Abstract
It has long been argued that cell cycle regulators such as cyclins, cyclin-dependent kinases and their inhibitors affect the fate of neuronal progenitor cells. Recently, we identified that cyclin D2, which localizes at the basal tip of the radial glial cell (i.e., the neural progenitor in the developing neocortex), functions to give differential cell fates to its daughter cells just after cell division. This basally biased localization is due to transportation of cyclin D2 mRNA via its unique cis-regulatory sequence and local translation into cyclin D2 protein at the basal endfoot. During division of the neural progenitor cells, cyclin D2 protein is inherited by the daughter cell that retain the basal process, resulting in asymmetric distribution of cyclin D2 protein between the two daughter cells. Cyclin D2 is similarly localized in the human fetal cortical primordium, suggesting a common mechanism for the maintenance of neural progenitors and a possible scenario in evolution of primate brains. Here we introduce our recent findings and discuss how cyclin D2 functions in mammalian brain development and evolution.
Keywords: asymmetric cell division, cyclin D2, localization, mRNA, post-transcriptional regulation, radial glia
Introduction
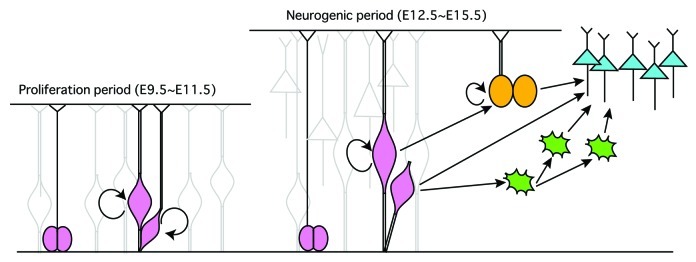
Mammalian brains are characterized by a large neocortex containing numerous neurons and glia generated from neural stem/progenitor cells (NSPCs) situated in the inner wall of the neural tube termed the ventricular zone (VZ). During cortical development, NSPCs are highly polarized stretching to both the ventricular (apical) surface and the pial (basal) surface of the cortical primordium. NSPCs initially divide symmetrically to increase their numbers from embryonic day nine (E9) to E11 in mice (the proliferative period).1,2 As development proceeds to the neurogenic period (starting around E11 in mice), NSPCs become longer and thinner to form “radial glia (RG)” as they support radial migration of cortical neurons.3,4 The RG cells divide asymmetrically and produce one apical progenitor cell (AP) and one neuronal cell or intermediate progenitor cell (IP).3,5 APs continue to divide asymmetrically, thereby increasing the number of neuronal cells while maintaining the number of APs. Newly produced neurons migrate out of the VZ to form the cortical plate (CP), while IPs divide symmetrically in the upper region of the VZ (i.e., the subventricular zone; SVZ) and generate a pair of IPs or neurons1,6,7 (Fig. 1). Asymmetric cell division of NSPCs is critical for establishing the architecture of the mammalian cerebral cortex.3,8 During the asymmetric division of APs, cell structures such as the basal process and apical membrane are inherited by one of the two daughter cells, and it is proposed that these components may function as cell fate determinants.4,9,10 Many molecules are localized in the apical region and affect cell fates (e.g., numb, prominin1, Par complex proteins).10-13 However, relatively little information is available about molecules in the basal side.
Figure 1. Schematic depiction of cortical development in mammals. At the early stage of corticogenesis (E9.5~11.5), neuroepithelial cells divide symmetrically to yield more progenitors, resulting in a thickened pseudo-stratified sheet where the mitotic cells are concentrated mainly on the apical side of the epithelium (the proliferation period). Later in corticogenesis (E12.5~15.5), neuroepithelial cells become long and thin radial glia (RG) and start to divide asymmetrically (the neurogenic period). Radial glia produce apical progenitors (APs) with self-renewing properties together with a terminally-differentiated neurons (blue) or intermediate progenitors (IPs, green), or outer radial glia (oRGs, yellow).
