Adult urinary incontinence (UI) is a highly prevalent condition, and one which can have a major impact on patients’ quality of life. It is also a major focus of a urologist’s workload. As a result, the Canadian Urological Association (CUA), with the aid of its Guidelines Committee, commissioned the development of a practice guideline document in 2005 first authored by Dr. Jacques Corcos. As per the CUA Guidelines Committee’s mandate, all guidelines are subject to revision after 5 years.
Methodology
A comprehensive review of the studies published from January 2005 and November 2011 was performed using PubMed, MEDLINE and The Cochrane Library databases. In addition, the bibliographies of all relevant articles were searched to avoid exclusion of significant articles. Focus was on systematic reviews, meta-analyses and evidence-based recommendations, when available. Data from the latest consensus of the International Continence Society (ICS), the International Consultation on Incontinence (ICI), the International Urogynecological Association (IUGA), the American Urological Association (AUA), the European Association of Urology (EAU), the Urinary Incontinence Treatment Network (UITN), the Society of Obstetricians and Gynecologists of Canada (SOGC) and the American Congress of Obstetricians and Gynecologists (ACOG) were also incorporated. This review does not address UI in children or patients with neurogenic bladder. All articles were reviewed using the Evidence-Based Medicine (EBM) levels, with a Modified Oxford grading System (Appendix A).
Introduction
UI can be defined as a complaint of any involuntary leakage of urine. It is estimated that 3.3 million (10%) Canadians experience incontinence, making it a highly prevalent condition, and one that is associated with a significant economic burden.1 Incontinence can be classified into three broad categories: stress, urge and mixed. Stress urinary incontinence (SUI) is defined as leakage associated with exertion, sneezing or coughing, and represents 50% of patients with incontinence in Canada.1,2 Urgency urinary incontinence (UUI) is leakage immediately preceded by or associated with a sudden desire to void, representing 14% of patients.1–3 Mixed urinary incontinence (MUI) is characterized by the combination of UUI and SUI and represents 32% of patients in Canada.1–3
Lower urinary tract symptoms (LUTS) include storage, voiding and post- micturition symptoms. Although the term LUTS was originally used to describe male bladder outlet symptoms, this term can also be used to describe symptoms both in men and women. These may arise from the bladder or bladder outlet (including the prostate) conditions, in addition to non-urinary tract sources, such as polypharmacy, polyuria, iatrogenic and psychogenic disorders.2
Overactive bladder (OAB) is a common condition defined as urgency, with (OAB wet) or without urgency incontinence (OAB dry), usually associated with increased daytime frequency and nocturia.2 In a recent Canadian population-based study, OAB symptoms were reported in 13.9% of respondents (13.1% of men and 14.7% of women). UI was reported by 28.8% of women with 68% having SUI, 21% MUI and 11% UUI. In men, 5.4% of respondents had UI (26% SUI, 15% MUI and 58% UUI). The prevalence of OAB symptoms was similar in both sexes; however, OAB with UUI is more common in women (7.1% vs. 3.3%). Furthermore, OAB symptoms are more significant with increasing age (23.8% for >60 years old vs. 12.2% for <60 years old.)4
Less common categories of urinary incontinence include total incontinence (associated with urinary tract fistula or ectopic ureter), functional (associated with psychiatric or mobility disorder), uncategorized, overflow, post-micturition dribble, radiotherapy and climacturia.
Specific considerations in men
OAB symptoms are a key component of LUTS in many men with or without bladder outlet obstruction (BOO). Although a very important element in some, prostatic enlargement is not the sole factor contributing to LUTS, and many men may present with primary idiopathic OAB. Even in those men with BOO, treatment aimed at relieving the obstruction leads to resolution of OAB symptoms in only 35% of men.5 This supports the need for increased awareness of the existence of primary OAB in men presenting with LUTS, and must be considered in their management.
Post-prostatectomy urinary incontinence (PPUI) is usually due to direct damage to the external urethral sphincter. The incidence of SUI after treatment of prostatic benign disease either using classical trans-urethral resection or laser therapy is estimated to be less than 1% to 3%.6 The incidence of post-radical prostatectomy urinary incontinence (PPI) is variable depending on the definition, the evaluation tool, and the collection method used. In general, 1% to 40% of patients suffer from PPI.7 It may be caused by bladder dysfunction, sphincter dysfunction or a combination of both. Bladder dysfunction presenting as OAB wet may contribute to PPUI but is rarely the sole cause (<10%). Sphincteric deficiency remains the major cause of UI in more than two-thirds of patients.8 A combination of both is present in at least a third of patients.7 Only 6% to 9% of PPUI will ultimately require surgical intervention.9 New laparoscopic and robotic-assisted prostatectomy techniques use many of the same surgical principles as open surgery. It was initially thought that these techniques would lead to improved postoperative continence, however, comparative systematic reviews haven not demonstrated this.6,10 Operative skills, bladder neck preservation and neurovascular bundle sparing technique may help to minimize the postoperative morbidity, however no significant benefits have been confirmed to date.11 The Canadian Agency for Drugs and Technologies for Health (CADTH) recently reported the comparison of postoperative morbidities between different surgical techniques of radical prostatectomy (open vs. laparoscopic vs. robot-assisted). There was a statistical difference in UI at 12 months postoperative in post-learning curve procedures between open versus robot-assisted techniques, but the results were inconclusive between laparoscopic versus robot-assisted. However, since these data were extracted from observational studies, with discrepant populations (in terms of age, follow-up and availability of preoperative data), the authors concluded that the general impact of these findings is likely to be small.12
Specific considerations in frail elderly patients
UI is a frequent and disabling condition affecting 30% to 60% of patients over 65 years old, and increasing exponentially with age.13 The prevalence is even higher for the frail and elderly (range: 43%–77%, median 58%).13 The frail older person can be defined as “a clinical phenotype combining impaired physical activity, mobility, balance, muscle strength, motor processing, cognition, nutrition, and endurance; associated high medication use and being homebound or in a care institution and a high risk of intercurrent disease, increased disability, hospitalization and death.”14,15 Because of these special features, the management of UI in this population will be addressed. The predominant type of UI in elderly women is MUI (level of evidence 1).16 A number of patients with OAB symptoms have detrusor overactivity that may be combined with impaired contractility (33%).17
Evaluation
The evaluation of a patient with UI should be systematic and include history, medical history, review of systems, social history, physical examination, investigations and treatment expectations (level of evidence 2, grade B).18 Important elements to consider on history include a review of storage, voiding and post-micturition symptoms, the type and severity of incontinence and degree of bother (level of evidence 3, grade B).19 Additional information includes the presence of pain, hematuria, recurrent infections, symptomatic pelvic organ prolapse in women, failed previous anti-incontinence surgery, previous pelvic radiation therapy or surgery and suspected fistula.20
The physical examination evaluates the general status (mental status, obesity, physical dexterity and mobility), abdominal examination (masses, bladder overdistension, scars), pelvic examination in women (perineum and external genitalia including tissue quality and sensation, vaginal size, vaginal examination with a speculum for vaginal prolapse), bimanual pelvic and anorectal examination for pelvic mass and pelvic muscle function, cough stress test (grade C),19 a focused neurological examination when indicated and a digital rectal examination (DRE) in men (level of evidence 4, grade C).
