Abstract
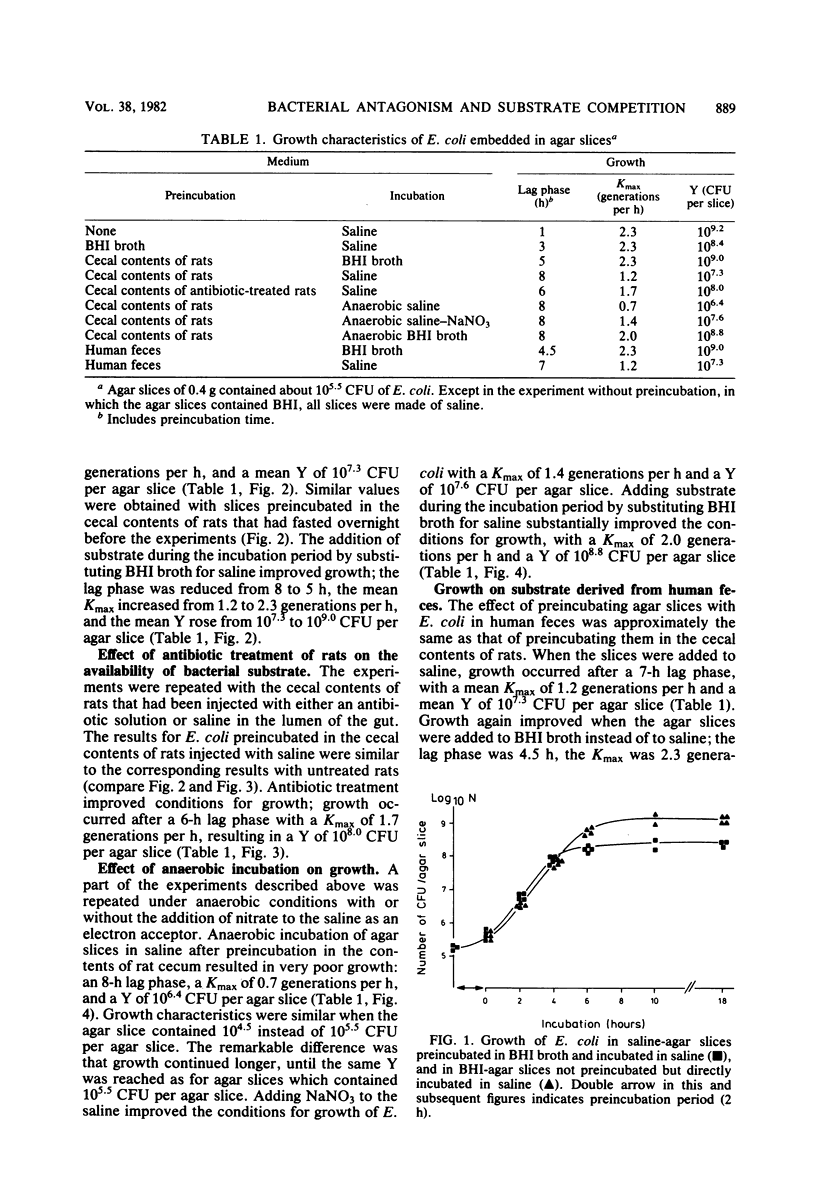
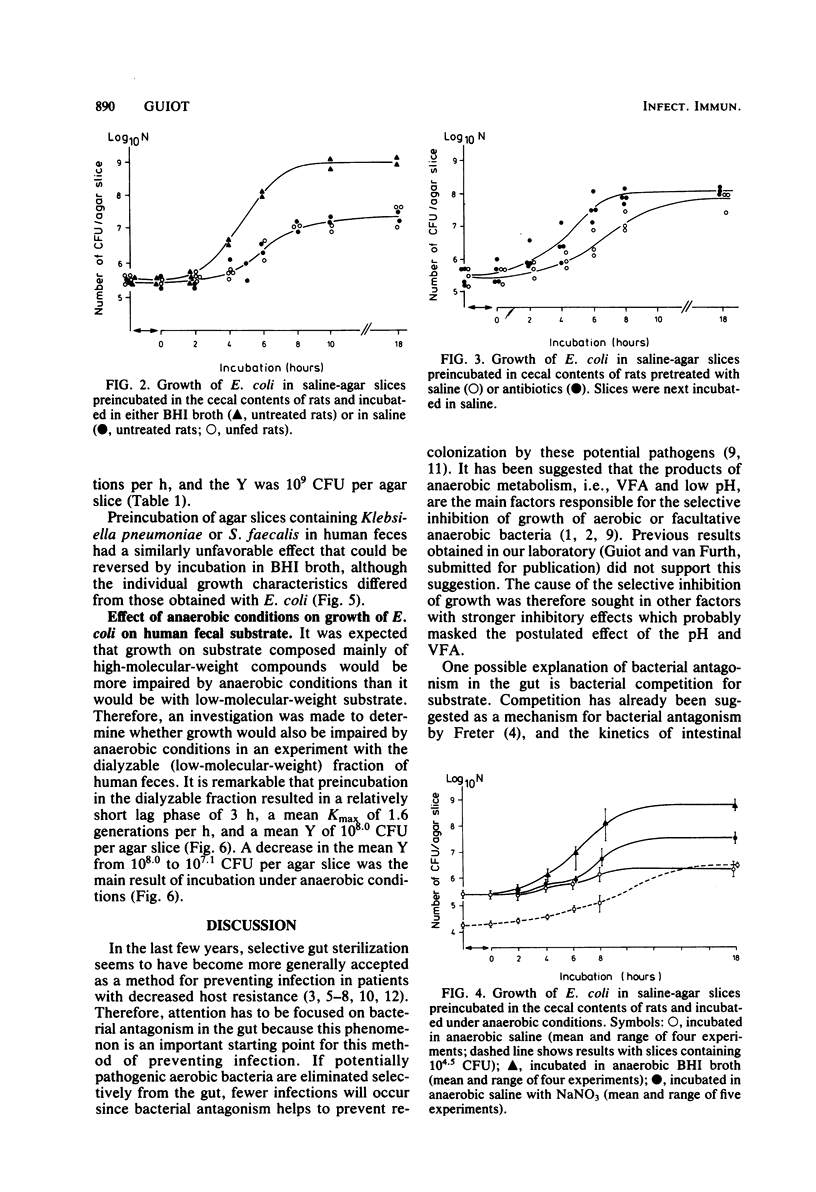
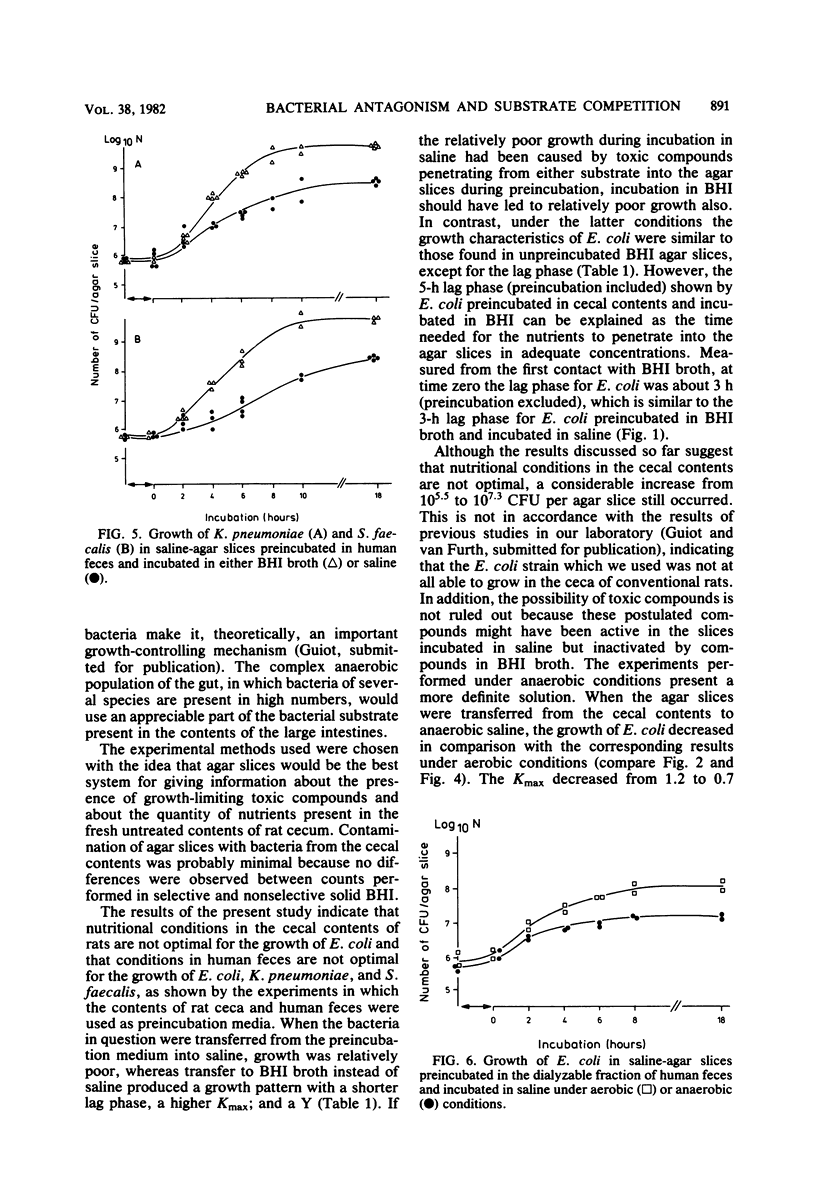
Small slices of agar, each containing about 10(5.5) Escherichia coli organisms, were preincubated either in the contents of rat cecum or in brain heart infusion broth for 2 h and then were transferred to a small sample of saline. The purpose of these experiments was to examine the ability of E. coli to grow on the substrate that penetrated from the contents of rat cecum into the agar slices. It appeared that preincubation in brain heart infusion broth gave rise to abundant growth, whereas only poor growth occurred after preincubation in the contents of rat cecum. This poor growth was completely reversed by adding brain heart infusion. The same experiments were repeated under anaerobic conditions, in which growth on substrate that penetrated from the contents of rat cecum into the agar slices was extremely poor. This extremely poor growth under anaerobic conditions was reversed to abundant growth by adding brain heart infusion broth. The addition of nitrate as an electron acceptor also stimulated growth of E. coli. Similar results were obtained with other bacteria and with human feces. The results can be interpreted as a demonstration that under anaerobic conditions such as occur in large intestines, bacterial antagonism is caused