Abstract
Individuals with Parkinson’s disease (PD) typically have difficulty rising from a chair. A major contributing factor may be altered anticipatory postural control; this hypothesis has been fueled by reports of altered function of the supplementary motor area in PD, an area linked to the preparation of movements. This study tested the hypothesis that individuals with PD would exhibit altered anticipatory postural control which would include a reduced preparatory hip flexion and decreased forward displacement of the COM prior to lift-off of the buttocks from the chair. Ten male subjects with PD and 10 male age-matched controls were instructed to rise from a chair without the use of their arms at their comfortable pace on two separated days during on and off-medication states. Body movements were recorded with an optoelectronic device, in addition to forces under the buttocks and each foot to calculate lower extremity joint angles, joint moments and net body centre of mass displacement (COM). The sit-to-stand (STS) duration was the same for the PD-on and controls, but greater for the PD-off group. The PD groups (on and off) used a hip flexion strategy (greater preparatory hip flexion displacement and forward COM displacement, reduced knee extensor moments) compared to the controls. Contrary to predictions, subjects with PD exaggerated, rather than reduced, the movement preparation of the STS using a hip flexion strategy. Possible underlying causes of this flexion strategy could include compensation for poor lower extremity muscle strength and a need for greater postural stability during the lift-off phase.
Keywords: Parkinson’s disease, postural control, basal ganglia, movement
Introduction
Rising from a chair is a daily function required for independent living. This task is particularly difficult for elderly individuals with musculoskeletal or neurological disorders. In a survey of 379 elderly persons with a diagnosis of arthritis, Parkinson’s disease (PD), multiple sclerosis, and other neurological disorders, 42% reported having difficulty with rising from a chair in the home (Munton et al., 1981). Eighty-one percent of the 101 individuals with PD surveyed in a study reported having difficulty with rising from a chair (Brod et al. 1998).
A recent analysis of sit-to-stand in healthy adults found that the task involves a distinct anticipatory postural component to move the centre of mass (COM) over the base of support, prior to a centrally programmed sequence of events to raise the body from the chair (Goulart and Valls-Sole 1999). Anticipatory postural control is the activation of postural adjustments before voluntary movement to minimize potential disturbances to balance that the movement may cause (Shumway-Cook and Woollacott, 1995). It has been speculated that the inability to rise from a chair in persons with PD may be a result of altered anticipatory postural control and consequently a failure to bring the COM adequately forward over the feet prior to the lift-off of the buttocks from the chair (Morris 2000; Morris et al. 1997). Such hypotheses have been fueled by reports of altered anticipatory postural control during tasks such as arm movements while standing in PD subjects (Latash et al. 1994; Rogers et al. 1987; Traub et al. 1980), in addition to altered supplementary motor area function in PD, which has been linked to the performance of preparatory postural control during tasks such as finger movements (Cunnington et al. 1996). However, no study has yet quantified the movement strategies used by individuals with PD to rise from a chair.
The purpose of this study was to describe the movement strategies underlying the ability of persons with PD to perform the STS. The kinematic and kinetic profiles underlying the movement to rise from a chair were compared among three groups: 1) individuals with PD in an “on-medication state” (PD-on), 2) in an “off-medication state (PD-off) and 2) age-matched controls. We hypothesized that individuals with PD would exhibit altered anticipatory postural control which would include a reduced preparatory hip flexion and decreased forward displacement of the COM prior to lift-off of the buttocks from the chair.
Methods
Subjects
Ten male subjects with PD (mean ± 1 standard deviation; age=64.1 ± 10.1 years; mass=84.0 ± 4.5 kg; height=173.9 ± 5.7 cm; time since diagnosis=4 ± 1.5 years) were recruited from local PD support organizations and medical clinics. Male subjects were selected as the incidence of PD in males has been reported to be as much as twice as in females (Twelves et al. 2003). Ten male age-matched control subjects (age=65.5 ± 12.4 years; mass=80.1 ± 9.7 kg; height=176.5 ± 8.7 cm) were recruited from the community. Inclusion criteria for subjects with PD included 1) clinical diagnosis of PD for a minimum of one year, 2) ability to rise from a chair, without the use of armrests in an off-medication state, and 3) no other neurological, orthopaedic, or cardiovascular condition(s) which could affect their ability to perform the STS task. The PD subjects fulfilled published criteria for clinically definite Parkinson’s disease (Calne et al. 1992) and other forms of Parkinsonism had been excluded. In addition, subjects with PD were all responsive to levodopa (mean daily dose = 440 mg CR levadopa and 115 mg carbidopa). Four subjects also used Ropinirole (mean daily dose = 4.8 mg) and one subject used Bromocroptine (mean daily dose = 3.75 mg). All subjects were informed of the research procedures before they gave written consent. The experimental protocol was approved by the local university and hospital ethics committees.