Initial investigations include a urinalysis and a 3-day voiding diary (grade C).2,21 In specific clinical situations, the following tests are recommended: symptoms/quality of life questionnaires (International Consultation on Incontinence Questionnaire [ICIQ] grade A, Incontinence Impact Questionnaire [IIQ]-7, Urogenital Distress Inventory [UDI]-6), pad weight testing, serum creatinine, uroflowmetry, post-void residual volume (PVR), methylene blue or pyridium pad testing, cystoscopy and urodynamic studies (UDS).18 Uroflowmetry and PVR assessment is recommended in the presence of significant voiding symptoms, symptomatic pelvic organ prolapse or bladder overdistension. A cystourethroscopy should be performed when the initial testing suggest other pathologies (e.g., LUTS combined with pain or discomfort, hematuria) or when a fistula is suspected. UDS is indicated when the diagnosis remains uncertain after history and physical examination, when the symptoms do not correlate with physical findings or after failed previous treatment (level of evidence 3, grade C).22
Specific considerations in men
In men with OAB, symptoms and quality of life assessment is recommended along with DRE and prostate-specific antigen. Measurement of PVR is an optional evaluation tool (level of evidence 4).
Most radical prostatectomy patients experience some incontinence immediately after catheter removal. In some men, continence can be achieved as early as a few weeks after surgery, but it may take up to 12 months to recover (level of evidence 4).7 The evaluation of PPI includes a thorough history, physical examination, urinalysis, urine culture, bladder diary, pad test (level of evidence 1–2, grade B) and an assessment of PVR (level of evidence 1–2, grade A).2,7 The 24-hour pad test most accurately reflects the severity of urinary incontinence.7,11,20 However, ICS standardized 1-hour pad test is more widely adopted in the clinical setting.9 A cystourethroscopy and UDS are recommended to assess lower urinary tract anatomy and function in patients who fail conservative and pharmacologic managements (level of evidence 2–3, grade B).23
Specific consideration in frail elderly patients
Due to the high prevalence of urinary incontinence in the elderly, screening for UI should be included in the annual health evaluation (grade A).15 The assessment must include an evaluation of comorbidities, medications, functional and cognitive impairment (grade B).24 In addition, fluid/volume status, accessibility to toilets and social or caregiver support should be assessed. The evaluation should initially focus on reversible conditions that can cause or exacerbate UI. These patients can be divided into three categories: lower urinary tract (symptomatic urinary tract infection [UTI], atrophic vaginitis, post-prostatectomy, stool impaction, drug side effects, diuretics, anticholonergics, psychotropics, narcotics/analgesics, alpha-adrenergic agonists and blockers, alcohol and caffeine), increased urine production (metabolic, excess fluid intake, volume overload) and impaired ability or willingness to reach a toilet (delirium, chronic illness, neurologic diseases and psychological).24,25
While evaluating UI in the frail elderly patient, it is reasonable to look for the 7 possible causes of transient UI described by the acronym and mnemonic DIAPPERS (Delirium, Infection, Atrophic vaginitis, Psychological, Pharmacologic, Excess urine output, Restricted mobility and Stool impaction). The treatment of these factors is straightforward and may improve patients’ symptoms (grade C).20
Treatment of UUI
Conservative therapy should be considered prior to the initiation of medical or surgical treatment of UUI. These include behavioural modifications such as scheduled voiding, fluid restriction when appropriate (grade B), smoking cessation (grade C), avoidance of caffeine and bladder training (grade A).2,26 Pelvic floor muscle training (PFMT) has been shown to be effective in improving UUI. In fact, it has been suggested to be better than oxybutinin as first-line therapy (grade B).20 If conservative measures alone are not effective, one should consider adding in pharmacological therapy. Antimuscarinics are appropriate as first- or second-line treatment for UUI (grade B). In clinical practice, patients are considered to have refractory UUI if they have failed at least 2 adequate treatments of antimuscarinic drugs. OnabotuliniumtoxinA (BoNT-A) (off-label), neuromodulation and surgical interventions, such as augmentation cystoplasty, are all acceptable options for a small percentage of patients who do not respond to conservative and drug therapies depending on availability of resources.
