Abstract
Pollen tubes navigate the route from stigma to ovule with great accuracy, but the cues that guide them along this route are not known. We reproduced the environment on the stigma of Nicotiana alata by immersing pollen in stigma exudate or oil close to an interface with an aqueous medium. The growth of pollen in this culture system mimicked growth on stigmas: pollen grains hydrated and germinated, and pollen tubes grew toward the aqueous medium. The rate-limiting step in pollen germination was the movement of water through the surrounding exudate or oil. By elimination of other potential guidance cues, we conclude that the directional supply of water probably determined the axis of polarity of pollen tubes and resulted in growth toward the interface. We propose that a gradient of water in exudate is a guidance cue for pollen tubes on the stigma and that the composition of the exudate must be such that it is permeable enough for pollen hydration to occur but not so permeable that the supply of water becomes nondirectional. Pollen tube penetration of the stigma may be the most frequently occurring hydrotropic response of higher plants.
Fertilization of the ovules of flowering plants occurs when desiccated pollen grains on the receptive surface of the female (the stigma) hydrate, germinate, and produce a tube that elongates directionally to penetrate the stigma (Knox, 1984; Dickinson and Elleman, 1994; Nasrallah et al., 1994). These events occur in the lipid-rich environment formed by the pollen coat or the stigma exudate, which is essential for successful fertilization (Pandey, 1963; Konar and Linskens, 1966b; Preuss et al., 1993; Goldman et al., 1994; Wolters-Arts et al., 1998). Cells of the “wet” stigmas of solanaceous plants release lipid droplets into the intercellular spaces of the stigma and directly onto its surface. The droplets accumulate and coalesce to form a transition layer of an oil-in-water emulsion between an aqueous phase within the stigma and a lipid phase on the surface (Konar and Linskens, 1966a; Herrero and Dickinson, 1979; Cresti et al., 1986; Kandasamy and Kristen, 1987). Pollen on solanaceous stigmas does not adhere to papillae but remains free within the exudate, where it hydrates despite the negligible amount of water present within the exudate itself (Konar and Linskens, 1966b) and the barrier to water movement that lipids are usually assumed to impose.
Following germination the directional growth of pollen tubes (which elongate by tip growth) into the stigma suggests that some external cue establishes their polarity. Light, tactile, electrical, and chemical cues have been suggested as polarizing agents in pollen tubes and other tip-growing cells (Heslop-Harrison and Heslop-Harrison, 1986; Reger et al., 1992; Cheung et al., 1995; Hülskamp et al., 1995; Malho and Trewavas, 1996; Kropf, 1997); however, although potential external guidance cues have been identified for pollen tubes, their roles in guidance within the pistil remain unclear (Hepler, 1997; Sommer-Knudsen et al., 1998).
By applying oils to exudate-free stigmas of transgenic Nicotiana tabacum plants, we recently demonstrated the importance of the chemical nature of the exudate by showing that stigma function, which is restored by the application of exudate, can also be restored by some triglycerides (Wolters-Arts et al., 1998). Results of the in vitro assay carried out with N. tabacum pollen (Wolters-Arts et al., 1998) suggest that the directional growth of pollen tubes into the stigma is dependent on the presence of a boundary between the exudate (or oil) and the aqueous environment of stigma cells. However, the nature of guidance in the N. tabacum in vitro assay was unclear because of the clustering of pollen grains and the possibility that some of the oils used were toxic. We report here the results of investigations of the growth of pollen tubes in an in vitro system in which pollen is immersed in exudate (or a functional substitute) close to an interface with an aqueous medium, thus reproducing one aspect of the stigma environment. Pollen in our system mimics pollen behavior on the stigma: it hydrates and germinates, and pollen tubes grow toward the aqueous medium, probably using a gradient in the concentration of water to set the direction of growth.
MATERIALS AND METHODS
Pollen Culture
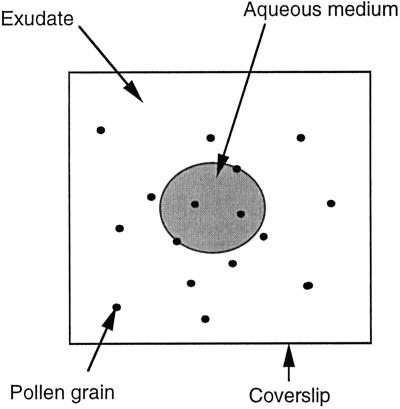
Nicotiana alata cv Link et Otto plants of the self-incompatibility genotypes S2S2 and S6S6 were used as sources of pistils, stigma exudate, and pollen. There was no difference in the growth of S2 and S6 pollen in exudate from S2S2 plants, which is consistent with the finding that the exudate has no role in the rejection of self-pollen in N. alata (Pandey, 1963) that occurs within the style (Lush and Clarke, 1997). Exudate was collected from mature stigmas of emasculated flowers with a micromanipulator (Leitz, Wetzlar, Germany) and used immediately. Oils tested as potential substitutes for the exudate were mineral oil (catalog no. M-5904, Sigma) and purified olive oil (Meadowlea Foods Ltd., Mascot, NSW, Australia). Cultures were established by placing a drop of exudate or oil (0.2–1.0 μL) on a glass microscope slide and inoculating it with freshly collected, air-dried pollen (approximately 100 grains/μL oil). A smaller drop of an aqueous medium was injected into the center of the oil-pollen mixture, and the culture was covered with a glass coverslip (2 × 2 mm) to produce a two-dimensional, two-phase culture in which a central aqueous phase was surrounded by exudate or oil (Fig. 1).