Protocol
Subjects with mild PD were tested on two different days and were randomly assigned to commence the first testing in either an on-medication (PD-on) or off-medication state (PD-off). On both days, the motor section of the UPDRS (Fahn et al. 1997) and Hoehn and Yahr scale (Hoehn and Yahr 1967) were assessed. Testing commenced between 8:00 a.m. and 10:00 a.m. and required an overnight withdrawal of medications for the off-state testing (CAPIT guidelines) (Langston et al. 1992). The subjects with mild PD confirmed that the on-state testing day was typical of their usual “on” functioning state. Control subjects were tested during a single morning test session. All subjects were asked to recall their physical activities and the time spent with each activity over the last week.
Subjects sat on an armless, backless, knee height chair instrumented with a six component forceplate (Bertec Corp, Columbus, OH) under the buttocks and two forceplates under each foot. The chair height was adjusted for each subject to allow for a 90° angle at the knee during sitting. The start position for each subject was with the feet 20 cm apart and with thigh support so that the distance between the anterior edge of the chair and the most anterior point of the patella was 20 cm. Subjects performed 5 sit-to-stand trials at their own pace (self-paced trials) on each day without the use of their arms. Two subjects required up to two additional trials to correct for obstructed body markers.
Infrared emitting markers were collected using an Optotrak (Northern Digital) imaging system to track bilateral fifth metatarsals, lateral malleolli, lateral knee joints, greater trochanters, iliac crests, clavicles and ears in the sagittal plane. Six seconds of Optotrak data were collected at a sampling rate of 60 Hz and synchronized to the forceplates, which were collected at 600 Hz. The sampling rates were selected to ensure adherence to the Nyquist Sampling Theorem (Winter and Patla, 1997).
Data reduction
Data were analyzed for five self-paced trials for PD-on, PD-off, and for controls. The first five trials that did not have any obstructed markers were used for the analysis. The dominant side (as determined by self-report) of the controls was used to compare to the most affected side (as determined by self-report) of the subjects with PD. Seven of the ten PD subjects were most affected on their dominant side.
Marker data and forceplate data were low pass filtered at 6 Hz and 50 Hz, respectively. Relative joint angles of the hip, knee and ankle were calculated from the markers. Net body centre of mass was also calculated from the markers, in conjunction with anthropometric data (Eng and Winter 1993). Synchronized segment kinematics and forceplate data were used to calculate net joint moments at the ankle, knee, and hip, using an inverse dynamic model (Eng and Winter 1995; Winter 1990) commencing at the foot segment. Since only one forceplate was used under the buttocks, the reaction force under the buttocks was resolved into a left and right component (reflecting the left and right buttock/thigh, respectively). The reaction force was partitioned to each side based on the frame-by-frame location of the medial-lateral centre of pressure measure relative to the left and right greater trochanter markers (i.e., if the centre of pressure was mid-way between the left and right greater trochanters, the reaction force would be divided in half for each buttock) (Equation 1 and 2).
| Equation 1 |
| Equation 2 |
Where FRb=right buttock reaction force, FLb=left buttock reaction force, F=total reaction force under the buttocks, GTL=left greater trochanter medial-lateral position, GTR=right greater trochanter medial-lateral position, and COP=medial-lateral centre of pressure under the buttocks.
Movement onset was identified from the forceplate under the buttocks as the initial increase in horizontal force beyond the quiet sitting baseline force level. Movement termination was identified as the point in time when the vertical movement of the right ear marker reached a plateau. The duration of the task was defined as the number of seconds to complete one STS maneuver. The STS was divided into two phases. The point at which the buttocks lift from the chair (lift-off) is a key event because body weight is transferred to the lower extremities and is also the time at which the hip and knee extensor moments peak (Ikeda et al. 1991; Schenkman et al. 1990). The first phase (preparation phase) was identified as movement onset to lift-off and the second phase (lift-off phase) from lift-off to movement termination with each phase comprising approximately half of the STS cycle (Table 1). Joint angles and moments were time-normalized and ensemble averaged across five trials (i.e., mean and standard deviation at each percent of the STS cycle) so that 0% represented movement onset, 50% represented lift-off, and 100% represented movement termination. The magnitude of the joint moments were normalized by body mass in kilograms. Net body centre of mass (COM) was derived using the location of the joint markers and anthropometric tables (Winter 1990). The horizontal COM moves continuously forward from onset of the STS movement to the end of the task. The forward COM displacements that occurred during the preparation phase and the lift-off phase were calculated.