The available pharmacological treatment includes oxybutinin immediate release (IR), extended release (ER) or transdermal, tolterodine (IR or ER), solifenacin, darifenacin, trospium chloride and fesoterodine. There is Level 1A evidence for each of these drugs showing superior efficacy versus placebo. Choice of agent may depend on physician experience and preference, formulary coverage, and/or patient preference and insurance coverage. A trial of 4 to 12 weeks is recommended to assess efficacy.2 Another antimuscarinic agent can be considered in cases of failure or intolerability. The agents differ by route and frequency of administration, receptor and organ selectivity, molecular size and lipophilicity and metabolism, all of which translate to various (often subtle) clinical differences (Table 1). Possible adverse effects are dry mouth, blurred vision, pruritus, tachycardia, somnolence, impaired cognition, headache and constipation. Antimuscarinics are contraindicated in patients with urinary retention, gastric retention and uncontrolled narrow-angle glaucoma.27
Table 1.
Antimuscarinic drugs for treating overactive bladder
| Molecule | Receptor selectivity | Mode of release and trade name | Start dose | Maximum dose | Notes |
|---|---|---|---|---|---|
| Oxybutinin | Slightly higher M1-M3 over M2 (clinical significance unclear) | IR (Ditropan) | 2.5 mg tid | 5 mg qid | Limited by dry mouth rates |
| ER (Ditropan XL) | 5 mg od | 30 mg od | Cognitive impairment | ||
| Transdermal patch (Oxytrol) | 3.9 mg/d 2 patches/week | Systemic side effects comparable to placebo | |||
| Transdermal gel (Gelnique) | 1 gm gel (∼4 mg oxybutynin daily) | Low rate of skin reaction | |||
| Tolterodine | None | IR (Detrol) | 1 mg bid | 2 mg bid | |
| None | ER (Detrol LA) | 2 mg od | 4 mg od | Well-tolerated | |
| Trospium chloride | None | IR (Trosec) | 20 mg bid | 20 mg bid | Low penetration across blood-brain barrier (quaternary amine) |
| Darifenacin | Relatively M3 selective | ER (Enablex) | 7.5 mg od | 15 mg od | Low cognitive impairment,28 high rate constipation |
| Solifenacin | Modest activity M3 over M2 and marginal for M1 | ER (Vesicare) | 5 mg od | 10 mg od | Higher rate dry mouth at 10 mg |
| Fesoterodine | None | ER (Toviaz) | 4 mg od | 8 mg od | |
A new drug will be available in near future. Mirabegron (B3-adrenoreceptor agonist) showed benefit over placebo and similar improvement as tolterodine. The adverse effects profile seems superior to antimuscarinics (mainly for cognitive impairment), however, an assessment of long-term safety profile on heart rate and blood pressure needs to be performed.31
The use of BoTN-A for refractory OAB is not approved by Health Canada at this time, but experience exists in other countries. The continence rates following BoTN-A injection varies between 29% to 87%.32,33 It has a variable duration of action (mean 6–9 months) with loss of efficacy seen within the first year of injection. Repeat injections appear to have a maintained efficacy without increase in adverse events (grade B).34 This treatment is not yet approved for idiopathic OAB in Canada.35 The optimal dose, site of injection, appropriate population and long-term safety remain unclear. The need for post-treatment catheterization is 0% to 25% for 100 units.36,37 BoTN-A must be used with caution in conditions that interfere with neuromuscular transmission (myasthenia gravis, Eaton- Lambert syndrome, Charcot-Marie-Tooth and aminoglycoside treatment).
Sacral neuromodulation (level of evidence 1–3) (grade A) is approved by Health Canada for refractory UUI. The cure rate of UUI is 39% and an improvement of greater than 50% is seen in 67% of patients. Long-term success has been evaluated with more than 10 years follow-up with sustained results.38 Other possible complications are pain at implantation site (2.5%), lead migration (0.6%), wound problems (7%), bowel dysfunction (6%), infection (2.5%) and generator problems (5%).35 It is an expensive treatment and proper patient selection is paramount.39,40
Percutaneous tibial nerve stimulation (PTNS) is another option for refractory UUI with a response rate of 54% to 81%.41,42 New evidence suggests that PTNS lacks side effects and that a 12-week treatment offers a durable response even at 12 month of follow-up; however, maintenance treatment appears to be necessary for sustained efficacy (level of evidence 1, grade B).35,42,43 Unfortunately, this treatment is labour intensive, the cost is higher than anticholinergic therapy and the long-term outcome is unavailable.