Figure 1.
Diagrammatic representation of the culture system (not to scale).
Cultures were photographed as soon as the coverslip was in place. Subsequent photographs were taken within 1 h and thereafter at intervals of 1 to 2 h. The drop of aqueous medium fragmented during establishment of some of the cultures, enabling rapid hydration of pollen grains associated with satellites of the main body of aqueous medium. Such grains were readily detected from photographs and were excluded from all analyses. Cultures were incubated at 25°C to 30°C. Aqueous media injected into oils were based on a pollen tube growth medium developed for N. alata (12.5% PEG 6000, 0.15 m Suc, 1.0 mm CaCl2, 1.0 mm KCl, 0.8 mm MgSO4, 1.6 mm H3BO3, 0.03% casein acid hydrolysate, and 25 mm Mes, pH 5.9). An olive oil:medium emulsion (1:1) was made by sonication for 20 min.
The aqueous medium was modified by omission of components. To ensure that Ca2+ was not present as a contaminant in a buffered solution of PEG, EGTA was added to the solution at a concentration (1 mm) just sufficient to prevent the growth of pollen tubes within the buffered PEG. Addition of Ca2+ to the buffered PEG containing 1 mm EGTA alleviated the inhibition of pollen tube growth.
Hydration and germination were monitored using an inverted microscope (model IM35, Zeiss). The degree of hydration of pollen grains was measured as the ratio of the minor axis of the grain to the major (longitudinal) axis. Water uptake was calculated from the increase in the volume of individual grains (Heslop-Harrison, 1987; Dickinson and Elleman, 1994). Germination was defined as the emergence of the pollen tube. Pollen tubes within a 150o arc centered on the pollen grain and widening toward the aqueous medium were defined as growing toward the aqueous medium. Distances were measured from the interface to the closest part of the pollen grain.
All experiments were conducted at least twice. In each experiment with exudate there was a minimum of four replicate cultures, and in experiments with olive oil there was at least eight. Cultures established with exudate were controls for the initial experiments with olive oil and mineral oil, and cultures with complete aqueous medium were controls for experiments with modified media. Data were pooled for statistical analysis (χ2).
The permeability of mineral and olive oils to water was compared by covering 1% agarose gels (in 3.5-cm-diameter Petri dishes, depth 1 mm) with layers of oil (depth 1 mm) and monitoring evaporation by weight (open dishes held in laboratory, four measurements within a 7.5-h period, four replicates of each treatment). Controls were gels with no covering layer of oil and layers of oil without any underlying gel.
Pollen Hydration and Growth on Stigmas
Growth of compatible pollen was examined by light microscopy (Photomakroscop M400, Wild-Leitz, Wetzlar, Germany, or Zeiss inverted microscope) and by epifluorescence microscopy (model BH2 photomicroscope, Olympus). Hydration and germination of individual pollen grains placed on stigma papillae with a micromanipulator were monitored with the inverted microscope in flowers cut from plants and with petals partially removed but otherwise intact (total of 20 grains observed). Pollen grains (total of 10) coated with exudate were also observed on papillae projecting from the exudate. These grains were coated with exudate by soaking bulk pollen in exudate and then draining excess exudate away with the tissues. Individual grains were extracted from the mass of pollen grains and positioned with a micromanipulator. Hydration of grains within the exudate (or a blend of exudate and oil) was observed on stigmas flooded with a suspension of pollen in mineral oil to overcome problems of refraction. A total of 20 grains were observed. In parallel with observations of individual grains, pollen was removed from stigmas (two per harvest time) by washing with mineral oil at intervals after pollination, and germinated grains were counted. Pollen tube penetration of washed stigmas was assessed by examining the surface of stigmas and stigma squashes (Martin, 1959) stained with aniline blue (0.1%, Merck, Darmstadt, Germany).
The effect of the RH surrounding the stigma on pollen germination and growth was studied using cut styles. The lower 17 mm of each style was sealed in a small vial (cut end in water), and the small vial was enclosed in a larger vial partly filled with pure water or a saturated solution of a salt. Solutions in the large vials produced a RH around the upper 3 mm of the style (including the stigma) ranging from 76% to 100%. Styles were incubated in the laboratory at approximately 20°C. Styles remained competent to support pollen germination and tube growth for more than 24 h after cutting. Treatments were replicated three times and the experiment was repeated five times.
RESULTS
Hydration and Germination of Pollen Grains in Isolated Exudate and on Stigmas
Exudate was collected from stigmas using a micromanipulator and placed on a microscope slide. Examination of the exudate by light microscopy showed that it was not an emulsion and that there was no change in its volume following exposure to air in the laboratory for 24 h. Dry pollen of N. alata is hydrophobic and dispersed readily in the isolated exudate, but pollen grains did not hydrate. These observations are consistent with previous findings that the exudate on solanaceous stigmas is lipidic and that it is not a reservoir of compounds required for pollen germination (Konar and Linskens, 1966b).
A culture system was developed in which pollen grains surrounded by exudate were supplied with a nearby source of water and other requirements for the germination and growth of pollen tubes (Fig. 1). A sharp interface formed between the aqueous medium and the exudate, with pollen grains located in both phases of the culture (exudate and aqueous) and spanning the interface between them. Following placement of the coverslip, there was no further movement of either pollen grains or the interface. The progressive hydration and germination of pollen was followed by photomicroscopy (Fig. 2, A–C). Pollen in the aqueous medium hydrated before the first inspection of cultures, within approximately 45 s of injection of the aqueous medium. Based on the difference in the average dimensions of pollen grains before and after hydration, the rate of uptake of water into grains in the aqueous phase was approximately 500 fL s−1. Grains spanning the interface hydrated within 3 to 4 min.