Table 1.
Timing of kinematic and kinetic parameters
| Variable | PD-off | PD-on | Control |
|---|---|---|---|
| STS duration (sec) | 1.97 (0.27) | 1.86 (0.37) | 1.89 (0.37)a |
| Preparation phase (% STS) | 46.0 (8.7) | 45.2 (4.2) | 44.4 (6.5) |
| Peak hip flexor moment (%) | 26.3 (2.6) | 27.8 (5.9) | 26.4 (5.7) |
| Peak hip extensor moment (%) | 48.4 (1.9) | 48.0 (2.1) | 49.2 (1.2)b |
| Peak knee extensor moment (%) | 49.4 (1.9) | 49.7 (2.2) | 52.3 (2.6)c |
| Peak ankle dorsiflexor moment (%) | 42.7 (8.4) | 43.4 (9.8) | 43.6 (4.2) |
| Peak hip flexion angle (%) | 44.5 (1.8) | 45.3 (6.3) | 49.9 (8.1)c |
| Peak ankle dorsiflexion angle (%) | 56.7 (3.9) | 57.0 (2.9) | 58.7 (3.9)a |
control group different from the PD-off group, Tukey’s Test, p < 0.05
control group different from the PD-on group, Tukey’s Test, p < 0.05
control group different from the PD-on/PD-off groups, Tukey’s Test, p < 0.05
Statistical analysis
The data were normally distributed as quantified with the Shapiro-Wilks statistic. Paired t-tests were used to determine whether subject characteristics (e.g., mass, activity level) were similar between the PD and control group. Randomized blocked (for subject) ANOVAs were used to compare the three groups (PD-on, PD-off and age-matched controls) for 1) duration of STS, 2) magnitude and timing of peak lower extremity moments and joint angle displacements and 3) magnitude of the COM displacement during the preparation phase and lift-off phase (see Table 1 and 2 for variables). An alpha level of .05 was used to identify significance and Tukey’s Test was used for pair-wise post-hoc analyses among the three groups.
Table 2.
Magnitude of kinematic and kinetic parameters
| Variable | PD-off | PD-on | Control |
|---|---|---|---|
| Peak hip flexor moment (Nm/kg) | 0.26 (0.23) | 0.28 (0.21) | 0.24 (0.09) |
| Peak hip extensor moment (Nm/kg) | 0.40 (0.17) | 0.42 (0.23) | 0.42 (0.22) |
| Peak knee extensor moment (Nm/kg) | 0.94 (0.12) | 1.02 (0.22) | 1.13 (0.20)c |
| Peak ankle dorsiflexor moment (Nm/kg) | 0.11 (0.005) | 0.13 (0.009) | 0.11 (0.005) |
| Hip flexion angular displacement (°) | 23.6 (10.0) | 21.5 (14.0) | 17.0 (8.6)a |
| Hip extension angular displacement (°)* | 60.2 (15.6) | 54.7 (23.3) | 56.5 (15.7)a |
| Knee extension angular displacement (°) | 76.8 (7.1) | 76.7 (6.5) | 75.3 (7.3) |
| Ankle dorsiflexion angular displacement (°) | 7.9 (2.4) | 6.9 (1.2) | 7.5 (3.7) |
| Ankle plantarflexion angular displacement (°) | 12.9 (4.2) | 12.7 (4.3) | 15.0 (2.6)b |
| COM displacement - preparation phase (cm) | 13.6 (1.8) | 14.2 (1.4) | 12.6 (1.9)c |
| COM displacement – lift-off phase (cm) | 11.1 (2.4) | 10.4 (2.2) | 12.5 (1.8)c |
control group different from the PD-off group, Tukey’s Test, p < 0.05
control group different from the PD-on group, Tukey’s Test, p < 0.05
control group different from the PD-on/PD-off groups, Tukey’s Test, p < 0.05
hip motion was between the pelvis and thigh segment (i.e., not trunk and thigh)
Results
Subject characteristics and clinical tests
The modified Hoehn & Yahr scale was a median of 2. The motor UPDRS score (maximum of 108) was a mean of 11 (on-state) and of 17.4 (off-state). Thus, the severity of PD was mild in these individuals likely due to the inclusion criteria which required subjects to be able to rise from a chair in an off-state. There was no difference in the Hoehn & Yahr scale between an on-state and off-state. The presence of mild PD would reduce the possible effects of deconditioning, inactivity, or joint contractures which are often associated with advanced stages of PD.
