Abstract
Objective
To compare the mobility status (admission and discharge status, in addition to change in status) between patients with stroke and traumatic brain injury (TBI) during inpatient rehabilitation and to determine the relationship between mobility status and outcome variables including length of stay.
Design
Prospective study using a consecutive sample
Setting
Freestanding tertiary rehabilitation centre
Participants
A total of 210 stroke and traumatic brain injury patients consecutively admitted for inpatient rehabilitation.
Main Outcome Measures
Clinical Outcome Variable Scale, a 13 item scale of mobility status (measured on admission and discharge from inpatient rehabilitation) and rehabilitation length of stay.
Results
With age and time since injury controlled in the model, the TBI group demonstrated a significantly higher mobility status on admission and discharge over the stroke group, but the change (improvement) in mobility status was not different. The admission mobility status accounted for 61% and 60% of variability of the discharge mobility status for the stroke and TBI groups, respectively. The admission mobility status accounted for 40% and 50% of the variability in rehabilitation length of stay for the stroke and TBI groups, respectively. Either the admission mobility status or the physical therapist’s prediction of the discharge status could be used to determine the actual discharge mobility status, although the physical therapist’s predictions were more accurate than using a statistical model.
Conclusions
The TBI group demonstrated a higher mobility status at admission and discharge from inpatient rehabilitation than the stroke group, however, the rate of improvement (improvement in mobility status per day) was not different between groups. Admission mobility status using the Clinical Outcome Variable Scale, was an excellent predictor of discharge mobility status and rehabilitation length of stay in stroke and TBI patients.
Keywords: Brain Injuries, rehabilitation, outcome assessment
INTRODUCTION
Stroke and traumatic brain injury (TBI) are two of the most prevalent neurological conditions affecting the central nervous system1 and are the most common disorders for which patients receive inpatient neurological rehabilitation. These two acquired brain injuries are significant sources of adult disability and mobility is a major domain which is affected. Mobility has been defined as “movement of the person from one postural position to another or from one location to another within walking or wheeling distance”.2 Persons with acquired brain injury who have impaired mobility are more likely to experience falls and to be discharged to a long term care facility rather than home. 3,4,5
Given that a large proportion of inpatient rehabilitation is aimed at maximizing mobility, it is critical that appropriate assessments be used to monitor the progress and effectiveness of treatments aimed at improving this domain. In addition, it would be helpful for clinicians to understand whether factors such as age, time since injury or type of brain injury might influence their patient’s mobility during rehabilitation. Functional scores such as the Functional Independence Measure (FIM) do include measures of mobility but these measures are part of the total multidisciplinary FIM score which also includes domains in self-care, communication, and cognition. The multidimensional nature of the FIM motor sub-score has been recognized, for example, Chae et al.8 suggested that the sphincter component of the FIM motor score may represent a distinct entity from the other motor components. Furthermore, the performance of self-care tasks may differ from that of general mobility since self-care involves to a greater extent factors such as hand dominance, visuospatial orientation, and cognition.
One might argue that evaluation of a single domain such as mobility is not reflective of the multidisciplinary rehabilitation process.9 Although the need for multidisciplinary outcomes cannot be understated, measures of mobility status such as the Clinical Outcome Variable Scale (COVS)2, the Duke Mobility Skills Profile10 or Mobility Scale for Acute Stroke11 can assist clinicians to develop specific goals, as well as to evaluate specific interventions, aimed at improving mobility
Differences in mobility status between patients with stroke and TBI have not been well established over the rehabilitation inpatient stay. Although the motor presentation of these two groups can be similar in some cases, the nature of the injury and the characteristics of the patients could result in differences in mobility status over the course of rehabilitation (e.g., the admission and discharge status, and change in mobility status). For example, patients with TBI are generally younger and healthier than stroke patients prior to their injury and one might expect greater recovery in the younger TBI patient as demonstrated by a higher discharge or greater change in mobility status. Surprisingly, Juneja et al.6 reported no differences for admission and discharge status for balance (Berg Balance Scale) and function (Functional Independence Measure) between patients with TBI and stroke, however, the groups were very small (less than 16 in each group). In contrast, Carey et al.7 reported that a TBI group demonstrated a higher admission mobility status and greater improvement in mobility status over a stroke group as evaluated by a 2-item mobility score from the Level of Rehabilitation Scale (LORS-II) from an impressive population of 2951 stroke and 429 TBI patients.
Documenting differences in mobility status between stroke and TBI patients would help clinicians to understand the effects that the pathology itself has on functional outcome and assist them in addressing the specific needs of each group. In addition, differences in mobility status could have implications for the inpatient caseload and resource management. For example, if one group does have lower admission mobility status, this group may require greater resources such as nursing and therapy staff to ensure that these patients are safe during all their activities (e.g., transfers) undertaken during their stay.