in a high degree by competition for substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. I. FACTORS WHICH INTERFERE WITH THE INITIATION OF INFECTION BY ORAL INOCULATION. J Exp Med. 1964 Nov 1;120:805–816. doi: 10.1084/jem.120.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B. M., Dankert J. Volatile fatty acids and aerobic flora in the gastrointestinal tract of mice under various conditions. Infect Immun. 1979 Mar;23(3):559–563. doi: 10.1128/iai.23.3.559-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker A. W., Rozenberg-Arska M., Sixma J. J., Verhoef J. Prevention of infection by trimethoprim-sulfamethoxazole plus amphotericin B in patients with acute nonlymphocytic leukaemia. Ann Intern Med. 1981 Nov;95(5):555–559. doi: 10.7326/0003-4819-95-5-555. [DOI] [PubMed] [Google Scholar]
- FRETER R. In vivo and in vitro antagonism of intestinal bacteria against Shigellaflexneri. II. The inhibitory mechanism. J Infect Dis. 1962 Jan-Feb;110:38–46. doi: 10.1093/infdis/110.1.38. [DOI] [PubMed] [Google Scholar]
- Guiot H. F., Furth R. Partial antibiotic decontamination. Br Med J. 1977 Mar 26;1(6064):800–802. doi: 10.1136/bmj.1.6064.798-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiot H. F., van der Meer J. W., van Furth R. Selective antimicrobial modulation of human microbial flora: infection prevention in patients with decreased host defense mechanisms by selective elimination of potentially pathogenic bacteria. J Infect Dis. 1981 May;143(5):644–654. doi: 10.1093/infdis/143.5.644. [DOI] [PubMed] [Google Scholar]
- Gurwith M. J., Brunton J. L., Lank B. A., Harding G. K., Ronald A. R. A prospective controlled investigation of prophylactic trimethoprim/sulfamethoxazole in hospitalized granulocytopenic patients. Am J Med. 1979 Feb;66(2):248–256. doi: 10.1016/0002-9343(79)90539-4. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- Sleijfer D. T., Mulder N. H., de Vries-Hospers H. G., Fidler V., Nieweg H. O., van der Waaij D., van Saene H. K. Infection prevention in granulocytopenic patients by selective decontamination of the digestive tract. Eur J Cancer. 1980 Jun;16(6):859–869. doi: 10.1016/0014-2964(80)90140-1. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Hargadon M. T., Fortner C. L., Young V. M., Wiernik P. H. A comparison of trimethoprim-sulfamethoxazole plus nystatin with gentamicin plus nystatin in the prevention of infections in acute leukemia. N Engl J Med. 1981 Apr 30;304(18):1057–1062. doi: 10.1056/NEJM198104303041801. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis J. M. Determination of the colonization resistance of the digestive tract of individual mice. J Hyg (Lond) 1974 Jun;72(3):379–387. doi: 10.1017/s0022172400023615. [DOI] [PMC free article] [PubMed] [Google Scholar]