The bradykinesia component of the UPDRS (items #23,24,25,26,31) was a mean of 4.7 and 7.2 in the on-state and off-state, respectively out of a possible 36. Tremor was 1.5 and 2.6, in the on-state and off-state, respectively out of a possible 28 (item #20 and 21). Rigidity was 2.2 and 3.7, in the on-state and off-state, respectively out of a possible 20 (item #22).
All subjects scored 0 (normal) on item #27 of the UPDRS (rise from a chair with arms folded where 0=normal and 4=unable to arise without help) except for one subject who scored 1 (‘slow, or may need more than one attempt’) as he required more than one attempt to rise from the chair in both an on and off medication state. The number of hours of physical activity (generally moderate activities were reported, e.g., walking, golf, gardening) was similar between groups with the means of 2.7±2.1 and 2.6±1.7 hours per week for the PD and control groups, respectively. There were no differences between the PD and control group for age, activity level, mass, or height.
STS kinematics and kinetics
The kinematic and kinetic variables are presented in Tables 1 and 2. For all three groups (PD-on, PD-off, controls), a peak hip flexor moment at 26–27% of STS resulted in about 20° hip flexion, which was followed by a peak ankle dorsiflexor moment at 45–50% of STS and resulted in 7° dorsiflexion motion. This was followed by a peak knee and hip extensor moment near lift-off (50%), which extended the hip and knee into standing.
Time to rise from a chair
Significant differences were found among the three groups for duration of the STS and the post-hoc analyses found that the duration of the STS for the PD-off was significantly slower than PD-on and controls (Table 1). The duration of the STS for PD-on and the controls was the same (1.86 and 1.89 seconds, respectively).
Timing of the joint moments and angles during the rise from a chair
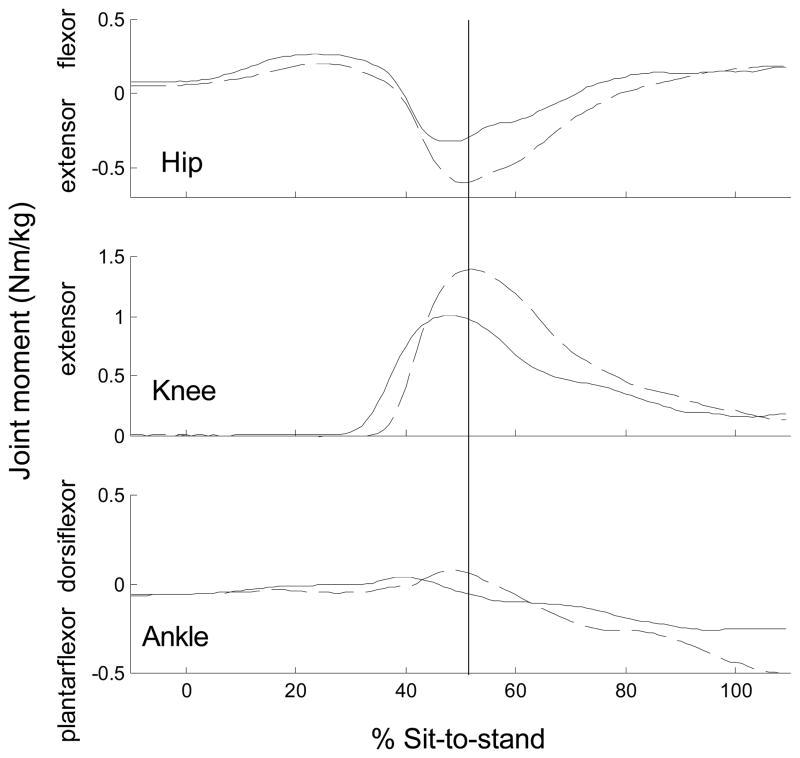
In general, the timing of the joint moments and angular displacements with respect to the lift-off was earlier for the PD-off and PD-on groups compared to the control group as demonstrated by an earlier peak hip and knee extensor moment and earlier peak hip flexion and ankle dorsiflexion angle (Table 1). Of functional importance, the peak knee extensor moment was reached immediately prior to lift-off for subjects with PD (49%), but post lift-off (52%) for controls (i.e., occurred after body weight was transferred to the limbs) (Figure 1). Three PD-off subjects did not have a distinct hip flexor moment peak.
