgomery AM, Silletti S. Inside-out regulation of L1 conformation, integrin binding, proteolysis, and concomitant cell migration. Mol Biol Cell. 2010;21:1671–85. doi: 10.1091/mbc.E09-10-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 99.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 100.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 101.Le Bras GF, Taubenslag KJ, Andl CD. The regulation of cell-cell adhesion during epithelial-mesenchymal-transition motility and tumor progression. Cell Adhes Migr. 2012 doi: 10.4161/cam.21326. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huszar M, Pfeifer M, Schirmer U, Kiefel H, Konecny GE, Ben-Arie A, et al. Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J Pathol. 2010;220:551–61. doi: 10.1002/path.2673. [DOI] [PubMed] [Google Scholar]
- 103.Tischler V, Pfeifer M, Hausladen S, Schirmer U, Bonde AK, Kristiansen G, et al. L1CAM protein expression is associated with poor prognosis in non-small cell lung cancer. Mol Cancer. 2011;10:127. doi: 10.1186/1476-4598-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Geismann C, Arlt A, Bauer I, Pfeifer M, Schirmer U, Altevogt P, et al. Binding of the transcription factor Slug to the L1CAM promoter is essential for transforming growth factor-β1 (TGF-β)-induced L1CAM expression in human pancreatic ductal adenocarcinoma cells. Int J Oncol. 2011;38:257–66. [PubMed] [Google Scholar]
- 105.Geismann C, Morscheck M, Koch D, Bergmann F, Ungefroren H, Arlt A, et al. Up-regulation of L1CAM in pancreatic duct cells is transforming growth factor beta1- and slug-dependent: role in malignant transformation of pancreatic cancer. Cancer Res. 2009;69:4517–26. doi: 10.1158/0008-5472.CAN-08-3493. [DOI] [PubMed] [Google Scholar]
- 106.Schäfer H, Struck B, Feldmann EM, Bergmann F, Grage-Griebenow E, Geismann C, et al. TGF-β1-dependent L1CAM expression has an essential role in macrophage-induced apoptosis resistance and cell migration of human intestinal epithelial cells. Oncogene. 2012 doi: 10.1038/onc.2012.44. In press. [DOI] [PubMed] [Google Scholar]
- 107.Gavert N, Vivanti A, Hazin J, Brabletz T, Ben-Ze’ev A. L1-mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol Cancer Res. 2011;9:14–24. doi: 10.1158/1541-7786.MCR-10-0406. [DOI] [PubMed] [Google Scholar]
- 108.Huber MA, Azoitei N, Baumann B, Grünert S, Sommer A, Pehamberger H, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang SE, Xiang B, Zent R, Quaranta V, Pozzi A, Arteaga CL. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009;69:475–82. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]