Some recent reports have suggested that admission mobility status is a potentially good predictor of functional improvements and of the inpatient rehabilitation length of stay for patients with stroke and traumatic brain injury.7,8,11 Such predictive abilities are becoming more critical in times where bed shortages and limited resources are common. If one group does have greater potential for functional recovery, perhaps, this should be taken into account when limited resources are encountered.
The purpose of this study was to compare mobility status (admission, discharge and change in mobility status) as measured by the COVS2, a 13 item scale of mobility status, between stroke and TBI patients during rehabilitation. In addition, we assessed the relationship between admission mobility status and rehabilitation outcomes, including length of hospital stay and discharge mobility status following inpatient rehabilitation. The hypotheses were that 1) the mobility status (admission, discharge and improvement) will be sensitive to specific types of acquired brain injuries and 2) the mobility status at admission will be predictive of the mobility status at discharge, expected change in mobility status (improvement) and length of the inpatient hospital stay and these relationships would be similar for both stroke and TBI patients. Because much of the physical therapy practice in neurorehabilitation focuses on improving mobility, we also postulated a third hypothesis that physical therapists would be as accurate in predicting the discharge mobility status of stroke and TBI patients compared to a statistical prediction model.
METHODS
Sample
Information was gathered from consecutive admissions to an adult tertiary rehabilitation centre in Vancouver, Canada, for acquired brain injury resulting from stroke or traumatic brain injury. Stroke was defined by an acute event of cerebrovascular origin, causing focal or global dysfunction lasting more than 24 hours. TBI was defined as an insult to the brain caused by an external physical force which resulted in impairment of cognitive abilities and/or physical functioning. The following information was recorded for each patient: time since injury prior to the rehabilitation admission, sex, age, length of inpatient rehabilitation stay (LOS), admission mobility status and discharge mobility status. Mobility status were evaluated within five days of admission and discharge. Criteria for rehabilitation admission included the ability to tolerate a full daily activity program which included at least two hours of physical therapy and occupational therapy, in addition to activities with other disciplines (e.g., speech, psychology, recreation, social work). Time of discharge was decided by the rehabilitation team and was considered when patients reached their functional goals.
Instrument
The instrument used to assess mobility status was the COVS2 (Appendix), which was originally adapted from the physical mobility items from the Patient Evaluation Conference System and the Health Status Rating Scale.12 The COVS was developed as an assessment representative of mobility status. These 13 items all involve postural or locomotor control and include tasks of bed mobility, sitting balance, ambulation, wheelchair mobility and arm function. The items are scored on a 7 point scale based on standardized descriptors (Appendix) with the maximum total mobility status of 91.
The COVS has demonstrated high inter-rater and intra-rater reliability as tested on a population of rehabilitation patients with neurological conditions and good concurrent validity with measures such as the Health Status Rating Form and the Kenny Self Care Evaluation.2 However, the predictive validity of the COVS has not been reported.
Following training and instruction of the COVS, eight therapists of the acquired brain injury team took part in rating of two patients on videotapes. These tapes were developed as a training tool for the COVS and are pre-judged as to the scores for each component (i.e., criterion-referenced measure). All 13 components and total scores were within the 95th percent confidence interval of the criterion standard, establishing the accuracy of the therapist’s ratings.
Procedures
The four COVS components which included “right” and “left” side function were modified to “most affected” and “least affected” side based on the arm function component so that subjects could be pooled together. The data was found to be normally distributed. One way ANOVAs were used to determine differences in the mobility status (admission, discharge and change in mobility status) between the TBI and stroke groups, in addition to subject characteristics (e.g., age, time since injury). Step-wise linear regression analysis was performed for the stroke and TBI groups separately. The first linear regression analysis was used to determine whether the discharge mobility status (dependent variable) could be predicted using the independent variables of admission mobility status, in addition to information readily available on admission (sex, age, and time since injury). A second linear regression was used to determine whether the admission mobility status (independent variable) was predictive of the LOS (dependent variable) and whether this prediction could be improved using other readily available admission information. A third linear regression was performed to determine factors which influence the change in mobility status (dependent variable) from admission to discharge. The final linear regression examined the relationship between the therapist’s predicted mobility discharge status on admission and the actual discharge status. Data were analyzed using SPSS 9.0 for Windowsa. A predictor was entered into the model at a significance of p < .01 and was eliminated at p > .05. The cumulative percent of variance accounted for by the addition of independent variables to the model was assessed by the adjusted R2, thereby indicating whether the model was a good predictor of the dependent variable. The adjusted R2 allows comparisons to be made between models with different numbers of predictor variables and sample sizes.13 As the units of measure for the independent variables differed (e.g., years, days, mobility status score), standardized (Beta-weights), rather than unstandardized regression coefficients were presented as a measure of the relative contribution of each variable in the model.13
RESULTS
A total of 136 patients with stroke and 74 patients with TBI were entered into the study over a 3 year period. Of the stroke group, 59% resulted from a left cerebrovascular accident. As expected, there were differences in some of the variables due to the nature of the neurological injury. The TBI group was younger and had experienced a longer acute hospital stay prior to admission to the rehabilitation facility since unconsciousness in moderate or severe TBI can be prolonged (Table 1). This stroke group was younger than a typical stroke population as a result of the admission criteria which required the ability to tolerate an relatively intense rehabilitation program. For both groups, there was a dominance of males. The length of stay was not different between the two groups (Table 1).