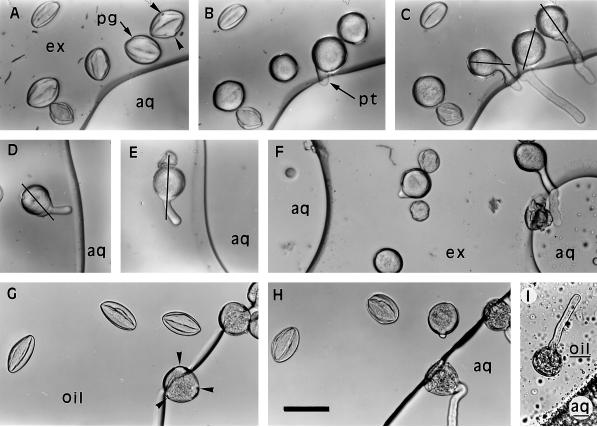
Figure 2.
Hydration, germination, and directional growth of pollen tubes in exudate or olive oil close to an interface with an aqueous medium. A to C, Progressive hydration and germination of pollen grains in exudate 50 min, 3 h, and 4.5 h, respectively, after injection of the aqueous medium. Pollen grains closest to the interface hydrated and germinated first. The pollen tube emerged from the aperture closest to the interface and grew toward the aqueous medium. D and E, Growth of two tubes in exudate toward the interface 5 h after the injection of aqueous medium. The direction of pollen tube growth was determined by both the position of the germinal aperture and the axis of polarity of the tip (photographed 5 h after establishment of the interface). F, Pollen in exudate between two interfaces with aqueous medium showing growth of tubes toward the nearest interface (photographed after 6 h). G and H, Pollen in olive oil 5 min and 5 h, respectively, after injection of aqueous medium. Pollen spanning the interface germinated first and tubes emerged into the aqueous medium. I, Pollen in an emulsion of aqueous medium in olive oil close to a boundary with a predominantly aqueous phase. The pollen tube did not grow toward the boundary (photographed after 5 h). aq, Aqueous medium; aq, predominantly aqueous phase; ex, stigma exudate; oil, predominantly oil phase; pg, pollen grain; pt, pollen tube. Arrowheads indicate apertures in pollen grain; lines indicate extended short axes of pollen grains based on photographs of partially hydrated grains. Bar in H = 50 μm and refers to the entire figure.
We were most interested in pollen grains that hydrated and germinated when surrounded by exudate. We used the photographic record of each pollen grain to exclude the possibilities that the grain hydrated when in transient contact with the aqueous medium during establishment of the culture or in a small satellite of aqueous medium produced by fragmentation of the main body of medium. Part of one such time series of photographs is shown in Figure 2, A to C. Only grains that were observed to hydrate progressively when surrounded by exudate are included in the following results.
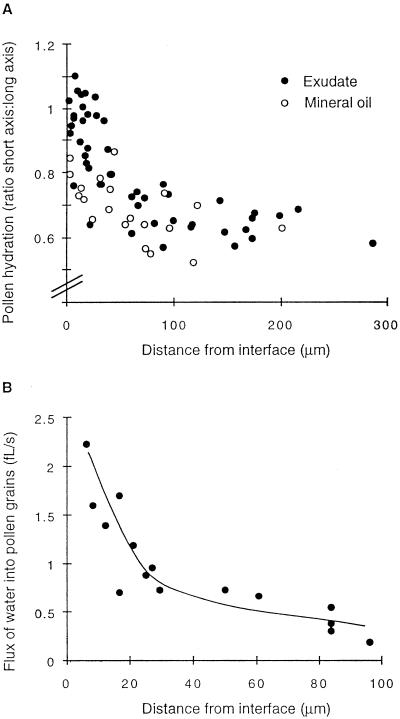
Pollen in exudate hydrated more slowly than pollen in direct contact with the aqueous medium. There was some variation between grains, but in general the closer grains were to the interface with the aqueous medium the more rapidly they hydrated (Figs. 2 and 3A). The flux of water into pollen grains was estimated for a subset of grains photographed at higher magnification (×25). The flux into grains 10 μm from the interface was approximately 1.5 fL s−1 (Fig. 3B). Grains between two interfaces hydrated more rapidly than grains a similar distance from a single interface (Fig. 2); these were excluded from our analyses. Some small, irregularly shaped grains did not hydrate (Fig. 2).
Figure 3.
Hydration of pollen grains surrounded by exudate or mineral oil but close to an interface with an aqueous medium. Each point represents a pollen grain. A, The degree of hydration of pollen grains in exudate or mineral oil 3 h after the establishment of the interface with the aqueous medium. B, Variation in the flux of water through exudate to pollen grains with distance from the interface (estimated during the first 90 min of pollen hydration).
The cytoplasm of cultured pollen grains started to stream at about the time grains became fully rounded. Germination of grains within the aqueous medium or spanning the interface started 30 min after injection of the aqueous medium. Germination of grains within the exudate started later and was more delayed the farther the grains were from the interface (Fig. 2, B and C). In one experiment, for example, the germination rate of pollen 3 h after injection of the aqueous medium was 80% for grains between 1 and 20 μm from the interface, 30% for grains between 20 and 40 μm, and 0% for grains farther than 40 μm. Grains up to 60 μm from the interface usually germinated within 6 h, but grains farther away, although they progressively hydrated, rarely germinated. The pollen tube emerged from the pore closest to the interface (Fig. 2, A and C, grain on extreme right) in more than 95% of grains.