Cyclin D2 and Mammalian Brain Development
Mouse cyclin D2 was first identified in a screen for delayed early response genes induced by colony-stimulating factor 1, and recognized as a member of a family that include at least two other related genes, cyclin D1 and D3.14 Cyclin D2 protein forms a complex with cyclin-dependent kinases (Cdk) 4 or 6 and translocates to the nucleus where the tumor suppressor protein Rb is phosphorylated to activate transcriptional factor E2F. This cascade of events progresses the cell cycle from G1- to S-phase.15,16
It is well known that neuronal progenitor cell fate can be affected by cell cycle regulators including cyclins,17-19 Cdks20 and their inhibitors.21-24 In the developing central nervous system (CNS), mRNA of cyclin D2 shows a unique localization, to the surface of the neural tube, not seen for other Cyclins.25,26 Because of this unique localization pattern, cyclin D2 was initially thought to be expressed in post-mitotic neurons25,26 but recent work identified that the mRNA and protein localized at the tip of the AP (i.e., endfoot).19,27 As with other cyclins, cyclin D2 is also localized at the nucleus of mitotic cells in the VZ and SVZ, and was assumed to have a function in cell cycle progression.27 In cyclin D2 knockout mice, the brain size is smaller and adult neurogenesis is dramatically impaired.28-31 Cyclin D2 is essential for expansion of the NSPCs in both embryonic and adult brains, but what is the significance of the biased localization of cyclin D2 in the basal endfoot of the APs?
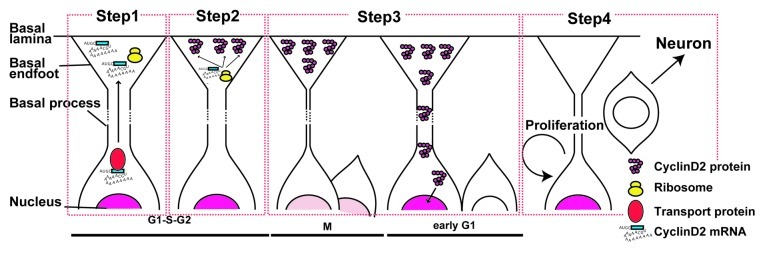
We have recently shown that overexpression of cyclin D2 increases the population of APs, while the loss of cyclin D2 function increases the neuronal population.19 This indicates that cyclin D2 being localized to the endfoot of APs is an example of a “basal fate determinant.” This is unique in that the mechanism for fate determination of APs is at the subcellular level (Fig. 2). Cyclin D2 mRNA is continuously transferred toward the basal side up to the endfoot via its unique 50-bp cis-element (Step 1), and is locally translated into the protein (Step 2). During asymmetric cell division, one of the daughter cells inherits its basal process, which automatically leads to asymmetrical inheritance of cyclin D2 protein between the daughter cells (Step 3). The daughter cell with cyclin D2 will become an AP, and the other without cyclin D2 will become a neuronal cell or an IP (Step 4).
Figure 2. Schematic depiction of cyclin D2 mRNA and protein dynamics during the cell cycle and its putative role as a fate determinant. Pink in the nucleus indicates cyclin D2 protein. (Step 1) Cyclin D2 mRNA is transported to the basal endfoot during G1, S- to G2-phase due to the cis-transport element that resides in the 3'UTR region of cyclin D2 mRNA (blue box in mRNA) together with the transportation machinery that recognizes the cis-element (red circle). (Step 2) Transported mRNA is locally translated into protein via ribosomes localized at the basal endfoot. (Step 3) During mitosis, cyclin D2 protein is inherited by one of the daughter cells with its basal process. In early G1-phase inherited Cyclin D2 creates clear asymmetry of the cyclin D2 protein level between two daughter cells. (Step 4) The daughter cell that has inherited cyclin D2 with the basal process remains as a progenitor, whereas the other daughter without the basal process proceeds differentiation.