Augmentation cystoplasty can be considered in special circumstances after failing all other options. Patients should be aware of the potential need for intermittent catheterization. However, only 50% of patients will be satisfied with the outcome of this procedure (level of evidence 3, grade C).20
Specific considerations in men
The management of male UUI follows the same general principles as above. Antimuscarinic agents may be used alone as first line therapy when primary idiopathic OAB exists. Because OAB increases with age, as does benign prostatic obstruction (BPO), a significant number of patients will have both conditions. In these patients, the BPO should be treated first with either alpha-blockers or 5-alpha-reduc-tase inhibitors (5-ARIs), or a combination of the two (level of evidence 2, grade B). If storage symptoms persist after a trial of alpha-blockers for 4 to 6 weeks, an antimuscarinic therapy can be instituted safely if the PVR is low (<200 mL) and the maximum urinary flow rate is >5 mL/s (level of evidence 2, grade B).44,45 Antimuscarinics have been shown not to significantly increase the PVR or the rate of acute urinary retention.46
Specific considerations in frail elderly
Pharmacologic therapy must be approached very carefully in the geriatric population due to the increase in potential side effects in this patient population. For UUI or MUI, antimuscarinic drugs can be prescribed with varying success rates (level of evidence 2, grade B).30 When starting therapy, important factors, including polypharmacy, pharmacokinetics, adverse drug reactions, drug-drug interactions and drug-disease interactions, must be considered.14,15 Moreover, dose titration regimen and evaluation of the balance between clinical benefits and side effects should be considered. There is potential of a negative impact on cognitive function with any antimuscarinic.28,47
Other etiologies may contribute to OAB symptoms in this patient population, including BOO, detrusor underactivity or detrusor hyperactivity with impaired contractility, with an elevated PVR. The treatment of these conditions should be aimed first toward ensuring bladder emptying (grade B).48
Treatment of SUI
Women
Initial management should start with lifestyle advice, physical therapies, scheduled voiding, behavioural therapies and medication. Lifestyle advice includes caffeine reduction (grade B), weight loss for the obese person (grade A), control of constipation (grade C), decrease in fluid intake in patients who are overhydrating and efforts to decrease chronic cough (smoking cessation) (grade C).2,19 PFMT should be offered as first-line therapy for SUI (grade A).49 Intensive and supervised PMFT is recommended if available (grade A).50 The benefit of biofeedback is unknown (grade B). Vaginal cones can be offered for first-line treatment for SUI or MUI, but its use may be limited due to discomfort.20 (grade B) Pessaries may be considered in the treatment of SUI even without concomitant pelvic organ prolapse (level of evidence 3, grade C).16,51 Behavioural therapy improves symptoms at 3 months, but this difference is not sustained at 12 months (level of evidence 2).52 Oral estrogen replacement is not recommended for SUI (level of evidence 2, grade B).49 Initial conservative management should be attempted for 8 to 12 weeks and thereafter the patient should be reassessed for success.2
When urethral hypermobility (UH) is present, the surgical treatment options include retropubic suspension, bladder neck slings and synthetic midurethral sling (MUS) (grade A). When intrinsic urethral deficiency is the primary cause, treatment options include bulking agents (grade B), bladder neck slings (grade A)/retropubic MUS and artificial urinary sphincter (grade B).2
Open retropubic colposuspension is effective for primary SUI which has longevity (grade A).53 In the past decade, open colposuspension has been largely replaced by MUS. The risk of voiding dysfunction and need for prolapse surgery is higher with open colposuspension than MUS, including the retropubic approach (level of evidence 1).
Bladder neck slings (BNS) can be performed with a variety of materials, including fascia (autologous or cadaveric), porcine dermis and mesh. Autologous fascial sling (AFS) is an effective treatment for SUI that has longevity and may be more effective than other biological and synthetic slings (grade A). The porcine dermal graft appears to lose tensile strength over time and is associated with a decreased cure rate compared to AFS and MUS (Table 2).54 BNS leads to the development of more storage LUTS than MUS.
Table 2.
Cure/dry rates of different anti-incontinence procedures for SUI
| Category | Procedure | Objective cure rate (short term) | Objective cure rate (long term) | Level of evidence | Comments |
|---|---|---|---|---|---|
| BNS | AFS | 90% | 82% after 48 mo59 | A | |
| CFS | 74% | 80% up to 43 mo59 | B | ||
| Porcine dermis | 73% | 54% at 36 mo54,61 | B | Not recommended | |
| MUS | Retropubic (TVT) | 88% | 90% at 10 y62 | A | Similar subjective cure rate (83%), TOT less complications |
| TOT | 84% | 84% at 5 y62 | B | ||
| Open colposuspension | Burch | MMK, needle suspension and paravaginal defect repair not recommended | |||
| MMK | 85–90% | 70% at 5 y63 | A |
SUI: stress urinary incontinence; BNS: bladder neck sling; MUS: midurethral sling; AFS: autologous sling; TVT: transvaginal tape; TOT: transobturator tape; MMK: Marshall-Marchetti-Krantz.
Retropubic MUS is more effective than Burch colposuspension, but equally as effective as fascial slings (level of evidence 2) (Table 1).55 AFS may result in more de novo storage LUTS than retropubic MUS. Retropubic and transobturator MUS are equally effective at 6 to 12 months and complication rates are comparable (level of evidence 2).56,57 MUS are contraindicated with urethrovaginal fistula, urethral diverticulum, intra-operative urethral injury, active UTI and untreated urinary malignancy. Retropubic slings have higher rates of bladder perforation, while transobturator tapes are associated with higher rates of groin pain and mesh exposure.58 Intraoperative cystoscopy is recommended during the placement of any MUS to assess for any injury to the bladder or urethra.59 Patients must be counselled preoperatively that the use of mesh in any pelvic floor surgery can be associated with complications ranging from minor to major (such as erosion), and these complications may necessitate repeat surgical intervention. Patients should be invited to read the Food and Drug Administration and/or Health Canada advisory on these products.