When two pores were approximately equidistant from the interface, the tube was equally likely to emerge from either pore. The position of tube emergence from most grains ensured that the tubes would reach the interface if they grew approximately radially outward from grains. However, except for pollen tubes in which the germinal pore was immediately adjacent to the aqueous medium (Fig. 2C, extreme right), tubes grew more directly toward the interface than would be expected from radial growth (Fig. 2, C–E). Tubes then passed into the aqueous medium. The accumulated results of seven experiments with exudate and the complete aqueous medium are summarized in the first row of Table I and show that the directional growth of pollen tubes toward the aqueous medium was highly reproducible.
Table I.
Direction of growth of pollen tubes emerging from grains immersed in lipid near an interface with an aqueous medium of varying composition
| Type of Lipid | Composition of Aqueous Medium | Tubes Growing Toward Aqueous Medium |
|---|---|---|
| % | ||
| Exudate | Complete | 99 (72) |
| Olive oil | Complete | 97 (115) |
| Complete, in dark | 97 (33) | |
| Exudate | PEG + Suc + Mes | 98 (56) |
| Olive oil | PEG + Mes | 97 (39) |
| PEG + Mes + 1.0 mm EGTA | 100 (33) | |
| PEG | 97 (37) | |
| Suc + salts + borate + amino acids + Mes | 79 (29) |
Numbers in parentheses show total number of tubes (pooled for all experiments) emerging within 6 h of the establishment of an interface between the lipid and the aqueous medium. In all treatments the direction of pollen tube growth was statistically different (P < 0.005) from random growth. Growth in the absence of PEG was statistically different (P < 0.005) from all other treatments.
All tubes from grains spanning the interface of the aqueous medium and the exudate emerged into the aqueous medium (total of 49 tubes observed). Pollen tubes from grains within the aqueous medium had no consistent directional orientation (data not shown). Pollen between two aqueous interfaces produced tubes that grew toward the nearest interface (Fig. 2F). Emulsion-sized droplets of an aqueous solution were observed within the exudate 1 to 2 h after cultures were established and became more common as experiments proceeded. The droplets were confined to localized regions of the exudate and usually derived from burst grains or tubes. The growth of pollen tubes emerging in regions of emulsion was not directional. The observations that follow are restricted to events occurring within 6 h of the introduction of the aqueous medium and to regions of the exudate (or substitute) in which emulsion-sized droplets were absent.
The hydration of pollen grains and growth of pollen tubes in the culture system were compared with growth on N. alata stigmas. Germination of pollen on stigmas was monitored by examining grains removed by washing the stigma in mineral oil and staining for the presence of pollen tubes within the stigma. Germination was first detected in grains removed from the stigma 30 min after pollination. The first tubes within the stigma were also detected after 30 min, but the main period of penetration into the stigma occurred between 2 and 4 h after pollination. The majority (>90%) of pollen tubes grew directly into the stigma. Hydration and germination of individual grains on the stigma were also observed. Some mature stigma papillae projected above the surface of the exudate, but pollen grains placed on these papillae never hydrated, even if the grains were first coated thinly with exudate (Fig. 4, A–D). Grains within the exudate could not be directly observed because of light refraction, but hydration and germination of pollen on the stigma were observed in stigmas gently flooded with a suspension of pollen in mineral oil. Grains within the exudate/oil and close to the stigma (Fig. 4, E and F), including those not in direct contact with papillae, hydrated. Pollen tubes grew toward the stigma (data not shown).
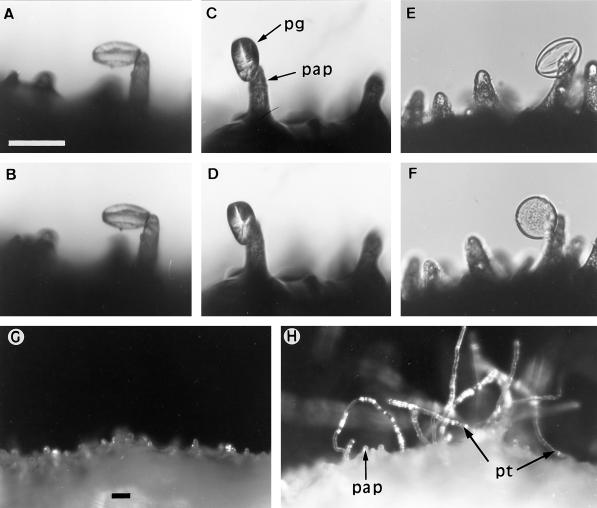
Figure 4.
Hydration of pollen grains and growth of pollen tubes on stigmas. A to D, Pollen on stigma papillae projecting through the exudate into air at the RH prevailing in the laboratory. Dry pollen (A and B) and pollen presoaked in exudate and drained (C and D) did not hydrate. Photographs were taken 1 h (A and C) and 18 h (B and D) after pollination. E and F, Pollen on stigma flooded with mineral oil at the start of the experiment and photographed 30 min and 4 h after pollination did hydrate. G and H, Growth of pollen tubes on stigmas held at controlled RH, photographed 16 h after pollination. G, Stigma at 88% RH. Only stigma papillae are visible above the exudate. H, Stigma at 100% RH. Pollen tubes have grown from the stigma into the surrounding air. pap, Papillar cell; pg, pollen grain; pt, pollen tube. Scale bar in A = 50 μm and refers to A through F; scale bar in G = 50 μm and refers to G and H.