Figure 1.
Typical joints moments during the sit-to-stand. Solid line = PD-on state (ensemble-average of 5 trials for a subject with PD), Dashed line = control subject (ensemble-average of 5 trials for a control subject). 0% = start of sit-to-stand. 100%=end of sit-to-stand. Vertical line=lift-off.
Magnitude of the joint moments and angles
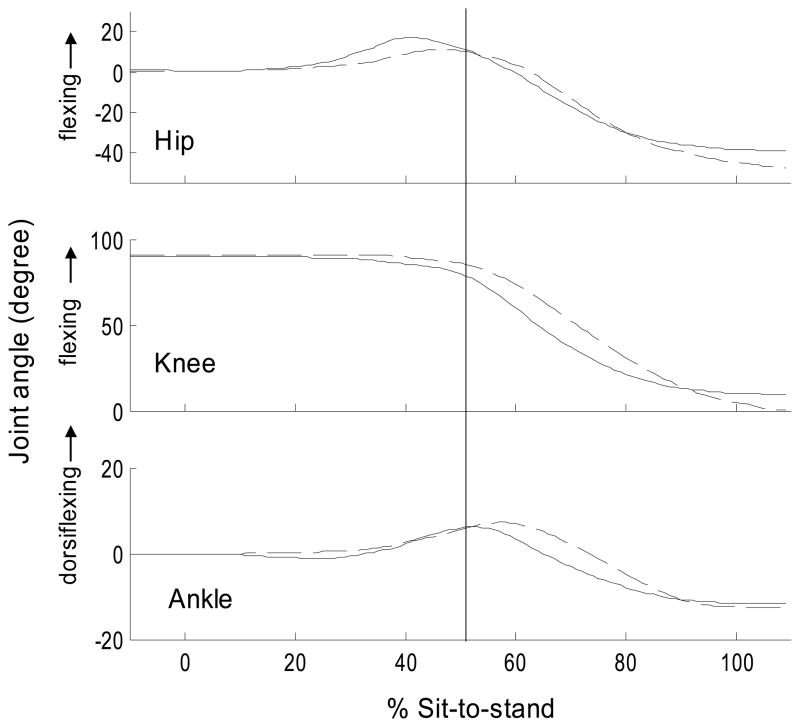
Although the mean hip flexor moment for the PD-on and PD-off groups was greater in magnitude compared to the mean of the control group, these comparisons did not meet statistical significance (p< 0.07). Hip flexion displacement, however, was significantly greater and was 39% and 26% greater in the PD-off and PD-on groups, respectively, compared to the control group (Table 2). The knee extensor moment was 20% and 10% lower in the PD-off and PD-on, respectively, compared to controls, but the hip extensor moment was similar across groups (Table 2). Ankle moment and angle magnitudes were similar across the groups, except for the ankle plantarflexion displacement which was greater in the control compared to PD-on group.
Centre of mass displacement
The PD-off and PD-on groups displaced their net COM 8% and 13% more forward, respectively, compared to the controls during the preparation phase and then 7% and 17% less forward, compared to the controls during the lift-off phase (Table 2). The COM displacement for the controls was similar in magnitude between the preparation and lift-off phase, while the PD groups had greater COM displacement for the preparation compared to lift-off phase.
Discussion
Time to rise from a chair
This study found that the time to rise from a chair was not significantly different between PD-on and controls. Therefore, kinematic and kinetic differences identified between these two groups cannot be attributed to the speed of rising. This is important to note since speed of rising has been reported to be an important factor that influences joint angles and moments used during the STS task (Pai and Rogers 1991).
The finding that the PD-on group did not move slower than controls is likely due to the mild degree of PD in these subjects. The mean Hoehn & Yahr score was only 2.1 out of a maximum of 5. Secondly, the total UPDRS motor score, out of a maximum of 108 points, averaged only 11.0 for PD-on and 17.4 for PD-off.