Although we showed that cyclin D2 affects the fate of APs, the exact molecular mechanism is still unknown. A correlation between G1-phase lengthening and neurogenesis has been noted32-37 (data controversial to this has recently been reported, though)38 If the lengthening of G1-phase causes neuronal differentiation, the biased localization of cyclin D2 will provide a shorter G1-phase to the daughter cell that inherits the basal process which in turn biases the fate of that daughter cell to a progenitor. Although this is an interesting scenario, time-lapse studies using slice culture suggest that inheritance of the basal process does not always lengthen the total cell cycle compared with the other daughter cell39,40 (personal communication with Dr. Matsuzaki). Another model could be that cyclin D2 controls cell fate in a manner other than controlling the cell cycle itself. For example, cyclin D2 is known to have a function in exporting the Cdk inhibitor p27(kip1) out of the nucleus, thereby promoting degradation.41,42 Since p27(kip1) promotes neurogenesis and radial migration of postmitotic neurons,21,22 inherited cyclin D2 may inhibit neurogenesis and promote cell proliferation19 via a p27(kip1)-dependent mechanism. There are many other reports showing that cell cycle regulators may function as cell fate determinants by a role independent of cell cycle regulation.20,21,43,44 Furthermore, another detailed analysis suggests that not only G1-phase but also S-phase is correlated with the differentiation state of NSPCs.38 Thus, the physiological functions of cyclin D2 in aspects of fate determination in vivo still remain to be elucidated.
Cyclin D2 and Brain Evolution
As described above, we have reported a new physiological function of cyclin D2 in the neuronal development of the mouse. The next question we focused on was “Is this mechanism conserved among species?”. In humans, we found an accumulation of cyclin D2 protein at the basal side of the cortical primordium at gestation week 16.19 We also noted that the cis-acting element we identified in mice for basal transportation is highly conserved in human (74% match in NCBI database). Therefore, it is probable in the human cortical primordium that cyclin D2 mRNA transported within the basal process toward the basal endfoot and locally translated into protein in a similar manner. It is of note that the basal transport cis-element we have identified appears to be unique to mammals, as similar sequences are not found in avians nor amphibians (NCBI database). Indeed, accumulation of cyclin D2 mRNA in the basal side of the chick forebrain is not observed (unpublished results). Acquisition of the genomic DNA sequences corresponding to the basal transportation regulatory element in the 3'UTR of cyclin D2 mRNA might be a critical diversification point in vertebrate brain evolution.
Recent progress in live imaging studies has revealed a new population of proliferative progenitors that have basal processes but no apical processes. These neural progenitor cells locate in the outer subventricular zone (OSVZ) of the fetal cortex of human and ferret, and are thus called OSVZ radial glia-like cells (oRG).45-47 Originally, oRG is believed to exist only in primates or gyrencephalic mammals, several groups have recently reported that there is a population of oRGs also in non-gyrencephalic mammals, including mice and marmosets.39,40,48 In the human fetal cortex, oRGs show a clear correlation between Hes1 expression and basal process inheritance.46,47 This indicates that the basal process may be required for receiving the Notch signal, a pivotal mechanism in maintaining the progenitor state. Furthermore, the basal process is reported to receive from the meninges a retinoic acid signal that controls the proliferation of the progenitor cells.49 Even though it is obvious that there is a clear relationship between brain size and percentage of oRGs out of all proliferating progenitors,50 more studies are required to understand the physiological significance of oRGs. Interestingly, cyclin D2-positive cells are observed in the OSVZ, and the dotted staining of cyclin D2 is frequently seen in the basal side but not in the apical side19 making it likely that these cells are oRGs.
In the mouse, cyclin D2 is also expressed in IPs and is shown to be required for their proliferation.27 This is further confirmed by our group; gain or loss of cyclin D2 function experiments drastically increased or decreased, respectively, the population of Tbr2-positive IPs in SVZ.19 Therefore, cyclin D2 is very important for proliferation not only of APs, but also of IPs. Taken together, it is reasonable to assume that cyclin D2 has proliferative activity also in oRGs.