Table 1.
Summary statistics for the 210 subjects
| TBI (74) | Stroke (136) | p-value | |
|---|---|---|---|
| Time since Injury (days) | 61.8 (38.5) | 39.4 (27.0) | < 0.001 |
| % male | 60 | 65 | |
| Age (years) | 34.3 (14.0) | 54.0 (10.9) | < 0.001 |
| Length of Stay (days) | 54.8 (30.4) | 54.3 (25.6) | 0.92 |
| Admission Mobility Status* | 59.3 (20.8) | 49.7 (17.3) | 0.001 |
| Discharge Mobility Status | 77.6 (15.0) | 71.8 (13.3) | 0.006 |
| Change in Mobility Status | 18.3 (12.7) | 22.1 (10.7) | 0.16 |
COVS mobility status: minimum score=7; maximum score=91
The mean admission mobility status was 52 ± 19 (± one standard deviation) and discharge mobility status was 74 ± 14. For both admission and discharge, there was a significant type of injury effect on mobility status (Table 1). The TBI group was admitted and discharged with a higher mobility status, but the actual change in mobility status (improvement) was not different between the two groups. The covariates of age and time since injury partially accounted for the differences between the TBI and stroke groups. However, the admission and discharge mobility status were still significantly different between these two groups (p < .05) when these covariates were entered into the ANOVA model.
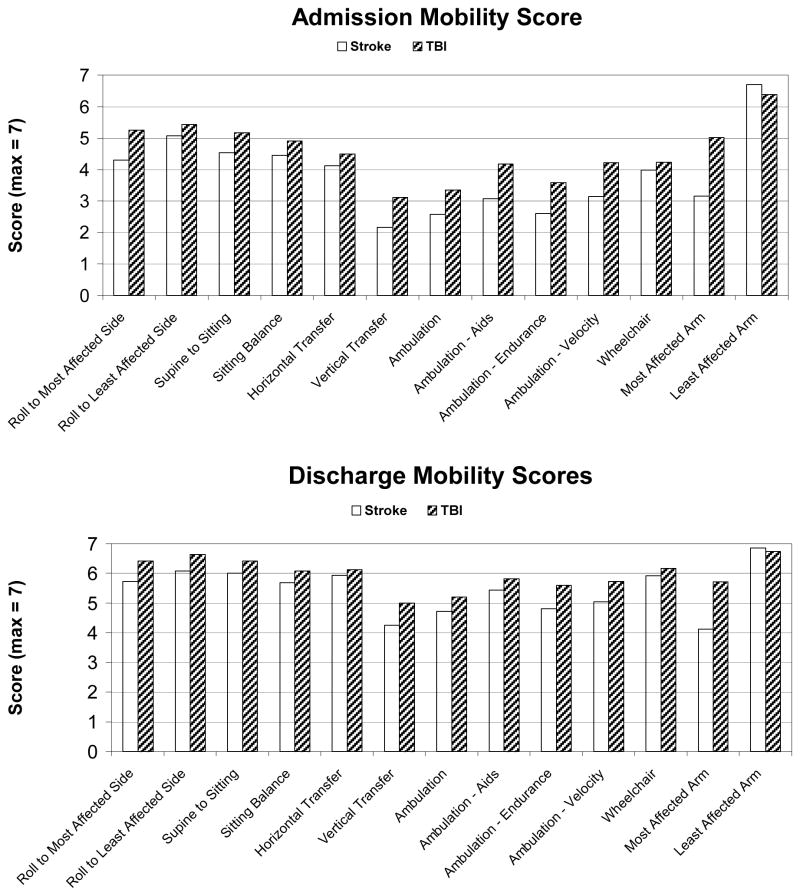
Improvement in all 13 mobility items was demonstrated from admission to discharge (Figure 1). Only 4 patients (2%) reached the maximum total score of 91 by the time of discharge. As expected, function of the least affected arm was scored close to normal (7) on admission and discharge. Rolling to the least affected side was the next highest score with 20% and 44% of patients attaining the maximum score of 7 on admission and discharge, respectively. The vertical transfer and the ambulation items were scored the lowest on admission, but showed the greatest improvement. The vertical transfer requires that the patient rise from a supine position on the floor to a sitting or standing position using a chair or wheelchair for support. This challenging task assesses an important aspect of independent ability for community living as it is indicative of whether a subject could get themselves up if they fell to the ground. This task was awarded a full score of 7 to only 4% of the patients on admission while 20% reached this milestone on discharge. The TBI group scored higher for all items over the stroke group at the time of admission and discharge except for the least affected arm function (Figure 1). The item demonstrating the greatest discrepancy between the two groups was the arm function of the most affected arm with the TBI group scoring above the stroke group.