Altered movement strategy
We had hypothesized that individuals with PD would show a reduced preparatory postural component to move the COM over the base of support prior to the lift-off of the buttocks from the chair. This hypothesis was based on clinical speculation (Morris 2000; Morris et al., 1997), in addition to the literature which has demonstrated impaired anticipatory postural control during arm movements while standing in PD subjects (Latash et al. 1994; Rogers et al. 1987; Traub et al. 1980). Furthermore, several studies have shown altered function of the supplementary motor area in PD due to its indirect connections with the basal ganglia (Cunnington et al. 1996; Grafton et al. 1995). The supplementary motor is one structure thought to have a major role with the preparation of movement (Brinkman 1984; Cunnington et al. 1996) and also specifically with anticipatory postural adjustments (Massion 1992; Massion et al. 1989). However, our results did not support the hypothesis that individuals with PD do not move their COM adequately over the base of support prior to lift-off. Contrary to this hypothesis, we found that these subjects with PD exhibited an exaggerated hip flexion strategy and moved their COM further forward during the preparation phase than the controls. The finding that anticipatory postural control was not lacking may have been partly a result of the mild disease severity (median Hoehn & Yahr score of 2) in this subject group. The mild PD disease state was a result of the protocol which required subjects to complete the STS task. Secondly, we felt that this mild disease state was an advantage as it reduced potential secondary complications (e.g., joint contractures, inactivity, fear of falling) from influencing the task performance. In fact, our protocol did detect differences between the controls and PD-groups, however, the increased preparatory postural component in the PD groups was an unexpected finding.
The hip flexion strategy found in these PD subjects may be a compensation for the early changes within the trunk segment (e.g., greater stiffness and reduced flexibility (Bridgewater and Sharpe 1998)) which precede the characteristic kyphosis of late PD. However, none of these mild PD subjects yet displayed any observable change to their trunk posture and all PD subjects were evaluated as normal on the UPDRS trunk posture subscore.
The hip flexion strategy could potentially compensate for the inability to generate lower extremity muscle force. This strategy places the COM further ahead and has the potential to redistribute the lower extremity moments. Alexander et al. (1997) reported that frail older adults who used a hip strategy had reduced knee and hip extensor moments while Doorenbosch et al. (1994) found that this strategy reduced the knee extensor moment, but increased the hip extensor moment in young adults. We recently reported that individuals with mild PD have substantial hip and knee extensor muscle weakness compared to age-matched controls (Inkster et al. 2003) while others have reported a reduced rate of muscle force generation in PD (Corcos et al. 1996; Stelmach and Worringham 1998). A reduced ability to generate force in PD could originate from both peripheral and central mechanisms. Reports of firing irregularities of single motor units in PD have been attributed to an alteration of central input to the motor neuron pool (Dengler et al. 1990; Glendinning and Enoka 1994). In addition, increased type I fibres and decreased type II fibres have been reported in individuals with PD, suggesting peripheral muscle changes (Edstrom 1970; Rossi et al. 1996) resulting from the disease process or perhaps from impaired mobility and the resulting sedentary lifestyle.
Given that impaired balance is characteristic of individuals with PD, it is also possible that the hip flexion strategy is preferred as it reduces the balance demands by increasing the time that the COM stays within the base of support (Coghlin and McFadyen 1994). Schultz et al. (1992) also suggested that postural stability at lift-off is a major determinant of the ability to rise from a chair and their biomechanical model showed that greater forward movement of the upper body over the feet was one effective method of increasing this stability.
Other key motor characteristics of the STS movement strategy in the PD subjects concur with the hip flexion strategy. The earlier peak knee extensor and hip extensor moment observed in the PD groups may be necessary to augment an upward projection of the body, else the exaggerated hip flexion could potentially result in a fall forward. The lower knee moment found in the PD groups could also be a result of the hip flexion strategy because it has been previously reported that a hip flexion strategy reduces the knee extensor moment, increases activation of the hamstring muscles and reduces activation of the rectus femoris muscles (Doorenbosch et al. 1994).
Clinical implications
Despite quantitative differences between subjects with PD and controls in STS performance, scoring on item 27 of the UPDRS (rise from a chair) was normal for nine of the ten subjects with PD and did not indicate signs of motor impairment. Thus, although these subjects had only mild PD and the UPDRS chair rise task scored normal, a movement analysis detected changes in the time to rise from a chair, the timing and magnitude of the joint moments and angles, and the COM trajectory. One of the problems with the UPDRS is that is relies on gradients of performance that must be visually discriminated. A “slow” rise from a chair is categorized as a grade 1 on item 27 of the UPDRS while a “normal” rise from a chair is categorized as a 0. However, there are many possible subdivisions of “slow” which cannot be accounted for in this UPDRS item. Given that significant changes in STS performance can go undetected using only the UPDRS subscore, clinicians may want to augment their assessment using timed measures (e.g., mean time for five STS movements), as are traditionally used to evaluate gait performance.