In an attempt to reduce morbidity associated with synthetic MUS, single incision slings were developed. These slings have potential advantages of reduced operative time, minimal dissection and less postoperative pain. However, available data and short-term results are insufficient to allow any recommendation.60
Currently, the Marshall-Marchetti-Krantz (MMK), the needle suspension procedure and paravaginal defect repair are not recommended for SUI (grade A). Laparoscopic colposuspension shows comparable subjective outcome, but poorer objective outcome compare to open colposuspension and MUS in the short- and medium-term (level of evidence 2). It is not recommended for routine surgical treatment of SUI (grade A). However, it might be considered in women who need a concomitant laparoscopic surgery and, in these cases, experienced laparoscopic surgeons should perform it (grade D).20
Periurethral bulking agents are an option for patients who do not wish to undergo more invasive surgery. Other indications include elderly and patients with a high anesthetic risk. The benefit of bulking agents is limited and short-term. Patients should be aware that repeat injections are likely to be required and that efficacy (48% at 12 to 23 months to 32% at 24 to 47 months) is inferior to conventional surgical techniques and diminishes over time (dry rate on a 24-hour pad test) (grade B).49,59
Data regarding use of the artificial sphincter for female SUI are limited, but the procedure may be considered with nonfunctioning urethras secondary to trauma to the pelvic nerves, severe intrinsic sphincter deficiency with multiple prior failed surgical procedures and significant SUI with poor bladder contractility (level of evidence 3, grade B).59 Erosion (28%), infection and device malfunction are potential complications.
Post-prostatectomy
As a conservative and preventive management of PPI, PFMT is recommended for the initial treatment for PPUI.9 PMFT can be either self-administered or assisted by a physical therapist. Duration benefit may be modest and short (<12 months after surgery) (level of evidence 2, grade B).11 Preoperative biofeedback-assisted behavioural training can shorten the time to regain continence postoperatively and can reduce the prevalence of severe persistent incontinence 6 months after prostatectomy (level of evidence 2, grade B).64–66 However, these observations are inconsistent. Postoperative electrical stimulation or biofeedback does not appear to improve continence outcomes over PMFT (level of evidence 2, grade B).66
A surgical intervention is offered usually 6 to 12 months after prostate surgery.6 Surgical choice is dependent on the severity of incontinence and patient expectations, and includes male sling, para-urethral inflatable devices or artificial urinary sphincter (AUS). In patients with previous bladder neck stricture, bladder neck patency must be established for a period of 6 months before undergoing surgery for PPUI (level of evidence 4, grade C).
Currently, for patients with mild to moderate PPI (24-hour pad test <400 g/d), the male sling appears to be an evolving option.6,7,9,67 The bone anchor (InVance, AMS, Minnetonka, MN) has demonstrated success rates (cured or improved) at medium term follow-up of 40% to 88%.6,67 Transobturator slings (AdVance, AMS, Minnetonka, MN) have success rates of 62% to 91%.6 Adjustable retropubic male slings (Argus, Red Leaf Medical, Mississauga, ON) have shown similar success rates at mid-term follow-up.6 Common complications associated with slings include transient acute urinary retention (0 to 15%), perineal/scrotal pain/numbness (16% to 72%) that usually resolve over time (within 3 months), erosion (0 to 2%), sling/perineal infection (2% to 12%) and de novo detrusor overactivity (0 to 14%).6,7 Several parameters are of paramount importance to have a successful implantation of a male sling. Patients with severe incontinence, previous radiation and prior failed AUS generally have worse outcomes (level of evidence 3, grade C).7 Male slings have potential advantages compared with AUS (physiologic voiding, less expensive, possible option for poor cognition patients and no manual dexterity is required).
Alternatively, the para-urethral inflatable devices may improve continence in the short- and medium-term. The results are variable and complications rate are high. For these reasons, making recommendation on para-urethral devices is impossible (level of evidence 3, grade D).7,68
The AUS remains the gold standard for PPUI with severe UI and after radiation therapy (level of evidence 1, grade A).11 Patients must be aware that radiation may increase the risk of complications (level of evidence 3, grade C). The long-term success of this procedure (59% to 90%) is well-established and outweighs the risk of revision (8% to 45%) and explantation (7% to 17%). The freedom of revision at 5 years is estimated at 50% to 75% (level of evidence 2). There is a high satisfaction rate of 87% to 90%.11 Age is not a contraindication, however, cognitive impairment and lack of manual dexterity may restrict AUS insertion (level of evidence 3, grade C).
Treatment of MUI
The initial management of MUI should focus on the predominant component, stress or urgency, and should be conservative in nature (level of evidence 3, grade C).2,69 Surgery for SUI is not contraindicated in patients with MUI and OAB or detrusor overactivity (grade B).2 It is important to counsel patients that the OAB component of UI may not improve with surgery (grade B).2 However, improvement or cure of OAB symptoms after sling anti-incontinence surgery ranges from 50% to 74%.70 The subjective and objective cure rate is lower than in patients with pure SUI (level of evidence 3).71 Due to the paucity of data, it is not possible to use preoperative criteria to predict which patients will have a better outcome (level of evidence 3).72
Treatment of UI in frail elderly patients
In the frail elderly patient, the degree of bother to the patient/caregiver, the goals of care, the level of cooperation and the life expectancy must be considered when planning management strategies (grade C).15 The treatment expectations from the patient/caregiver should be defined by using the continence paradigm.73 It is defined as four states in which patients can be categorized: incontinent (wet), contained incontinence (pads), dependant continence (assistance, behavioural treatment and medications) and independent continence (dry). Conservative and behavioural treatments should be considered: lifestyle changes (grade C), bladder training for fit patient (grade B) and prompted voiding for frail (grade A–B).15 Pelvic floor muscle exercises can be offered to cognitively intact patients, but data on their efficacy are lacking (grade C).15 For female patients with SUI or MUI with predominantly stress, who failed conservative management, age is not a contraindication for surgical treatment (grade C).74 Bulking agents may be effective in some older women with genuine SUI (grade C). In a subset of patients (minimal mobility, advanced dementia and nocturnal urinary incontinence) contained continence is sometimes the only available choice.14
Conclusion
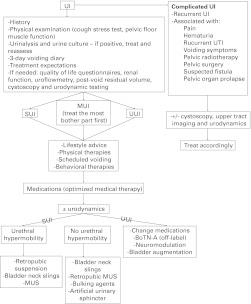
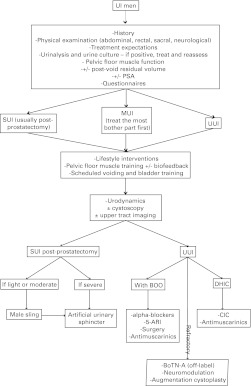
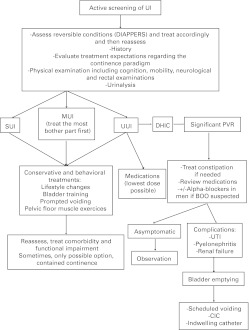
UI in adults is an evolving and burgeoning medical and surgical field. This guideline provides a concise approach to its evaluation and discusses the various treatment options. Algorithms summarize the management of UI (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
Management of urinary incontinence in women. UI: urinary incontinence; UTI: urinary tract infection; SUI: stress urinary incontinence; UUI: urgency urinary incontinence; MUI: mixed urinary incontinence; MUS: midurethreal sling.