Oil, Water, and PEG Are the Only Requirements for Directional Growth in Culture
Our results show that the presence of an immiscible, aqueous medium within isolated stigma exudate establishes the conditions needed for the guidance of pollen tubes toward the aqueous medium. To investigate the role of components of the culture system in directing pollen tube growth toward the aqueous medium, we tested potential substitutes for the exudate (olive oil and mineral oil) and omitted components of the aqueous medium.
The time course of hydration and germination in olive oil was the same as in the exudate. The first grains to hydrate were those closest to the interface with the aqueous medium, and their tubes grew toward the aqueous medium (Fig. 2, G and H). These results were highly reproducible, as shown by the summary of results presented in Table I. All of the more than 800 tubes observed emerging from grains spanning the interface grew directly into the aqueous medium (Fig. 2H). Growth of tubes in the absence of a directional supply of aqueous medium was investigated by replacing olive oil with a 1:1 emulsion of olive oil and medium and establishing cultures as described above. These cultures had a predominantly oil phase and a predominantly aqueous phase, but the boundary between the phases was less distinct and stable than in the usual cultures. Pollen in the predominantly oil phase of these cultures often produced tubes that did not grow toward the boundary between phases (Fig. 2I), but the results were not quantified because of the instability of the boundary. The predominantly water phase was opaque (Fig. 2I), precluding observations.
When mineral oil was substituted for exudate, only grains that were located in the aqueous medium or that spanned the interface became fully hydrated, even after prolonged incubation (Fig. 3A). All of the grains spanning the interface (35 were observed) produced tubes that grew directly into the aqueous medium. A possible explanation for the failure of pollen to hydrate and germinate in mineral oil is that this oil is less permeable to water than olive oil. The permeability of the two oils was assessed by measuring the effectiveness of a covering layer (1 mm deep) of each oil in reducing evaporation from gels. After 7.5 h, the mass of gels exposed to air had decreased by 457 ± 3 mg (± se), whereas the mass of gels covered by olive oil or mineral oil had decreased by 4.6 ± 0.01 and 0.75 ± 0.06 mg, respectively. There was no change in the mass of the pure oils.
The data presented show that stigma-specific compounds within the exudate are not required for tube growth toward an interface with an aqueous medium. Directional growth of pollen tubes also occurred in cultures incubated in the dark (Table I), showing that any changes in the direction or wavelength of light caused by the presence of the interface were not the signal for directional growth. Water itself and any of the compounds dissolved in the aqueous medium are potential sources of a chemical guidance cue within the oil. To identify this cue, we modified the composition of the aqueous medium. No observations were possible when pure water was substituted for the usual medium because most of the pollen grains burst before germinating. The released cell contents formed an emulsion around any grains that remained intact in the oil.
Directional growth of pollen tubes in exudate persisted when the aqueous medium was simplified to a buffered solution of PEG and Suc; growth of tubes in olive oil persisted when the aqueous medium was a buffered solution of PEG (Table I). Although Ca2+, a potential guidance cue, was not added to the simple media used in these experiments, its presence as a contaminant was not precluded. To minimize the possibility that Ca2+ was responsible for guidance, EGTA was added to a buffered solution of PEG at a concentration just sufficient to prevent the germination of pollen added directly to the medium. Some grains within the oil germinated and these tubes grew toward the EGTA-containing medium (Table I), where they burst. Tubes also grew toward a pure solution of PEG and toward an aqueous medium containing all components except PEG (but at a lower frequency, Table I). The incidence of pollen grain and tube bursting was higher in all of the modified media than in the complete medium; pollen grains in regions of emulsion created by this bursting were not included in the results.
Disruption of Normal Water Gradients at the Stigma Alters the Direction of Pollen Tube Growth
Results with pollen in culture suggested that a gradient of water within the exudate could direct pollen tubes into the stigma. To investigate the role of water gradients at the stigma, pollinated stigmas were enclosed in a RH from 76% to 100%. At a RH ≤ 88%, most pollen tubes grew directly into the stigma; at a RH > 88%, the incidence of tubes projecting through the exudate and into the surrounding air increased with increasing RH (Fig. 4, G and H).
DISCUSSION
The Directional Growth of Pollen Tubes on Stigmas Is Reproduced in Culture
The cues that guide pollen tubes into and through the style function by establishing an axis of polarity. Attempts have been made to identify chemical guidance cues by observing the responses of pollen tubes growing in gelled media to additives diffused into the gels. The additives tested include defined compounds such as sources of Ca2+, undefined compounds from tissue slices and pistil extracts, and purified components of the pistil (Brink, 1924; Tsao, 1949; Rosen, 1971; Heslop-Harrison and Heslop-Harrison, 1986; Mascarenhas, 1993; Cheung et al., 1995).
The present culture system represents a departure from most previous assays because it reproduces a key element of the environment on solanaceous stigmas. Pollen was immersed in natural exudate or a functional substitute, where it hydrated only when a source of water and other requirements for germination and growth were introduced to the system as a physically distinct aqueous phase. The low density of pollen ensured that the pathway of water to pollen was through oil and not from grain to grain. Hydrated pollen germinated and tubes grew directionally toward the aqueous phase. The conclusion that growth is directional is based on observations of individual tubes and thus avoids a problem present in many assays for directional growth: that differential growth at high and low concentrations of the test compound is difficult to distinguish from attraction or repulsion of pollen tubes (Rosen, 1971; Heslop-Harrison and Heslop-Harrison, 1986). The growth of a small number of N. alata tubes parallel to or away from the aqueous interface (Table I) showed that pollen tubes can extend into the oil phase; therefore, some form of guidance must operate to direct the majority of tubes to the interface. Another common problem in experiments on directional growth is that the results are not quantified; our quantification of the directional growth of N. alata pollen tubes showed that it was highly reproducible (Table I).