Conclusions
It is important to understand why persons with PD have difficulty with rising from a chair in order to develop appropriate rehabilitation interventions. From this study, we believe that these individuals with mild PD demonstrated compensatory motor strategies, possibly due to a reduced ability to generate force in the lower extremities and a need for greater postural stability during the lift-off phase.
Figure 2.
Typical joint angles during the sit-to-stand. Solid line = PD-on state (ensemble-average of 5 trials for a subject with PD), Dashed line = control subject (ensemble-average of 5 trials for a control subject). The identical subjects are displayed in Figure 1. 0% = start of sit-to-stand. 100%=end of sit-to-stand. Vertical line=lift-off. For display purposes, the hip and ankle start joint angle was set to 0° and the knee at 90°. Note the hip motion was between the pelvis and thigh segment (i.e., not trunk and thigh).
Acknowledgments
This study was supported by operating grants from CIHR (MT-14318) and the BC Medical Services Foundation, in addition to salary support to JJE from CIHR and the Michael Smith Foundation for Health Research.
References
- Alexander NB, Schulz AB, Ashton-Miller JA, Gross M, Giordani B. Muscle strength and rising from a chair in older adults. Muscle Nerve Suppl. 1997;5:56–59. [PubMed] [Google Scholar]
- Bridgewater KJ, Sharpe MH. Trunk muscle performance in early Parkinson’s disease. Phys Ther. 1998;78:567–576. doi: 10.1093/ptj/78.6.566. [DOI] [PubMed] [Google Scholar]
- Brinkman C. Supplementary motor area of the monkey’s cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod M, Mendelsohn GA, Roberts B. Patient’s experiences of Parkinson’s disease. J Gerontol B Psychol Sci Soc Sci. 1998;53:213–222. doi: 10.1093/geronb/53b.4.p213. [DOI] [PubMed] [Google Scholar]
- Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32:S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- Coghlin SS, McFadyen BJ. Transfer strategies used to rise from a chair in normal and low back pain subjects. Clin Biomech. 1994;9:85–92. doi: 10.1016/0268-0033(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Chen C-M, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson’s disease: Relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Thickbroom GW, Laing BA, Mastaglia FL, Bradshaw JL, Phillips JG. Effects of magnetic stimulation over supplementary motor area on movement in Parkinson’s disease. Brain. 1996;119:815–822. doi: 10.1093/brain/119.3.815. [DOI] [PubMed] [Google Scholar]
- Dengler R, Konstanzer A, Gillespie J, Argenta M, Wolf W, Struppler A. Behavior of motor units in Parkinsonism. Adv Neurol. 1990;53:167–173. [PubMed] [Google Scholar]
- Doorenbosch CAM, Harlaar J, Roebroeck M, Lankhorst JG. Two strategies of transferring from sit-to-stand; the activation of monoarticular and biarticular muscles. J Biomech. 1994;27:1299–1307. doi: 10.1016/0021-9290(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Edstrom L. Selective changes in the sizes of red and white muscle fibres in upper motor lesions and Parkinsonism. J Neurol Sci. 1970;11:537–550. doi: 10.1016/0022-510x(70)90104-8. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Winter DA. Estimations of the horizontal displacement of the total body centre of mass: Considerations during standing activities. Gait Posture. 1993;1:141–144. [Google Scholar]
- Eng JJ, Winter DA. Kinetic analysis of the lower limbs during walking: What information can be gained from a three-dimensional model? J Biomech. 1995;28:753–758. doi: 10.1016/0021-9290(94)00124-m. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Members of the UPDRS Development Committee. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park: MacMillan Health Care Information; 1997. pp. 153–163.pp. 293–304. [Google Scholar]
- Glendinning DS, Enoka RM. Motor unit behavior in Parkinson’s disease. Phys Ther. 1994;74:61–70. doi: 10.1093/ptj/74.1.61. [DOI] [PubMed] [Google Scholar]
