Abstract
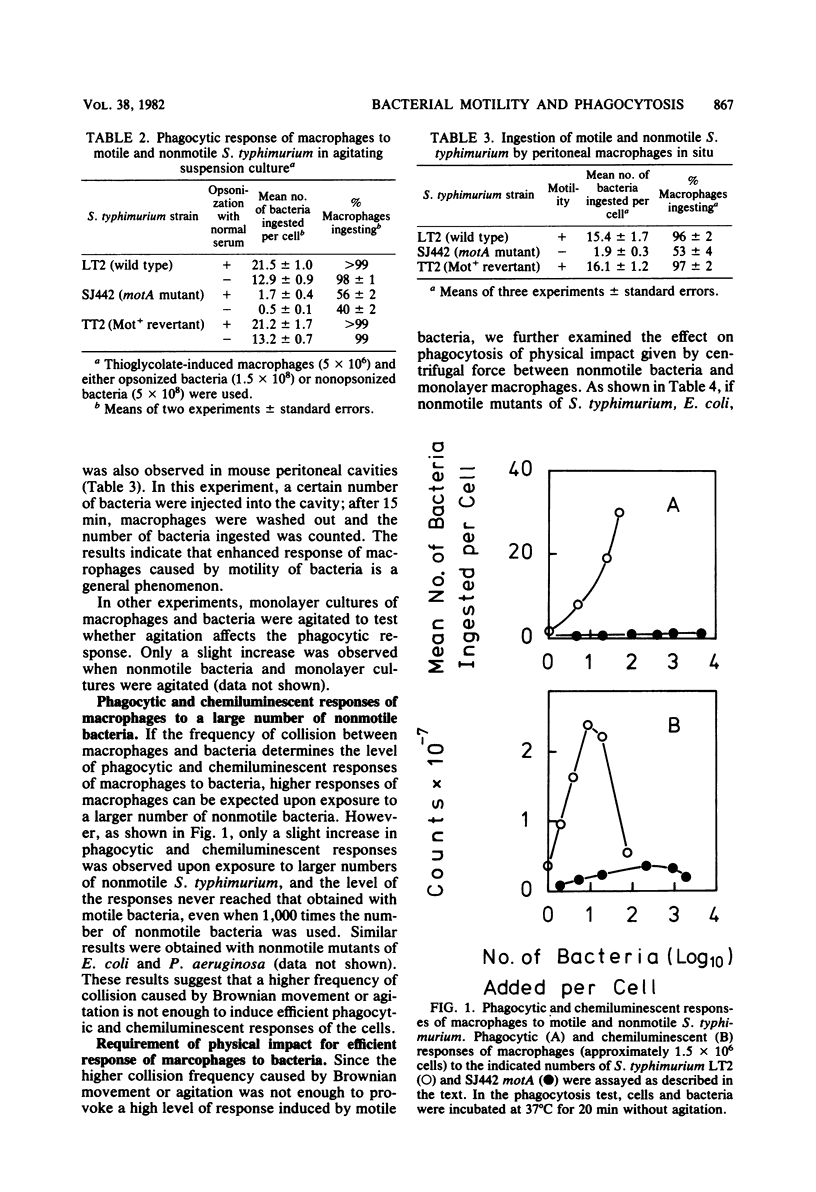
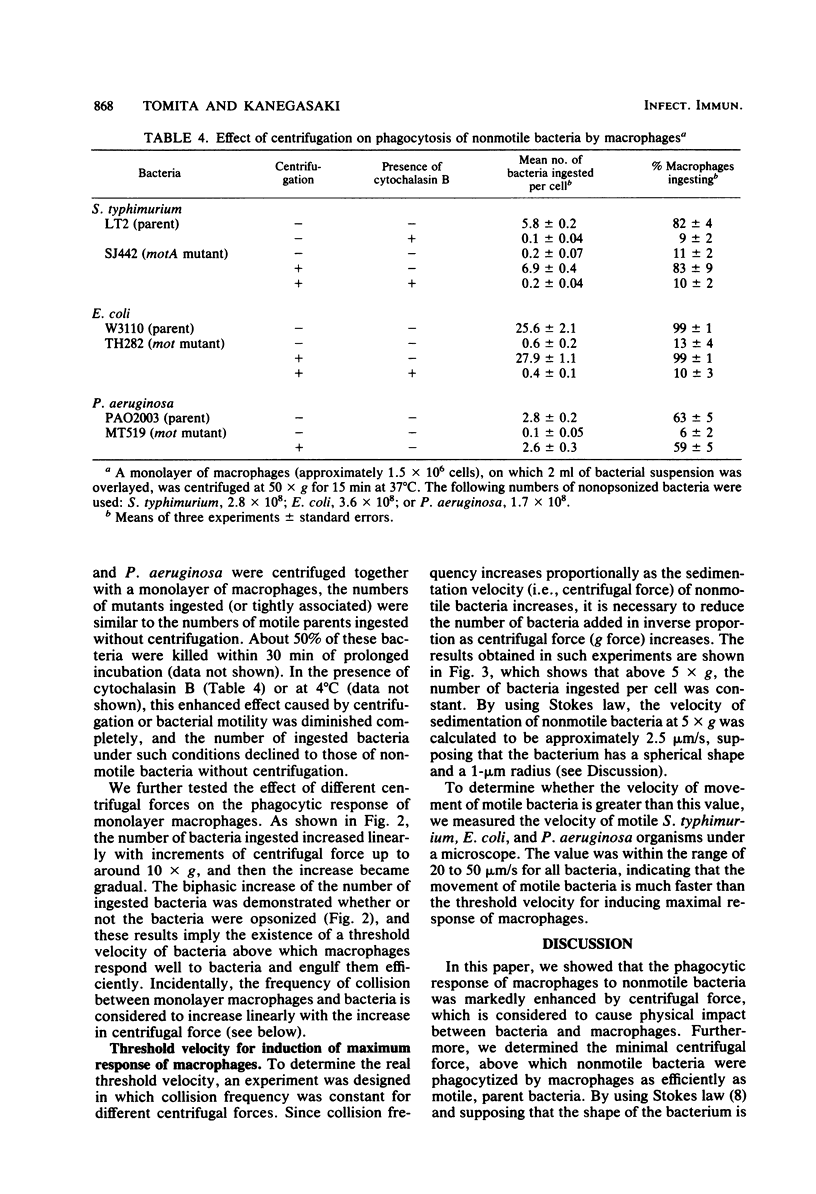
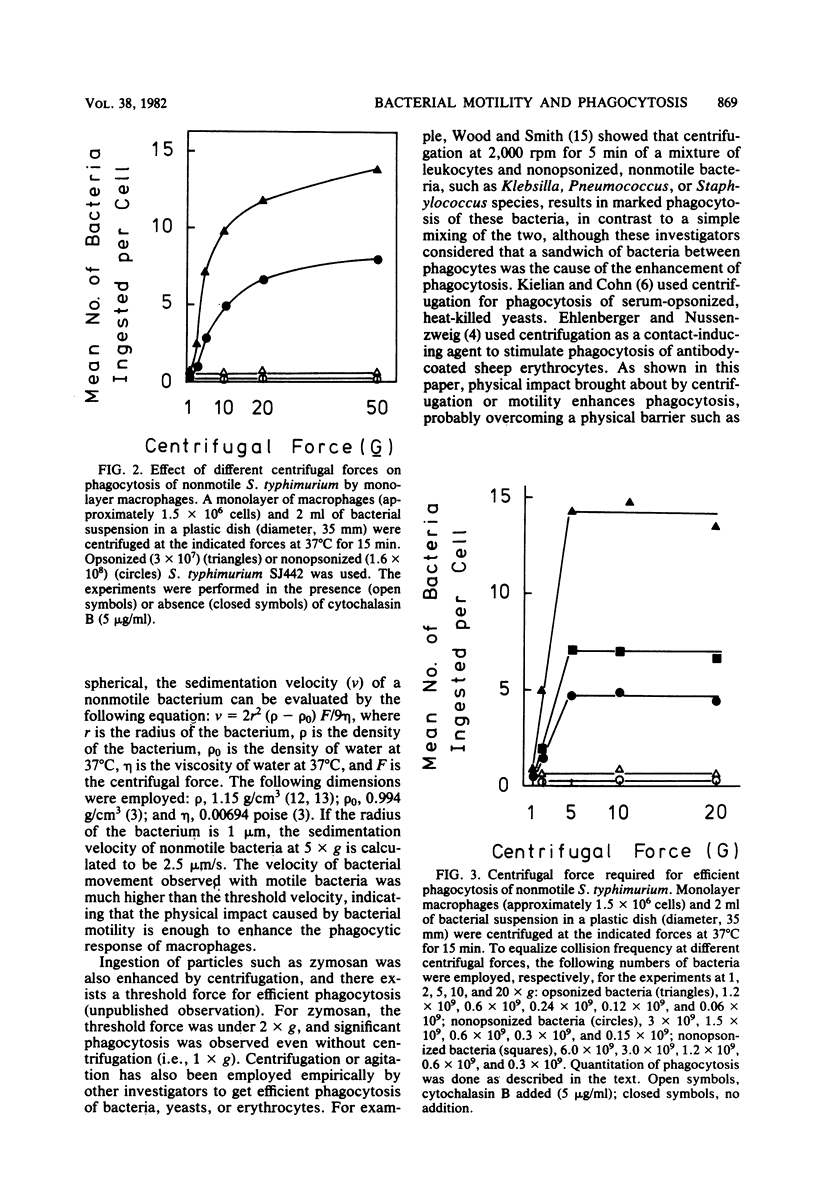
The mechanism of enhanced phagocytic and chemiluminescent responses of macrophages caused by bacterial motility (T. Tomita, E. Blumenstock, and S. Kanegasaki, Infect. Immun. 32:1242, 1981) was studied. Both responses increased up to a certain level with an increased number of motile bacteria, such as Salmonella typhimurium, Escherichia coli, or Pseudomonas aeruginosa, added. In contrast, only a slight increase was observed with the motility (mot) mutants of these bacteria, even when 4,000 bacteria per single macrophage were added. If nonmotile bacteria were centrifuged together with a monolayer culture of macrophages, the number of bacteria ingested per macrophage increased dramatically. This phenomenon was not observed in the presence of cytochalasin B or at a low temperature, and about half of the associated bacteria were killed within 30 min of prolonged incubation, indicating that the bacteria were not simply embedded on the macrophage surface. An observed biphasic increase of ingestion with an increase in centrifugal force suggested