Stem cells, which can divide and renew themselves throughout life, differentiate into the specialized tissues that are needed during development and into the cell types that are necessary to repair adult tissues. Although stem cells can be considered bona fide immortal cells in that they can renew themselves and regenerate tissues throughout a person’s lifetime, we should acknowledge that these cells gradually lose their unique ability to effectively maintain tissues and organs. From a simplistic perspective, it therefore seems reasonable to argue that “we are as old as our adult stem cells are” because endogenous stem cells themselves become damaged as we age. A number of possible mechanisms for this damage are being investigated. For example, studies are being conducted to determine whether losses of tissue function are due to a decrease in the number of stem cells, to the inability of stem cells to respond adequately to signals from their surroundings (the “niche”), or to reduced signaling from the niche. Through these investigations, we hope to gain a greater understanding of the pivotal molecules and processes that allow human adult stem cells to regenerate tissues by dividing, proliferating and eventually differentiating to replace a wide range of cell types.1 We should acknowledge that allocating new or young stem cells into an old environment, e.g., the body of an aged patient, for tissue regeneration purposes is not likely to lead to the expected outcome if we cannot switch on the necessary supportive functions in aged niches. Thus, options to overcome the consequences of aging may involve supporting stem cell transplants in elderly patients by either co-transplanting components of the stem cell niche into these patients or rejuvenating the existing stem cell niche using drug therapy. Alternatively, the induction of the self-renewal and proliferation of endogenous adult stem cells using non-invasive and non-toxic therapies may eventually constitute a legitimate alternative to stem cell transplantation. But can we pharmacologically mobilize endogenous adult stem cells for repair and regeneration?2 Recent advances in neurogenesis indicate that this goal may now be achievable. Metformin, a traditional biguanide that is widely used in humans to treat type 2 diabetes and other metabolic disorders, may be able to harness endogenous repair mechanisms to promote regeneration in situations in which this process does not normally occur.3
It has been suggested that metformin improves several of the adverse neuroanatomical outcomes that are associated with Alzheimer disease (AD). Metformin has also been demonstrated to increase lifespan and delay the onset of cognitive impairment in a mouse model of Huntington’s disease. These effects of metformin on the nervous system may be associated with the well-recognized insulin-lowering effects of metformin because hyperinsulinemia is known to enhance the onset and progression of neurodegenerative processes.4 Accordingly, there is widespread interest in using metformin in individuals with early-stage AD. In a series of elegant experiments in culture and in animals, Freda Miller and her colleagues recently raised the alternative possibility that metformin’s ability to directly enhance neurogenesis might positively impact certain nervous system disorders in a manner that is independent of the drug’s effects on insulin sensitivity.3 Miller and her colleagues had previously shown that the transcriptional coactivator CREB-binding protein, also known as CREBBP or CBP, maximizes embryonic neural precursor cell development.5 These researchers also demonstrated that the effects of CBP on neurogenesis require CBP activation by atypical protein kinase C (aPKC). Because Wondisford and colleagues had demonstrated that metformin’s ability to suppress hepatic gluconeogenesis requires the phosphorylation of CBP at serine 436 via aPKC,6 Miller and her colleagues hypothesized that because the aPKC/CBP pathway is downstream of metformin’s primary target, AMPK, metformin treatment could activate the AMPK → aPKC/CBP axis in neural stem cells, thereby creating new neurons. Miller and her colleagues report in the July 6 issue of Cell Stem Cell that, in a series of experiments in culture, metformin treatment promotes neurogenesis in both mouse and human neural stem cells.3 Compared with stem cells from control mice, stem cells from metformin-treated mice exhibit a nearly doubled capacity to produce new neurons. Notably, in living mice, metformin treatment induces an increase of approximately 30% in the number of new neurons in the hippocampus, a cerebral region that is closely involved in forming new memories. The pro-neurogenesis activity of metformin requires the presence of normal levels of CBP, as demonstrated by the fact that metformin has no effect in animals with only one copy of the CBP gene. Perhaps more importantly, using a classic behavioral test in which mice were forced to learn the position of an escape platform hidden under the surface in a water-filled maze and then asked rapidly to learn a new position, Miller and her colleagues confirmed that mice that had been treated with metformin could form new memories more rapidly than mice that had been given a control substance. Moreover, analyses demonstrated that the enhanced spatial memory formation of metformin-treated mice (specifically, treated mice preferentially searched the new region when they were put back into the maze in which the platform had been removed, whereas control mice spent more time searching for the platform in its original quadrant) notably parallels a significant increase in the number of new adult neurons in the dentate gyrus. The ability of metformin to enhance new memory formation is directly dependent on the ability of the drug to promote neurogenesis because the pharmacological killing of neural precursor cells efficiently blocks the effect of metformin on memory formation and concomitantly reduces the number of new neurons.