- Goulart FR-P, Valls-Sole J. Patterned electromyographic activity in the sit-to-stand movement. Clin Neurophysiol. 1999;110:1634–1640. doi: 10.1016/s1388-2457(99)00109-1. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Waters C, Sutton J, Lew MF, Couldwell W. Pallidotomy increases activity of motor association cortex in Parkinson’s Disease: A positron emission tomographic study. Ann Neurol. 1995;37:776–783. doi: 10.1002/ana.410370611. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurol. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Ikeda ER, Schenkman ML, Riley PO, Hodge WA. Influence of age on dynamics of rising from a chair. Phys Ther. 1991;71:473–481. doi: 10.1093/ptj/71.6.473. [DOI] [PubMed] [Google Scholar]
- Inkster LM, Eng JJ, MacIntyre DL, Stoessl AJ. Leg muscle strength is reduced in PD and relates to the ability to rise from a chair. Mov Disord. 2003;18:157–162. doi: 10.1002/mds.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantations. Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- Latash ML, Alexander SA, Neyman I, Nicholas JJ. Anticipatory postual adjustments during self inflected and predictable perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1994;58:326–334. doi: 10.1136/jnnp.58.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J, Viallet F, Massarino R, Khalil The supplementary motor area is implicated in the coordination between posture and movement in man. C R Acad Sci III. 1989;308:417–23. [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Morris M. Movement disorders in people with Parkinson’s disease: A model for physical therapy. Phys Ther. 2000;80:578–597. [PubMed] [Google Scholar]
- Morris M, Bruce M, Smithson F, Collier J, Gardiner R, Dodd K, Bond J, Huxham F. Physiotherapy Strategies for People with Parkinson’s Disease. In: Morris ME, Iansek R, editors. Parkinson’s Disease: A Team Approach. Southern Health; Cheltenham: 1997. pp. 42–4. [Google Scholar]
- Munton JS, Ellis MI, Chamberlain MA, Wright V. An investigation into the problems of easy chairs used by the arthritic and the elderly. Rheumatol Rehabil. 1981;20:164–173. doi: 10.1093/rheumatology/20.3.164. [DOI] [PubMed] [Google Scholar]
- Pai Y-C, Rogers MW. Speed variation and resultant joint torques during sit-to-stand. Arch Phys Med Rehabil. 1991;72:881–885. doi: 10.1016/0003-9993(91)90004-3. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Kukulka CG, Soderberg GL. Postural adjustments preceding rapid arm movements in parkinsonian subjects. Neurosci Lett. 1987;75:246–251. doi: 10.1016/0304-3940(87)90305-3. [DOI] [PubMed] [Google Scholar]
- Rossi B, Siciliano G, Carboncini MC, Manca ML, Massetani R, Viacava P, Muratorio A. Muscle modifications in Parkinson’s disease: myoelectric manifestations. Electroencephalogr Clin Neurophysiol. 1996;101:211–218. doi: 10.1016/0924-980x(96)94672-x. [DOI] [PubMed] [Google Scholar]
- Schenkman M, Berger RA, Riley PO, Mann RW, Hodge WA. Whole-body movements during rising to standing from sitting. Phys Ther. 1990;70:638–651. doi: 10.1093/ptj/70.10.638. [DOI] [PubMed] [Google Scholar]
- Schultz AB, Alexander NB, Ashton-Miller JA. Biomechanical analyses of rising from a chair. J Biomech. 1992;12:1383–1391. doi: 10.1016/0021-9290(92)90052-3. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott MH. Motor control: Theory and Applications. Baltimore: Williams and Wilkins; 1995. [Google Scholar]
- Stelmach GE, Worringham CJ. The preparation and production of isometric force in Parkinson’s disease. Neuropsychologia. 1998;26:93–103. doi: 10.1016/0028-3932(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Traub MM, Rothwell JC, Marsden CD. Anticipatory postural reflexes in Parkinson’s disease and other akinetic-rigid syndromes and in cerebellar ataxia. Brain. 1980;103:393–412. doi: 10.1093/brain/103.2.393. [DOI] [PubMed] [Google Scholar]
- Twelves D, Perkins KSM, Counsell C. Systematic review of incidence studies in Parkinson’s disease. Mov Disord. 2003;18:19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor control of Human Movement. Toronto: John Wiley & Sons; 1990. [Google Scholar]
- Winter DA, Patla AE. Signal Processing and Linear Systems for the Movement Sciences. Waterloo: Ontario, Waterloo Biomechanics; 1997. [Google Scholar]