Fig. 2.
Management of urinary incontinence in men. UI: urinary incontinence; PSA: prostate-specific antigen; SUI: stress urinary incontinence; UUI: urgency urinary incontinence; MUI: mixed urinary incontinence; BOO: bladder outlet obstruction; DHIC: detrusor hyperactivity with impaired contractility; ARI: alpha-reductase inhibitors; CIC: clean intermittent catheterization.
Fig. 3.
Management of urinary incontinence in elderly. UI: urinary incontinence; SUI: stress urinary incontinence; UUI: urgency urinary incontinence; MUI: mixed urinary incontinence; BOO: bladder outlet obstruction; DHIC: detrusor hyperactivity with impaired contractility; UTI: urinary tract infection; CIC: clean intermittent catheterization.
Appendix a.
Level of evidence and grade of recommendations
| Level | Type of evidence |
|
| |
| 1 | Meta-analysis of randomised trials or at least one randomised trial |
| 2 | One well-designed controlled study without randomisation or at least one other type of well-designed quasi-experimental study |
| 3 | Well-designed non-experimental studies (comparative, correlation and case reports) |
| 4 | Expert committee reports or opinions or clinical experience of respected authorities |
|
| |
| Grade | Nature of recommendations |
|
| |
| A | Clinical studies of good quality and consistency addressing the specific recommendations and including at least one randomized trial Based on level 1 evidence (recommended) |
| B | Well-conducted clinical studies, but without randomised clinical trials |
| Consistent level 2 or 3 evidence (recommended) | |
| C | Made despite the absence of directly applicable clinical studies of good quality |
| Level 4 studies or majority evidence (optional) | |
| D | Evidence inconsistent/inconclusive (no recommendation possible) or the evidence indicates that the drug should not be recommended |
Footnotes
Competing interests: Dr. Tu: Advisor and speaker: Allergan, Astellas, Pfizer, Medtronic, Coloplast, Researcher: Astellas, Pfizer, Allergan, Coloplast, Gynecare, Cook Myosite, Mednotric; Dr. Carlson: Advisor and speaker: Allergan, AMS, Astellas, Pfizer, Researcher: Astellas, Cook Myosite, Pfizer; Dr. Gajewski: Medtronic, Pfizer, Lilly, Astellas, Watson, Allergan; Dr. Jolivet: Advisor and speaker: Pfizer, Astellas, GSK, Formator: Laborie, Researcher: Pfizer, Allergan, Astellas; Dr. Bailly: Advisor and speaker: Allergan, Astellas, Pfizer, Researcher: Pfizer.
This paper has been peer-reviewed.
References
- 1.The Canadian continence foundation Incontinence: A Canadian perspective. 2007. http://www.canadian-continence.ca/pdf/Research_paper_August2007.pdf (Accessed September 10, 2012).
- 2.Abrams P, Andersson KE, Birder L, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29:213–40. doi: 10.1002/nau.20870. [DOI] [PubMed] [Google Scholar]
- 3.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 4.Herschorn S, Gajewski J, Schulz J, et al. A population-based study of urinary symptoms and incontinence: the Canadian Urinary Bladder Survey. BJU Int. 2008;101:52–8. doi: 10.1111/j.1464-410X.2007.07198.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Kim HW, Lee SJ, et al. Comparison of doxazosin with or without tolterodine in men with symptomatic bladder outlet obstruction and an overactive bladder. BJU Int. 2004;94:817–20. doi: 10.1111/j.1464-410X.2004.05039.x. [DOI] [PubMed] [Google Scholar]
- 6.Welk BK, Herschorn S. The male sling for post-prostatectomy urinary incontinence: a review of contemporary sling designs and outcomes. BJU Int. 2012;109:328–44. doi: 10.1111/j.1464-410X.2010.10502.x. . Epub 2011 Oct 17. [DOI] [PubMed] [Google Scholar]
- 7.Herschorn S, Bruschini H, Comiter C, et al. Surgical treatment of stress incontinence in men. Neurourol Urodyn. 2010;29:179–90. doi: 10.1002/nau.20844. [DOI] [PubMed] [Google Scholar]
- 8.Porena M, Mearini E, Mearini L, et al. Voiding dysfunction after radical retropubic prostatectomy: more than external urethral sphincter deficiency. Eur Urol. 2007;52:38–45. doi: 10.1016/j.eururo.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 9.Bauer RM, Gozzi C, Hubner W, et al. Contemporary management of postprostatectomy incontinence. Eur Urol. 2011;59:985–96. doi: 10.1016/j.eururo.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–63. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Bauer RM, Bastian PJ, Gozzi C, et al. Postprostatectomy incontinence: all about diagnosis and management. Eur Urol. 2009;55:322–33. doi: 10.1016/j.eururo.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 12. The Canadian Agency for Drugs and Technologies in Health. http://www.cadth.ca (Accessed September 11, 2012).