There are other studies of pollen growing in oil. Maize pollen tubes supplied directly with water while immersed in mineral oil were observed to grow toward an aqueous phase (Kranz and Lörz, 1990). However, this work was a study of in vitro fertilization in maize and observations of directional growth were not investigated in depth. We reported that Nicotiana tabacum pollen tubes in some triglycerides appear to grow toward an aqueous phase (Wolters-Arts et al., 1998). Our interpretation of the N. tabacum assay is consistent with the general findings of that study, but the assay itself is open to other interpretations because water uptake may have been facilitated by direct transfer of water between the clustered grains. In addition, the direction of chemical gradients of charged or polar molecules in oil are not clearly defined if grains are clustered.
The Directional Supply of Water Is the Most Likely Guidance Cue
The directional growth of N. alata pollen tubes in our assay was not a response to touch because there were no directional surfaces in the culture system; nor was it a response to gravity, because it was always at right angles to the major axis of pollen tube growth or to light, which was not required for the response (Table I). The elimination of touch, gravity, and light as cues implies that the cue is a chemical one. The putative chemical cue is not a style-specific molecule, because pollen tubes surrounded by olive oil grew toward the aqueous medium, which was chemically defined. When the supply of water and nutrients provided by the aqueous medium was not directional, as in emulsions, growth was also not directional (Fig. 2I).
Chemical gradients, even those operating over the short distances (up to 60 μm or about 1.5 pollen grain diameters) reported here, are problematic as guidance cues. The rapidity of diffusion in liquids means that, for the gradient to be maintained, there must be both a continuously active source and a continuously active sink for the guidance molecule. Pollen grains are active sources of CO2 and sinks for O2. CO2 is more soluble in organic solvents, including oil, by a factor of 2 to 3 (Stephen and Stephen, 1963; Meyer and Canny, 1975). O2 is about 10 times more soluble in hydrocarbons than in water (Battino et al., 1983). Thus, gradients in the concentration of these gases in the oil surrounding pollen grains will be the same in all directions. Conversely, directional chemical gradients in the exudate (or oil) will be formed by charged or polar molecules for which the aqueous medium (or stigma) is the source and the pollen grains are the sinks. Such molecules include Ca2+ and sugars, both of which have been proposed as external cues for pollen tube guidance (Reger et al., 1992; Mascarenhas, 1993). However, cultured pollen tubes grew preferentially toward aqueous solutions that contained neither Ca2+ nor added sugars (Table I). Directional growth persisted when the aqueous medium was a solution of PEG in water, suggesting that a gradient within the oil in water or PEG was the cue.
The question of which molecule, water or PEG, was effective in guiding pollen tubes is difficult to resolve. In an ideal system, the response to a signal of varying strengths would be studied using pollen competent to respond to that signal. However, varying the availability of water alters the competence of pollen to respond, and varying the availability of PEG may also affect competence. Thus, in the absence of water or in the presence of pure water, N. alata pollen grains do not germinate. The addition of PEG to media for growing pollen tubes increases germination frequency and results in faster-growing tubes with morphologies more like those of tubes growing in styles (Jahnen et al., 1989; Read et al., 1993).
The reasons for the growth-promotory effects of PEG are not known but do not appear to be related to its effect on the osmotic potential of the growth medium (Jahnen et al., 1989). PEG may be a substitute for a compound(s) normally supplied by the style. When PEG was omitted from the aqueous medium, pollen tubes grew toward the interface with reduced fidelity (Table I). We interpret this reduction in fidelity as the consequence of a reduction in the competence of pollen tubes to respond to water as a signal. The probability that water was the guidance cue is supported by the presence of a gradient in the availability of water to pollen within the oil phase, as shown by the more rapid hydration of grains close to the interface (Fig. 3). Furthermore, pollen tubes on stigmas grew toward a water-saturated atmosphere (Fig. 4, G and H). The directional growth of pollen tubes into stigmas of other species is also disrupted in very humid atmospheres (Knox, 1984; Preuss et al., 1993; Dickinson and Elleman, 1994).
The timing of N. alata germination on the stigma was similar to that of pollen in culture, thus enabling a reconstruction of events on the stigma to be drawn. The flux of water into the first grains to germinate (after 30 min) on the stigma must, by analogy with grains in culture, be approximately 500 fL s−1. These rapid germinants will be in direct contact with the stigma's sources of water, the walls of the papilla cells and the fluid in intercellular spaces. The germination of most grains, however, is delayed until several hours after pollination, suggesting that fluxes of 1 to 2 fL s−1 are probably more typical on the stigma and that most grains are surrounded by exudate. The exudate thus acts as a throttle that reduces the rate of water uptake. Restricting the rate of water uptake by N. alata pollen does not appear to be of importance in itself, because there is no evidence that pollen that imbibed rapidly was functionally impaired either in vivo or in vitro. The biological significance of the low permeability of the exudate probably lies in the creation of an environment in which the supply of water is directional, thus establishing a cue for the guidance of hydrotropic pollen tubes toward the stigma, and in reducing water loss from the stigma and hydrating pollen (Konar and Linskens, 1966b).
Although it has long been proposed that roots are hydrotropic, some apparently hydrotropic responses of roots are in fact due to greater root proliferation in moist regions of soils; others may be due to altered responses to gravity or other cues in roots growing in dry soils (Leopold and LaFavre, 1989; Coutts and Nicoll, 1993; Takano et al., 1995). In addition, the water gradients required for hydrotropic responses may be rare in soils (Coutts and Nicoll, 1993). Little is known about the mechanism of root hydrotropism (Takano et al., 1997).