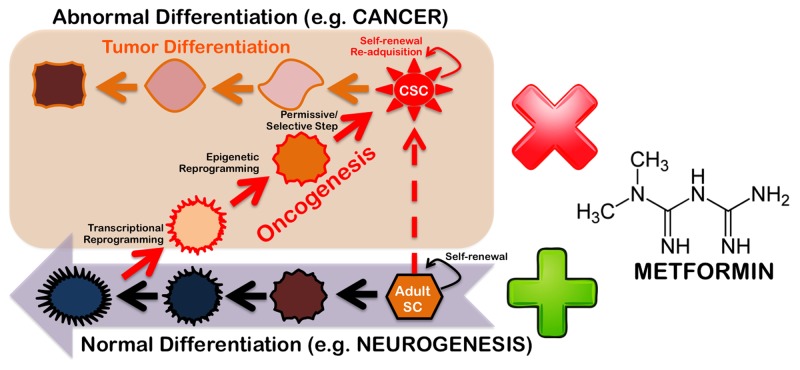
The implication that the use of metformin or metformin-like drugs might be a valuable pharmacological approach for nervous system therapy in disorders such as ischemic stroke and AD is strongly supported by the fact that the metformin dose used to treat the mice in Miller’s study was 200 mg/kg/day for up to 38 d, which is equivalent to 960 mg/day for a 60 kg person; therefore, metformin-enhanced neurogenesis was observed at a dose that was less than half the recommended safe dose for humans (2,550 mg/day for an average-sized person of 60 kg) and significantly lower than the dose that is commonly employed in diabetic patients (three 500 mg doses each day). From the perspective of future human studies, we wonder whether one can expect enhanced benefits from metformin in terms of neurogenesis and memory formation by keeping the metformin level more constant using new sustained-release formulations developed for dosing convenience. It also remains to be determined whether higher doses of metformin can more impressively promote neurogenesis and/or enhance spatial memory formation. Despite these gaps in our knowledge, the findings of Miller and her colleagues present new possibilities for the study of the gerosuppressant activity of metformin from a stem cell-centered perspective.7 Metformin has been shown to increase the lifespan of mouse models, both with and without cancer prevention; metformin also provides a metabolic barrier to the reprogramming of somatic cells into stem cells.8 We are thus beginning to delineate a new and complex scenario in which metformin-like drugs can specifically regulate the expression of cancer stem cell-specific genes to efficiently disrupt the stem cell compartment in multiple cancers while also controlling the balance of the self-renewal and differentiation of embryonic and adult stem cells.9 Because a fundamental principle of cell biology is that stem cells with greater potential for self-renewal and pluripotency will also have a higher probability of causing tumors,10 it is necessary to determine whether the pharmacological activation of the AMPK → aPKC/CBP axis via the systemic delivery of metformin might interfere with mechanisms that are important for stem cell-related tumorigenesis but are dispensable for adult stem cell development in mature tissues. If metformin can indeed uncouple tumorigenicity from pluripotency in stem cells (Fig. 1), new gerosuppressant approaches using metformin-like therapeutic drugs may be able to efficiently rejuvenate the tissue maintenance and repair processes driven by endogenous stem cells while diminishing tumorigenic predispositions in aging tissues.
Figure 1. Gerosuppressant metformin: “Learning” how to uncouple tumorigenesis from pluripotency.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21878
References
- 1.Rando TA. Nature. 2006;441:1080–6. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 2.Miller FD, et al. Cell Stem Cell. 2012;10:650–2. doi: 10.1016/j.stem.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, et al. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 4.de la Monte SM, et al. Curr Opin Investig Drugs. 2009;10:1049–60. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, et al. Dev Cell. 2010;18:114–25. doi: 10.1016/j.devcel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 6.He L, et al. Cell. 2009;137:635–46. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anisimov VN. Aging. 2012;2:760–74. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menendez JA, et al. Aging (Albany NY) 2011;3:348–62. doi: 10.18632/aging.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Barco S, et al. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoepfler PS. Stem Cells. 2009;27:1050–6. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]