Figure 1.
1a. Mobility status on admission for TBI versus stroke patients
1b. Mobility status on discharge for TBI versus stroke patients.
(n=74 TBI patients, 136 stroke patients)
For predicting the dependent variables of discharge mobility status and change in mobility status, the same significant independent variables were found when analyzing the TBI and stroke groups separately. Both the discharge mobility status and change in mobility status could be predicted by the admission mobility status (Table 2, Table 3) with adjusted R2 values ranging from .34 to .61. These models were improved slightly by the addition of the time since injury, but not sex or age. A higher admission mobility status and shorter time since injury prior to rehabilitation was associated with a higher discharge mobility status. A lower admission status and shorter time since injury was associated with a greater change in mobility status.
Table 2.
Standardized Regression Coefficients (β) and Adjusted Coefficients of Variation (R2) for Predicting Discharge Mobility Status for Stroke and TBI
| Group | Variable | β | R2 change | P value | Adjusted R2 |
|---|---|---|---|---|---|
| Stroke (n=136) | Admission Mobility Status | .783 | .613 | <.001 | .610 |
|
| |||||
| Admission Mobility status | .753 | <.001 | |||
| Time since injury | −.247 | .060 | < .001 | .668 | |
|
| |||||
| TBI (n=74) | Admission Mobility status | 0.78 | .606 | <.001 | .600 |
|
| |||||
| Admission Mobility status | 0.67 | <.001 | |||
| Time since injury | −0.33 | .097 | < .001 | .693 | |
Table 3.
Standardized Regression Coefficients (β) and Adjusted Coefficients of Variation (R2) for predicting the Change in Mobility Status for Stroke and TBI
| Group | Variable | β | R2 change | P value | Adjusted R2 |
|---|---|---|---|---|---|
| Stroke (n=136) | Admission Mobility Status | −.59 | .350 | <.001 | .344 |
|
| |||||
| Admission Mobility Status | −.63 | . | < .001 | ||
| Time since injury | −0.34 | 115 | < .001 | .464 | |
|
| |||||
| TBI (n=74) | Admission Mobility Status | −.71 | .509 | <.001 | .501 |
|
| |||||
| Admission Mobility Status | −.82 | < .001 | |||
| Time since injury | −0.31 | .084 | .001 | .580 | |
For the TBI group, the LOS could be significantly predicted by the admission status and the model improved by entering the time since injury and age. A lower admission status, longer time since injury and lower age was associated with a longer LOS (Table 4). These three factors could explain over 60% of the variability in LOS and the admission status alone accounted for over 50% of the variability. For the stroke group, the LOS could be predicted by the admission status, but not any of the other variables and accounted for 40% of the variability (Table 4).
Table 4.
Standardized Regression Coefficients (β) and Adjusted Coefficients of Variation (R2) for predicting the Length of Stay for Stroke and TBI
| Group | Variable | β | R2 change | P value | Adjusted R2 |
|---|---|---|---|---|---|
| Stroke (n=136) | Admission Mobility Status | −.631 | .398 | < .001 | .396 |
|
| |||||
| TBI (n=74) | Admission Mobility Status | −.714 | .510 | < .001 | .501 |
|
| |||||
| Admission Mobility Status | −.613 | < .001 | |||
| Time since injury | .306 | .087 | < .001 | .582 | |
|
| |||||
| Admission Mobility Status | −.657 | < .001 | |||
| Time since injury | .335 | < .001 | |||
| Age | −.174 | .028 | .05 | .604 | |
The prediction of the discharge mobility status with the actual discharge mobility status by the therapist was excellent and produced an R2 of 0.66 and 0.83 for the stroke and TBI groups, respectively (Table 5). These values were higher than the stepwise regression model which produced an R2 of 0.62 using the admission status and the time since injury.
Table 5.
Standardized Regression Coefficients (β) and Adjusted Coefficients of Variation (R2) for predicting the discharge mobility status for stroke and TBI from therapist estimations.
| Group | Variable | β | P value | Adjusted R2 |
|---|---|---|---|---|
| Stroke (n=136) | Therapists estimated discharge status | −.59 | <.001 | .657 |
| TBI (n=74) | Therapists estimated discharge status | −.71 | <.001 | .832 |
DISCUSSION
Mobility status between the TBI and stroke group
The COVS provides an outcome measure of mobility status and is indicative of the postural and locomotor control of the patient. The locomotion and transfer items of the FIM also represent a measure of mobility. However, the 13 items in the COVS reflect a broader skill range extending from a relatively low level (bed mobility and sitting balance) through to ambulatory tasks.