The directional growth of pollen tubes toward aqueous media results from the initial selection of the germinal pore for pollen tube emergence and from continuing minor adjustments to the axis of polarity of the growing tip (Fig. 2). The setting of and the subsequent adjustment to the axis of polarity can be explained by turgor-driven expansion of the cell wall of pollen tubes if the wall is weaker at higher water contents. Turgor pressure within the pollen grain could thus direct tube emergence from the pore closest to the source of water by overcoming the mechanical resistance of the wall at its most hydrated part. Growth of the tube would also be directed to the most hydrated parts. Stretching of the wall could result in the activation of channels in the underlying membrane (e.g. Ca2+ channels; Hepler, 1997), thus realigning the vesicle delivery system at the tip and reinforcing the directional response. Other explanations include the possibilities that vesicle docking at the plasma membrane is facilitated in the more hydrated parts of the membrane and that the vesicle delivery system is aligned as a result of a signal cascade initiated by differential hydration of wall or membrane components.
The Role of Lipids in Pollen Hydration
It has been suggested that the effects of lipids on pollen hydration and tube growth in species with dry stigmas are due to lipid or lipid fragments acting as signals for hydration or penetration (Preuss et al., 1993; Pruitt, 1997). The only oils known to be functional replacements for the exudate of solanaceous plants are also lipids (Wolters-Arts et al., 1998). Our finding that pollen of N. alata germinated when entirely surrounded by lipid (exudate or olive oil) but not when surrounded by mineral oil is also consistent with a specific role for lipids in germination. However, a simpler hypothesis is that the effects of lipids on pollen germination and tube growth are due to their physicochemical properties.
The failure of pollen surrounded by mineral oil to hydrate fully (Fig. 3) can be accounted for physicochemically if mineral oil has a lower permeability to water, as was suggested by the greater effectiveness of mineral oil than olive oil in reducing evaporation from an underlying water layer. Pollen grains at the interface of mineral oil and the aqueous medium hydrated fully, and these grains germinated to produce tubes that grew directly into the aqueous medium, as would be expected of pollen exposed to a very steep water gradient. The failure of other nonlipid oils to act as substitutes for the exudate (Wolters-Arts et al., 1998) may have been due to the physicochemical properties of these oils or to their toxicity to pollen. Pollen in vitro is notoriously sensitive to both organic and inorganic additives and rarely behaves as a perfect osmometer. The sensitivity of pollen precludes many potentially interesting experiments.
The ability of the stigma to support pollen hydration, germination, and tissue penetration by pollen tubes is unique in wild-type plants and in the Solanaceae is associated with the presence of a liquid, lipid exudate on the surface. The exudate has multiple roles in water management. The present work shows that it must be sufficiently permeable to water to establish hydraulic continuity between stigma and pollen so that pollen hydrates but not so permeable that the supply of water or a functional equivalent of PEG becomes nondirectional. Guidance toward the stigma by a water gradient may be the first step in a multistage process of guidance to the ovules.
ACKNOWLEDGMENTS
We thank A. Clarke, P. Gerola, J. Golz, M. Herrero, C. Land, B. McGinness, C. Schultz, T. Schultz, and T. Spurck for their contributions to this work.
Footnotes
This work was supported by a special research grant from the Australian Research Council to the Plant Cell Biology Research Centre.
LITERATURE CITED
- Battino R, Rettich TR, Tominaga T. The solubility of oxygen and ozone in liquids. J Phys Chem Ref Data (American Chemical Society) 1983;12:163–178. [Google Scholar]
- Brink RA. The physiology of pollen. IV. Chemotropism; effects on growth of grouping grains; formation and function of callose plugs; summary and conclusions. Am J Bot. 1924;11:417–436. [Google Scholar]
- Cheung AY, Wang H, Wu H-M. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell. 1995;82:383–393. doi: 10.1016/0092-8674(95)90427-1. [DOI] [PubMed] [Google Scholar]
- Coutts MP, Nicoll BC. Orientation of the lateral roots of trees. II. Hydrotropic and gravitropic responses of lateral roots of Sitka spruce grown in air at different humidities. New Phytol. 1993;124:277–281. doi: 10.1111/j.1469-8137.1993.tb03817.x. [DOI] [PubMed] [Google Scholar]
- Cresti M, Keijzer CJ, Tiezzi A, Ciampolini F, Focardi S. Stigma of Nicotiana: ultrastructural and biochemical studies. Am J Bot. 1986;73:1713–1722. [Google Scholar]
- Dickinson HG, Elleman CJ (1994) Pollen hydrodynamics and self-incompatibility in Brassica oleracea. In AG Stephenson, T-H Kao, eds, Pollen-Pistil Interactions and Pollen Tube Growth. American Society of Plant Physiologists, Rockville, MD, pp 45–61
- Goldman MHS, Goldberg RB, Mariani C. Female sterile tobacco plants are produced by stigma-specific cell ablation. EMBO J. 1994;13:2976–2984. doi: 10.1002/j.1460-2075.1994.tb06596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK. Tip growth in pollen tubes: calcium leads the way. Trends Plant Sci. 1997;2:79–80. [Google Scholar]