the existence of a threshold velocity for efficient phagocytosis. The minimum centrifugal force required for maximal response was determined under the conditions in which equalized collision frequency between bacteria and macrophages was maintained when different centrifugal forces were employed. From the value obtained (5 x g), the required rate of movement was calculated as approximately 2.5 microns/s, supposing that the bacterium is spherical and has a 1-micron radius. This value is much lower than the velocity of movement of motile bacteria (20 to 50 microns/s). The results indicate that physical impact caused by bacterial motility is enough to induce a high response of macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenstock E., Jann K. Natural resistance of mice to Salmonella typhimurium: bactericidal activity and chemiluminescence response of murine peritoneal macrophages. J Gen Microbiol. 1981 Jul;125(1):173–183. doi: 10.1099/00221287-125-1-173. [DOI] [PubMed] [Google Scholar]
- Craven R. C., Montie T. C. Motility and chemotaxis of three strains of Pseudomonas aeruginosa used for virulence studies. Can J Microbiol. 1981 Apr;27(4):458–460. doi: 10.1139/m81-070. [DOI] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Phagosome-lysosome fusion. Characterization of intracellular membrane fusion in mouse macrophages. J Cell Biol. 1980 Jun;85(3):754–765. doi: 10.1083/jcb.85.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E., Edebo L. Association of viable and inactivated Salmonella typhimurium 395 MS and MR 10 with HeLa cells. Infect Immun. 1976 Oct;14(4):851–857. doi: 10.1128/iai.14.4.851-857.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- McGee M. P., Myrvik Q. N. Phagocytosis-induced injury of normal and activated alveolar macrophages. Infect Immun. 1979 Dec;26(3):910–915. doi: 10.1128/iai.26.3.910-915.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Blumenstock E., Kanegasaki S. Phagocytic and chemiluminescent responses of mouse peritoneal macrophages to living and killed Salmonella typhimurium and other bacteria. Infect Immun. 1981 Jun;32(3):1242–1248. doi: 10.1128/iai.32.3.1242-1248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Jr, Smith M. R. Intercellular Surface Phagocytosis. Science. 1947 Jul 25;106(2743):86–87. doi: 10.1126/science.106.2743.86. [DOI] [PubMed] [Google Scholar]