Due to the nature of the injury, the TBI patient was younger and had experienced a longer time since injury prior to admission to rehabilitation. However, the greater mobility status on admission and discharge mobility for the TBI patients over the stroke patients was not simply due to the age or time since injury as significant differences in mobility status remained despite entering these covariates into the model. We suggest that these differences may be attributed from the specific pathology of the different brain injuries and their resulting clinical manifestations. Mobility limitations can result from a variety of factors including, but not limited to decreased muscle strength, spasticity, soft tissue or joint restrictions, impaired balance, visual impairment, pain, sensory or perceptual impairment, lack of motivation, vestibular dysfunction, and cognitive dysfunction.14,15,16 The mobility status on admission and discharge of the TBI group was better than the stroke group for almost all the postural and locomotor tasks. Thus, one might want to consider the greater physical needs of the stroke patient when planning inpatient caseloads and resources. The arm function items highlighted some of the specific differences between the TBI and stroke groups; the greatest discrepancy between the TBI group and stroke groups was found in the arm function of the most affected arm while the function of the least affected arm was the only item in which the stroke group scored higher than the TBI group. This latter observation is indicative of the frequent bilateral clinical presentation in TBI patients.
Ideally, a patient who is demonstrating substantial continuing recovery in either motor or cognitive function would not be discharged until he or she reached a plateau. In reality, a myriad of factors in addition to physical and cognitive function influences the discharge date, including motivation, family support and the availability of an appropriate residence and outpatient service. It was surprising that there were no differences in the rehabilitation length of stay for the TBI and stroke groups as others have reported a longer LOS for TBI compared to stroke patients.7,17 However, the LOS in this study was longer than that reported by these studies likely due to differences in policies, practices, and health care coverage available in Canada.
Given that the rate of change was similar for both the stroke and TBI groups (i.e., both the change in mobility status and LOS were not different between groups), this suggests that rehabilitation/recovery was as efficient (as measured by improvement per day) for both groups.
Relationship of admission and discharge mobility status
This study demonstrated that admission mobility status is an important predictor for the absolute discharge mobility status and the relative changes in mobility status during inpatient rehabilitation for acquired brain injury. Furthermore, these relationships were similar for both the TBI and stroke groups. Discharge planning commences as soon as a patient is admitted and predictions of mobility status could provide the clinical team with information in preparation for discharge, particularly with those individuals who are expected to require significant home or community needs.
Correlations between admission and discharge mobility status in this study were particularly high with the admission mobility status accounting for 60 to 61% of the variability in discharge status. This was not unexpected as previous investigators have reported that 50 to 70% of the variability in discharge FIM motor scores could be accounted for in models mainly weighted by the admission FIM motor score in TBI and stroke populations.17,18,19 Other investigators have been able to account for over 50% of the variability in discharge Barthel Index using the admission Barthel Index in stroke patients.20,21 Chae et al.8 suggested that dependency in physical activities of daily living after stroke is primarily determined by degree of motor impairment. The results of our study concur with previous findings that functional or motor status on admission is one of the most important predictors for the discharge status of the corresponding scale.
Higher admission mobility status was associated with higher discharge status, but with less change in mobility status. Jongbloed22 in a review of 33 stroke studies concluded that predictors for functional discharge were not necessarily the same as for functional improvement. As a longer time since injury was associated with less change in mobility status, it is possible that those patients who were admitted with a higher mobility status experienced substantial recovery while waiting in the acute care setting. A longer time since injury could reflect a greater severity of injury resulting in less potential for change. However, Cowen et al.18 controlled for injury severity using the Glasgow Coma Scale and CT findings, and still found that a longer time since injury was associated with less improvement in TBI patients.
Although these results may suggest that those with lower admission mobility status may have greater capacity for change in the rehabilitation setting, one must be cautious in this interpretation, as a change in status from 20 to 30 cannot be assumed to be an improvement in mobility status identical to a change from 70 to 80 due to the ordinal nature of the scale and the summation of different performance categories.23,24
Predictive analysis of the length of stay
A number of investigators have attempted to account for LOS with predictors which included demographics, functional status at admission, medical complications, and radiological information. It was surprising that admission mobility status alone could account for 40 to 50% of the variability in LOS, given the large number of factors which could potentially affect LOS. Only a few other investigators have reported such high correlations (e.g., accounting for over 40% of the variability) between functional motor measures and LOS11,17,18,25,26 while the majority of studies have reported more modest correlations.6,8,17,27,28,29,30,31 It is possible that a longer LOS results in better predictive abilities since patients may demonstrate greater recovery prior to discharge. Of the six studies (including this study)11,17,18,25,26 which have reported correlations which account for at least 50% of the LOS variability, four are studies undertaken outside of the United States where the LOS is longer than that commonly found in the United States.