- Herrero M, Dickinson H. Pollen-pistil incompatibility in Petunia hybrida: changes in the pistil following compatible and incompatible intraspecific crosses. J Cell Sci. 1979;36:1–18. doi: 10.1242/jcs.36.1.1. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. Pollen germination pollen tube growth. Int Rev Cytol. 1987;107:1–78. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y (1986) Pollen-tube chemotropism: fact or delusion? In M Cresti, D Romano, eds, Biology of Reproduction and Cell Motility in Plants and Animals. University of Siena, Italy, pp 169–174
- Hülskamp M, Schneitz K, Pruitt R. Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. Plant Cell. 1995;7:57–64. doi: 10.1105/tpc.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnen W, Lush WM, Clarke AE. Inhibition of in vitro pollen tube growth by isolated S-glycoproteins of Nicotiana alata. Plant Cell. 1989;1:501–510. doi: 10.1105/tpc.1.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy MK, Kristen U. Developmental aspects of ultrastructure, histochemistry and receptivity of the stigma of Nicotiana sylvestris. Ann Bot. 1987;60:427–437. [Google Scholar]
- Knox RB. Pollen-pistil interactions. In: Linskens H-F, Heslop-Harrison J, editors. Encyclopedia of Plant Physiology New Series, Vol 17. Heidelberg, Germany: Springer-Verlag; 1984. pp. 508–608. [Google Scholar]
- Konar RN, Linskens HF. The morphology and anatomy of the stigma of Petunia hybrida. Planta. 1966a;71:356–371. doi: 10.1007/BF00396321. [DOI] [PubMed] [Google Scholar]
- Konar RN, Linskens HF. Physiology and biochemistry of the stigmatic fluid of Petunia hybrida. Planta. 1966b;71:372–387. doi: 10.1007/BF00396322. [DOI] [PubMed] [Google Scholar]
- Kranz E, Lörz H. Micromanipulation and in vitro fertilization with single pollen grains of maize. Sex Plant Reprod. 1990;3:160–169. [Google Scholar]
- Kropf DL. Induction of polarity in fucoid zygotes. Plant Cell. 1997;9:1011–1020. doi: 10.1105/tpc.9.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold AC, LaFavre AK. Interactions between red light, abscisic acid, and calcium in gravitropism. Plant Physiol. 1989;89:875–878. doi: 10.1104/pp.89.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush WM, Clarke AE. Observations of pollen tube growth in Nicotiana alata and their implications for the mechanism of self-incompatibility. Sex Plant Reprod. 1997;10:27–35. [Google Scholar]
- Malho R, Trewavas AJ. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 1996;8:1935–1949. doi: 10.1105/tpc.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FW. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol. 1959;34:125–128. doi: 10.3109/10520295909114663. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP. Molecular mechanisms of pollen tube growth and differentiation. Plant Cell. 1993;5:1303–1314. doi: 10.1105/tpc.5.10.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer CP, Canny MJ. CO2 storage in Eucalyptus oil glands: a hypothesis disproved. Aust J Plant Physiol. 1975;2:647–658. [Google Scholar]
- Nasrallah JB, Stein JC, Kandasamy MK, Nasrallah ME. Signalling the arrest of pollen tube developments in self-incompatible plants. Science. 1994;266:1505–1508. doi: 10.1126/science.266.5190.1505. [DOI] [PubMed] [Google Scholar]
- Pandey KK. Stigmatic secretion and bud-pollinations in self- and cross-incompatible plants. Naturwissenschaften. 1963;50:408–409. [Google Scholar]
- Preuss D, Lemieux B, Yen G, Davis RW. A conditional mutation eliminates surface components from Arabidopsis pollen and disrupts cell signalling during fertilization. Genes Dev. 1993;7:974–985. doi: 10.1101/gad.7.6.974. [DOI] [PubMed] [Google Scholar]
- Pruitt RE. Molecular mechanics of smart stigmas. Trends Plant Sci. 1997;2:328–329. [Google Scholar]
- Read SM, Clarke AE, Basic A. Stimulation of growth of cultured Nicotiana tabacum W 38 pollen tubes by poly(ethylene glycol) and Cu(II) salts. Protoplasma. 1993;177:1–14. [Google Scholar]
- Reger BJ, Chaubal R, Pressey R. Chemotropic responses by pearl millet pollen tubes. Sex Plant Reprod. 1992;5:47–56. [Google Scholar]
- Rosen WG (1971) Pistil pollen interactions in Lilium. In J Heslop-Harrison, ed, Pollen: Development and Physiology. Butterworths, London, pp 239–254
- Sommer-Knudsen J, Lush WM, Clarke AE, Basic A. Reevaluation of the role of a transmitting tract-specific glycoprotein on pollen tube growth. Plant J. 1998;13:529–535. [Google Scholar]
- Stephen H, Stephen T (Eds) (1963) Solubilities of Inorganic and Organic Compounds, Vol 1: Binary Systems, Part 2. Pergamon Press, Oxford, UK
- Takano M, Takahashi H, Hirasawa T, Suge H. Hydrotropism in roots: sensing of a gradient in water potential by the root cap. Planta. 1995;197:410–413. [Google Scholar]
- Takano M, Takahashi H, Hirasawa T, Suge H. Calcium requirement for the induction of hydrotropism and enhancement of calcium-induced curvature by water stress in primary roots of pea, Pisum sativum L. Plant Cell Physiol. 1997;38:385–391. [Google Scholar]
- Tsao T-H. A study of chemotropisms of pollen tubes in vitro. Plant Physiol. 1949;24:494–504. doi: 10.1104/pp.24.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C. Lipids are required for directional pollen tube growth. Nature. 1998;392:819–821. doi: 10.1038/33929. [DOI] [PubMed] [Google Scholar]