In sum, cyclin D2 function is generally required for proliferation of various neural progenitors, i.e., APs, IPs and probably oRGs. There is little doubt that prolonged maintenance and the massive proliferation of NSPCs are essential in brain evolution, and that cyclin D2 is a key player.
Concluding Remarks
Asymmetric inheritance of cyclin D2 in dividing daughter cells of APs is the first description of a post-transcriptional, regulatory mechanism in the developing vertebrate CNS. This unique mechanism comes from the shape of the AP, which is highly polarized and has a long basal process. Although cell cycle regulators such as cyclins are one of the most well studied molecules, there is still little information about the molecular dynamics in vivo. There are many questions that remain to be elucidated about the physiological functions of cyclin D2 in the developing CNS. This is a first step toward the next cycle of research in cortical development and evolution.
Acknowledgments
We thank Dr. Fumio Matsuzaki and Dr. Tommy Lewis for critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21500
References
- 1.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, et al. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–50. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 4.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 5.Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17:648–57. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–45. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- 7.Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 8.Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/S0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 9.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–41. doi: 10.1016/S0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 10.Kosodo Y, Röper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–24. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa MR, Wen G, Lepier A, Schroeder T, Götz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- 13.Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development. 2002;129:4843–53. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- 14.Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–13. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 16.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–5. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 17.Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5:320–31. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Pilaz LJ, Patti D, Marcy G, Ollier E, Pfister S, Douglas RJ, et al. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci USA. 2009;106:21924–9. doi: 10.1073/pnas.0909894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsunekawa Y, Britto JM, Takahashi M, Polleux F, Tan SS, Osumi N. Cyclin D2 in the basal process of neural progenitors is linked to non-equivalent cell fates. EMBO J. 2012;31:1879–92. doi: 10.1038/emboj.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali F, Hindley C, McDowell G, Deibler R, Jones A, Kirschner M, et al. Cell cycle-regulated multi-site phosphorylation of Neurogenin 2 coordinates cell cycling with differentiation during neurogenesis. Development. 2011;138:4267–77. doi: 10.1242/dev.067900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, et al. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–24. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen L, Besson A, Roberts JM, Guillemot F. Coupling cell cycle exit, neuronal differentiation and migration in cortical neurogenesis. Cell Cycle. 2006;5:2314–8. doi: 10.4161/cc.5.20.3381. [DOI] [PubMed] [Google Scholar]
- 23.Vernon AE, Movassagh M, Horan I, Wise H, Ohnuma S, Philpott A. Notch targets the Cdk inhibitor Xic1 to regulate differentiation but not the cell cycle in neurons. EMBO Rep. 2006;7:643–8. doi: 10.1038/sj.embor.7400691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mairet-Coello G, Tury A, Van Buskirk E, Robinson K, Genestine M, DiCicco-Bloom E. p57(KIP2) regulates radial glia and intermediate precursor cell cycle dynamics and lower layer neurogenesis in developing cerebral cortex. Development. 2012;139:475–87. doi: 10.1242/dev.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross ME, Risken M. MN20, a D2 cyclin found in brain, is implicated in neural differentiation. J Neurosci. 1994;14:6384–91. doi: 10.1523/JNEUROSCI.14-11-06384.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross ME, Carter ML, Lee JH. MN20, a D2 cyclin, is transiently expressed in selected neural populations during embryogenesis. J Neurosci. 1996;16:210–9. doi: 10.1523/JNEUROSCI.16-01-00210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glickstein SB, Alexander S, Ross ME. Differences in cyclin D2 and D1 protein expression distinguish forebrain progenitor subsets. Cereb Cortex. 2007;17:632–42. doi: 10.1093/cercor/bhk008. [DOI] [PubMed] [Google Scholar]