Age was not a significant predictor for LOS or discharge mobility status in the stroke group, but was a significant predictor of LOS for the TBI group. Is it possible that the risk behavior of younger TBI patients resulted in more severe injuries (and consequently a longer LOS)? A post-hoc analysis found that age was not a significant predictor of the admission mobility status in the TBI group. However, there does not appear to be a consensus in the literature on the effect of age on LOS in stroke and TBI patients as some investigators have reported increasing LOS25,32, decreasing LOS17,33,34 or no relationship26,35,36 with age. The exact findings may be dependent on the age range of the selected group, practices and policies of the institutions or on the variables controlled. For example, Reeder et al.36 found no age effects for the LOS in TBI patients if injury severity and etiology were controlled.
Accuracy of the clinical prediction
The prediction of the discharge mobility status by the therapist at the time of admission was more accurate than that derived from a statistical model alone. The therapist’s score may be more accurate, likely due to greater available information (e.g., motivation, attention, comprehension) from the admission assessment which goes into the prediction. Interestingly, predictions for the stroke group were less accurate than for the TBI group. The evaluating therapists suggested that the generally less healthy state of the stroke patient contributed to some of the unpredictability of the stroke patient’s response and recovery to rehabilitation. In addition, they felt that perceptual impairments (e.g., neglect) were more profound in stroke patients and this factor also contributed to inaccuracies of their predictions. Korner-Bitensky et al.37 also reported excellent predictive accuracy by physical therapists using a similar mobility scale (Patient Evaluation Conference System) for stroke patients. These results demonstrate that predictions from the clinicians are valuable in planning treatment and discharge strategies.
Limitations
A limitation of this study is that it includes only participants from one rehabilitation program which may limit the generalizability of the findings. The facility in this study is a tertiary hospital which receives referrals from urban, suburban and rural communities and is typical of a Canadian rehabilitation program. However, predictors of LOS may in part depend on the policies and practices of specific institutions, resources available to the region, in addition to the type of medical coverage and services available.
Lastly, an argument can be made for transforming ordinals scales prior to their parametric analysis38, 39, however, Wright and Linacre39 also stressed that raw ordinal scores can be subjected to linear measures if tested for linearity and if missing data is not a problem.
Conclusions
Differences in mobility status were identified for patients with acquired brain injury whose conditions resulted from a stroke versus a traumatic brain injury. The TBI group demonstrated greater mobility status at admission and discharge from inpatient rehabilitation than the stroke group, but the change in mobility status was not different between groups. Admission mobility using the Clinical Outcome Variable Scale, was an excellent predictor of discharge mobility status and rehabilitation length of stay for both the stroke and TBI groups.
Acknowledgments
Financial Support: BC Health Research Foundation and the Canadian Institute of Health Research (operating grant # 57862).
Appendix
1/2. Roll in bed from supine lying (to affected and unaffected side, performed separately)
-
1
dependent
-
2
1 person assist (with/without device)
-
4
rolls by self nut needs assistance for final position (getting comfortable)
-
5
independent with device
-
6
independent without device, but slow awkward, requires more effort
-
7
normal
3. Gets to a sitting position from supine lying in bed
-
1
dependent
-
2
1 person assist (with/without device)
-
4
supervision/instruction for safety, or verbal cueing
-
5
independent with device
-
6
independent without device, but slow awkward, requires more effort
-
7
normal
4. Sitting balance - edge of bed, thighs supported, hands on lab, feet flat on floor
-
1
not able to sit unsupported
-
2
no displacement tolerated
-
3
move within base
-
5
move beyond base and return
-
6
tolerates quick push beyond base
-
7
normal
5. Horizontal transfer – from chair or wheelchair to bed/plinth
dependent
1 person assist with device
1 person assist without device
supervision, with or without device
independent with device
independent without device, but slow awkward, requires more effort