- 13.Offermans MP, Du Moulin MF, Hamers JP, et al. Prevalence of urinary incontinence and associated risk factors in nursing home residents: a systematic review. Neurourol Urodyn. 2009;28:288–94. doi: 10.1002/nau.20668. [DOI] [PubMed] [Google Scholar]
- 14.DuBeau CE. Beyond the bladder: management of urinary incontinence in older women. Clin Obstet Gynecol. 2007;50:720–34. doi: 10.1097/GRF.0b013e3180d0a4e7. [DOI] [PubMed] [Google Scholar]
- 15.DuBeau CE, Kuchel GA, Johnson T, 2nd, et al. Incontinence in the frail elderly: report from the 4th International Consultation on Incontinence. Neurourol Urodyn. 2010;29:165–78. doi: 10.1002/nau.20842. [DOI] [PubMed] [Google Scholar]
- 16.Goode PS, Burgio KL, Richter HE, et al. Incontinence in older women. Jama. 2010;303:2172–81. doi: 10.1001/jama.2010.749. [DOI] [PubMed] [Google Scholar]
- 17.Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987;257:3076–81. doi: 10.1001/jama.1987.03390220074024. [DOI] [PubMed] [Google Scholar]
- 18.Ghoniem G, Stanford E, Kenton K, et al. Evaluation and outcome measures in the treatment of female urinary stress incontinence: International Urogynecological Association (IUGA) guidelines for research and clinical practice. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:5–33. doi: 10.1007/s00192-007-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritel X, Fauconnier A, Bader G, et al. Diagnosis and management of adult female stress urinary incontinence: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur J Obstet Gynecol Reprod Biol. 2010;151:14–9. doi: 10.1016/j.ejogrb.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 20.Thuroff JW, Abrams P, Andersson KE, et al. EAU guidelines on urinary incontinence. Eur Urol. 2011;59:387–400. doi: 10.1016/j.eururo.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Holroyd-Leduc JM, Tannenbaum C, Thorpe KE, et al. What type of urinary incontinence does this woman have? JAMA. 2008;299:1446–56. doi: 10.1001/jama.299.12.1446. [DOI] [PubMed] [Google Scholar]
- 22.Amir B, Farrell SA. SOGC Committee opinion on urodynamics testing. J Obstet Gynaecol Can. 2008;30:717–27. doi: 10.1016/S1701-2163(16)32921-8. [DOI] [PubMed] [Google Scholar]
- 23.Azzouzi AR, Ballereau C, Desgranchamps F, et al. [Management of male urinary incontinence after radical prostatectomy (CTMH AFU 2006-1/5): incidence and role of urodynamic assessment and electromyography] Prog Urol. 2008;18:14–8. doi: 10.1016/j.purol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs CF, Johnson TM, 2nd, Ouslander JG. Office management of geriatric urinary incontinence. Am J Med. 2007;120:211–20. doi: 10.1016/j.amjmed.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 25.Resnick NM. An 89-year-old woman with urinary incontinence. JAMA. 1996;276:1832–40. doi: 10.1001/jama.1996.03540220056031. [DOI] [PubMed] [Google Scholar]
- 26.Nygaard I. Clinical practice. Idiopathic urgency urinary incontinence. N Engl J Med. 2010;363:1156–62. doi: 10.1056/NEJMcp1003849. [DOI] [PubMed] [Google Scholar]
- 27.Smith AL, Wein AJ. Urinary incontinence: pharmacotherapy options. Ann Med. 2011;43:461–76. doi: 10.3109/07853890.2011.564203. [DOI] [PubMed] [Google Scholar]
- 28.Kay G, Crook T, Rekeda L, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol. 2006;50:317–26. doi: 10.1016/j.eururo.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 29.Cardozo L. Systematic review of overactive bladder therapy in females. Can Urol Assoc J. 2011;5:S139–42. doi: 10.5489/cuaj.11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagg AS, Cardozo L, Chapple C, et al. Overactive bladder syndrome in older people. BJU Int. 2007;99:502–9. doi: 10.1111/j.1464-410X.2006.06677.x. [DOI] [PubMed] [Google Scholar]
- 31.Tyagi P, Tyagi V, Chancellor M. Mirabegron: a safety review. Expert Opin Drug Saf. 2011;10:287–94. doi: 10.1517/14740338.2011.542146. [DOI] [PubMed] [Google Scholar]
- 32.Cartwright R, Renganathan A, Cardozo L. Current management of overactive bladder. Curr Opin Obstet Gynecol. 2008;20:489–95. doi: 10.1097/GCO.0b013e32830fe38c. [DOI] [PubMed] [Google Scholar]
- 33.Rovner E, Kennelly M, Schulte-Baukloh H, et al. Urodynamic results and clinical outcomes with intradetrusor injections of onabotulinumtoxinA in a randomized, placebo-controlled dose-finding study in idiopathic overactive bladder. Neurourol Urodyn. 2011;30:556–62. doi: 10.1002/nau.21021. [DOI] [PubMed] [Google Scholar]
- 34.Smith CP. Botox(R) in urology--will it become standard of care for urge urinary incontinence? J Urol. 2010;184:2235–6. doi: 10.1016/j.juro.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 35.Apostolidis A. Neuromodulation for intractable OAB. Neurourol Urodyn. 2011;30:766–70. doi: 10.1002/nau.21123. [DOI] [PubMed] [Google Scholar]
- 36.Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol. 2010;184:2416–22. doi: 10.1016/j.juro.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Rickey LM, Kenton K. Botulinum toxin: new option for refractory lower urinary tract symptoms in women. Clin Obstet Gynecol. 2008;51:176–86. doi: 10.1097/GRF.0b013e3181621fd2. [DOI] [PubMed] [Google Scholar]