- 28.Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, Jaworski J, et al. The critical role of cyclin D2 in adult neurogenesis. J Cell Biol. 2004;167:209–13. doi: 10.1083/jcb.200404181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jedynak P, Jaholkowski P, Wozniak G, Sandi C, Kaczmarek L, Filipkowski RK. Lack of cyclin D2 impairing adult brain neurogenesis alters hippocampal-dependent behavioral tasks without reducing learning ability. Behav Brain Res. 2012;227:159–66. doi: 10.1016/j.bbr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto Y, Tsunekawa Y, Nomura T, Suto F, Matsumata M, Tsuchiya S, et al. Differential proliferation rhythm of neural progenitor and oligodendrocyte precursor cells in the young adult hippocampus. PLoS One. 2011;6:e27628. doi: 10.1371/journal.pone.0027628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glickstein SB, Monaghan JA, Koeller HB, Jones TK, Ross ME. Cyclin D2 is critical for intermediate progenitor cell proliferation in the embryonic cortex. J Neurosci. 2009;29:9614–24. doi: 10.1523/JNEUROSCI.2284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi T, Nowakowski RS, Caviness VS., Jr. Mode of cell proliferation in the developing mouse neocortex. Proc Natl Acad Sci USA. 1994;91:375–9. doi: 10.1073/pnas.91.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T, Nowakowski RS, Caviness VS., Jr. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–57. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci. 2003;116:4947–55. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- 35.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–50. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 36.Lange C, Calegari F. Cdks and cyclins link G1 length and differentiation of embryonic, neural and hematopoietic stem cells. Cell Cycle. 2010;9:1893–900. doi: 10.4161/cc.9.10.11598. [DOI] [PubMed] [Google Scholar]
- 37.Salomoni P, Calegari F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 2010;20:233–43. doi: 10.1016/j.tcb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Arai Y, Pulvers JN, Haffner C, Schilling B, Nüsslein I, Calegari F, et al. Neural stem and progenitor cells shorten S-phase on commitment to neuron production. Nat Commun. 2011;2:154. doi: 10.1038/ncomms1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci. 2011;31:3683–95. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Tsai JW, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–61. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susaki E, Nakayama K, Nakayama KI. Cyclin D2 translocates p27 out of the nucleus and promotes its degradation at the G0-G1 transition. Mol Cell Biol. 2007;27:4626–40. doi: 10.1128/MCB.00862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Susaki E, Nakayama KI. Multiple mechanisms for p27(Kip1) translocation and degradation. Cell Cycle. 2007;6:3015–20. doi: 10.4161/cc.6.24.5087. [DOI] [PubMed] [Google Scholar]
- 43.Ratineau C, Petry MW, Mutoh H, Leiter AB. Cyclin D1 represses the basic helix-loop-helix transcription factor, BETA2/NeuroD. J Biol Chem. 2002;277:8847–53. doi: 10.1074/jbc.M110747200. [DOI] [PubMed] [Google Scholar]
- 44.Bienvenu F, Jirawatnotai S, Elias JE, Meyer CA, Mizeracka K, Marson A, et al. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature. 2010;463:374–8. doi: 10.1038/nature08684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fietz SA, Kelava I, Vogt J, Wilsch-Bräuninger M, Stenzel D, Fish JL, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–9. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 46.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–61. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 47.Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.García-Moreno F, Vasistha NA, Trevia N, Bourne JA, Molnár Z. Compartmentalization of cerebral cortical germinal zones in a lissencephalic primate and gyrencephalic rodent. Cereb Cortex. 2012;22:482–92. doi: 10.1093/cercor/bhr312. [DOI] [PubMed] [Google Scholar]
- 49.Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reillo I, de Juan Romero C, García-Cabezas MA, Borrell V. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex. 2011;21:1674–94. doi: 10.1093/cercor/bhq238. [DOI] [PubMed] [Google Scholar]