normal
6. Vertical Transfer from supine on floor to chair or to stand
dependent
1 person assist with device
1 person assist without device
supervision, with or without device
independent (with/without device) in home, but slow awkward, requires more effort
independent in community
normal
7. Ambulation
no functional ambulation
1 person continuous physical assist
1 person intermittent physical assist
supervision
independent level surfaces only, assist with environmental barriers, stairs with railing
independent including environmental barriers, stairs without railing
normal
8. Ambulation – Aids
not walking
parallel bars/2 person continuous assist
walker (rollator and walk-canes)
2 aids
1 aid (except straight cane)
straight cane
no aids
9. Ambulation – Endurance
-
1
not walking
-
2
≤ 10 m ( 6 m = parallel bars)
-
3
≤ 50 m
-
4
≤ 100 m (86 m = timed 2 min walk)
-
5
≤ 500 m (300 m = park walk)
-
7
> 500 m (park walk and gym loop)
10. Ambulation - Velocity
-
1
0 metre/sec
-
2
≤ 0.1 m/s
-
4
≤ 0.3 m/s
-
5
≤ 0.6 m/s
-
6
≤ 0.9 m/s
-
7
> 0.9 m/s
11. Wheelchair Mobility
dependent
assistance
intermittent assist for distances > 30 m
supervision
independent indoors
independent outdoors except curbs and grass
independent operation of wheelchair
12/13. Arm Function (affected and unaffected performed separately) Starting Position: sitting at a table, in a wheelchair/chair
-
1
unable to actively move any part of the arm
-
2
some active movement – nothing useful
-
4
able to use arm as a stabilizer or as an assist
-
5
able to bring cup to the mouth
-
6
functional including fine movements (penny)
-
7
normal
Footnotes
SPSS Inc., 233 S. Wacker Dr, Chicago, IL, 60606
References
- 1.Wade DT, Langton Hewer R. Epidemiology of some neurological diseases with special reference to work load on the NHS. Int Rehabil Med. 1987;8:129–137. doi: 10.3109/03790798709166197. [DOI] [PubMed] [Google Scholar]
- 2.Seaby L, Torrance G. Reliability of a physiotherapy functional assessment used in a rehabilitation setting. Physiotherapy Canada. 1989;41(5):264–271. [Google Scholar]
- 3.Brosseau L, Potvin L, Philippe P, Boulanger Y-L. Post-stroke inpatient rehabilitation. II. Predicting discharge disposition. Am J Phy Med Rehabil. 1996;75:431–436. doi: 10.1097/00002060-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Prudham D, Evans JG. Factors associated with falls in the elderly: A community study. Age and Ageing. 1981;10:141–146. doi: 10.1093/ageing/10.3.141. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43:1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 6.Juneja G, Czyrny JJ, Linn RT. Admission balance and outcomes for patients admitted for acute inpatient rehabilitation. Am J Phys Med Rehabil. 1998;77:388–393. doi: 10.1097/00002060-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Carey RG, Siebert JH, Posavac EJ. Who makes the most progress in inpatient rehabilitation? An analysis of functional gain. Arch Phys Med Rehabil. 1988;69:337–343. [PubMed] [Google Scholar]
- 8.Chae J, Johnston M, Kim H, Zorowitz R. Admission motor impairment as a predictor of physical disability after stroke rehabilitation. Am J Phys Med Rehabil. 1995;74:218–223. doi: 10.1097/00002060-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Freeman JA, Hobart JC, Thompson AJ. Outcomes-based research in neurorehabilitation: the need for multidisciplinary team involvement. Disability and Rehabilitation. 1996;18:106–110. doi: 10.3109/09638289609166025. [DOI] [PubMed] [Google Scholar]
- 10.Duncan P. Center for Human Aging. Duke University; 1989. Duke Mobility Skills Profile. [Google Scholar]
- 11.Brock K, Robinson P, Simondson J, Goldie P, Nosworthy J, Greenwood K. Prediction of length of hospital stay following stroke. J Qual Clin Practice. 1997;17:37–46. [PubMed] [Google Scholar]
- 12.Tapping C. Instruments to Measure Health Status of Patients Receiving Physiotherapy. II. Ottawa (ON): Health and Welfare Canada; 1981. Toward Assessment of Quality of Care in Physiotherapy. [Google Scholar]
- 13.Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied Regression Analysis and Multivariable Methods. Pacific Grove: Duxbury Press; 1998. [Google Scholar]
- 14.Mayo NE, Korner-Bitensky NA, Becker R. Recovery time of independent function post- stroke. Am J Phys Med Rehabil. 1991;70:5–12. doi: 10.1097/00002060-199102000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Sakari-Rantala R, Era P, Rantanen T, Heikkinen E. Associations of sensory-motor functions with poor mobility in 75–80-year-old people. Scand J Rehab Med. 1988;30:121–127. doi: 10.1080/003655098444237. [DOI] [PubMed] [Google Scholar]
- 16.Shumway-Cook A, Woollacott MH. Theory and Practical Applications. Baltimore (MD): Williams and Wilkins; 1995. Motor control. [Google Scholar]
- 17.Heinemann AW, Linacre JM, Wright BD, Hamilton BB, Granger C. Prediction of rehabilitation outcomes with disability measures. Arch Phys Med Rehabil. 1994;75:133–143. [PubMed] [Google Scholar]
- 18.Cowen TD, Meythaler JM, DeVivo MJ, Ivie CS, Lebow J, Novack TA. Influence of early variables in traumatic brain injury on functional independence measure scores and rehabilitation length of stay and charges. Arch Phys Med Rehabil. 1995;76:797–803. doi: 10.1016/s0003-9993(95)80542-7. [DOI] [PubMed] [Google Scholar]
- 19.Heinemann A, Hamilton B, Linacre JM, Wright BD, Granger C. Functional status and therapeutic intensity during inpatient rehabilitation. Am J Phys Med Rehabil. 1995;74:315–326. doi: 10.1097/00002060-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Hertanu JS, Demopoulos JT, Yang WC, Calhoun WF, Fenigstein HA. Stroke rehabilitation: Correlation and prognostic value of computerized tomography and sequential functional assessments. Arch Phys Med Rehabil. 1984;65:505–508. [PubMed] [Google Scholar]
- 21.Jongbloed L, Jones W. Prediction of recovery after stroke: an examination of outcome criteria. Can J Rehab. 1988;2(2):87–92. [Google Scholar]
- 22.Jongbloed L. Prediction of function after stroke: a critical review. Stroke. 1986;17(4):765–776. doi: 10.1161/01.str.17.4.765. [DOI] [PubMed] [Google Scholar]
- 23.Merbitz C, Morris J, Grip J. Ordinal scales and foundations of misinference. Arch Phys Med Rehabil. 1989;70:308–312. [PubMed] [Google Scholar]
- 24.Wright BD, Linacre JM. Observations are always ordinal; Measurements, however, must be interval. Arch Phys Med Rehabil. 1989;70:857–860. [PubMed] [Google Scholar]
- 25.Brosseau L, Philippe P, Potvin P, Boulanger Y-L. Post-stroke inpatient rehabilitation. I. Predicting length of stay. Am J Phys Med Rehabil. 1996;75:422–430. doi: 10.1097/00002060-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 26.High WM, Hall KM, Rosenthal M, Mann N, Zafonte R, Cifu DX, Boake C, Bartha M, Ivanhoe C, Yablon S, Newton CN, Sherer M, Silver B, Lehmkuhl LD. Factors affecting hospital length of stay and charges following traumatic brain injury. J Head Trauma Rehabil. 1996;11:85–96. [Google Scholar]
- 27.Harvey RL, Roth EJ, Heinemann AW, Lovell LL, McGuire JR, Diaz S. Stroke rehabilitation: Clinical predictors of resource utilization. Arch Phys Med Rehabil. 1998;79:1349–1355. doi: 10.1016/s0003-9993(98)90226-x. [DOI] [PubMed] [Google Scholar]
- 28.Stineman MG, Williams SV. Predicting inpatient rehabilitation length of stay. Arch Phys Med Rehabil. 1990;71:881–7. [PubMed] [Google Scholar]
- 29.Stineman MG, Escarce JJ, Goin J, Hamilton BB, Granger CV, Williams SV. A case-mix classification system for medical rehabilitation. Med Care. 1994;32:366–379. doi: 10.1097/00005650-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Heinemann AW, Roth E, Kaplan P, Betts H. Multivariate analysis of improvement and outcome following stroke rehabilitation. Arch Neurol. 1987;44:167–72. doi: 10.1001/archneur.1987.00520230051013. [DOI] [PubMed] [Google Scholar]
- 31.Heinemann AW, Sahgal V, Cichowski K, Ginsburg K, Tuel S, Betts B. Functional outcome following traumatic brain injury rehabilitation. J Neurol Rehabil. 1990;4:27–37. [Google Scholar]
- 32.Kalra L. Does age affect benefits of stroke unit rehabilitation? Stroke. 1994;25:346–351. doi: 10.1161/01.str.25.2.346. [DOI] [PubMed] [Google Scholar]
- 33.Franchignoni F, Tesio L, Martino MT, Benevolo E, Castagna M. Length of stay of stroke rehabilitation inpatients: prediction through the Functional Independence Measure. Ann Ist Super Sanita. 1998;34:463–467. [PubMed] [Google Scholar]
- 34.Granger CV, Hamilton BB, Fiedler RC. Discharge outcome after stroke rehabilitation. Stroke. 1992;23:978–982. doi: 10.1161/01.str.23.7.978. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcomes. The Copenhagen stroke study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- 36.Reeder KP, Rosenthal M, Lichtenberg P, Wood D. Impact of age on functional outcome following traumatic brain injury. J Head Trauma Rehabil. 1996;11:22–31. [Google Scholar]
- 37.Korner-Bitensky N, Mayo N, Cabot R, Becker R, Coopersmith H. Motor and functional recovery after stroke: accuracy of physical therapists’ predictions. Arch Phys Med Rehabil. 1989;70:95–99. [PubMed] [Google Scholar]
- 38.Chang W, Chan C. Rasch analysis for outcomes measures: Some methodological considerations. Arch Phys Med Rehabil. 1995;76:934–939. doi: 10.1016/s0003-9993(95)80070-0. [DOI] [PubMed] [Google Scholar]
- 39.Wright BD, Linacre JM. Observations are always ordinal; measurements, however, must be interval. Arch Phys Med Rehabil. 1989;70:857–860. [PubMed] [Google Scholar]