- 38.Al-zahrani AA, Elzayat EA, Gajewski JB. Long-term outcome and surgical interventions after sacral neuromodulation implant for lower urinary tract symptoms: 14-year experience at 1 center. J Urol. 2011;185:981–6. doi: 10.1016/j.juro.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 39.Yamanishi T, Kamai T, Yoshida K. Neuromodulation for the treatment of urinary incontinence. Int J Urol. 2008;15:665–72. doi: 10.1111/j.1442-2042.2008.02080.x. [DOI] [PubMed] [Google Scholar]
- 40.Herbison GP, Arnold EP. Sacral neuromodulation with implanted devices for urinary storage and voiding dysfunction in adults. Cochrane Database Syst Rev. 2009:CD004202. doi: 10.1002/14651858.CD004202.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Ridout AE, Yoong W. Tibial nerve stimulation for overactive bladder syndrome unresponsive to medical therapy. J Obstet Gynaecol. 2010;30:111–4. doi: 10.3109/01443610903428922. [DOI] [PubMed] [Google Scholar]
- 42.Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol. 2010;183:1438–43. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Occhino JA, Siegel SW. Sacral nerve modulation in overactive bladder. Curr Urol Rep. 2010;11:348–52. doi: 10.1007/s11934-010-0132-6. [DOI] [PubMed] [Google Scholar]
- 44.Chapple C. Systematic review of therapy for men with overactive bladder. Can Urol Assoc J. 2011;5:S143–5. doi: 10.5489/cuaj.11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Athanasopoulos A, Chapple C, Fowler C, et al. The role of antimuscarinics in the management of men with symptoms of overactive bladder associated with concomitant bladder outlet obstruction: an update. Eur Urol. 2011;60:94–105. doi: 10.1016/j.eururo.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 46.Ng CK, Gonzalez RR, Te AE. Refractory overactive bladder in men: update on novel therapies. Curr Urol Rep. 2006;7:456–61. doi: 10.1007/s11934-006-0054-5. [DOI] [PubMed] [Google Scholar]
- 47.Herschorn S, Pommerville P, Stothers L, et al. Tolerability of solifenacin and oxybutynin immediate release in older (> 65 years) and younger (</= 65 years) patients with overactive bladder: sub - analysis from a Canadian, randomized, double-blind study. Curr Med Res Opin. 2011;27:375–82. doi: 10.1185/03007995.2010.541433. [DOI] [PubMed] [Google Scholar]
- 48.Taylor JA, 3rd, Kuchel GA. Detrusor underactivity: Clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006;54:1920–32. doi: 10.1111/j.1532-5415.2006.00917.x. [DOI] [PubMed] [Google Scholar]
- 49.Shamliyan TA, Kane RL, Wyman J, et al. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med. 2008;148:459–73. doi: 10.7326/0003-4819-148-6-200803180-00211. [DOI] [PubMed] [Google Scholar]
- 50.Dumoulin C, Hay-Smith J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2010:CD005654. doi: 10.1002/14651858.CD005654.pub2. [DOI] [PubMed] [Google Scholar]
- 51.Nager CW, Richter HE, Nygaard I, et al. Incontinence pessaries: size, POPQ measures, and successful fitting. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1023–8. doi: 10.1007/s00192-009-0866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter HE, Burgio KL, Brubaker L, et al. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609–17. doi: 10.1097/AOG.0b013e3181d055d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapitan MC, Cody JD, Grant A. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev. 2009:CD002912. doi: 10.1002/14651858.CD002912.pub4. [DOI] [PubMed] [Google Scholar]
- 54.Guerrero KL, Emery SJ, Wareham K, et al. A randomised controlled trial comparing TVT, Pelvicol and autologous fascial slings for the treatment of stress urinary incontinence in women. BJOG. 2010;117:1493–502. doi: 10.1111/j.1471-0528.2010.02696.x. [DOI] [PubMed] [Google Scholar]
- 55.Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143–55. doi: 10.1056/NEJMoa070416. [DOI] [PubMed] [Google Scholar]
- 56.Richter HE, Albo ME, Zyczynski HM, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066–76. doi: 10.1056/NEJMoa0912658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novara G, Artibani W, Barber MD, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2010;58:218–38. doi: 10.1016/j.eururo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 58.Ross S, Robert M, Swaby C, et al. Transobturator tape compared with tension-free vaginal tape for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2009;114:1287–94. doi: 10.1097/AOG.0b013e3181c2a151. [DOI] [PubMed] [Google Scholar]
- 59.Dmochowski RR, Blaivas JM, Gormley EA, et al. Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol. 2010;183:1906–14. doi: 10.1016/j.juro.2010.02.2369. [DOI] [PubMed] [Google Scholar]
- 60.Walsh CA. TVT-Secur mini-sling for stress urinary incontinence: a review of outcomes at 12 months. BJU Int. 2011;108:652–7. doi: 10.1111/j.1464-410X.2011.10333.x. [DOI] [PubMed] [Google Scholar]
- 61.Giri SK, Hickey JP, Sil D, et al. The long-term results of pubovaginal sling surgery using acellular cross-linked porcine dermis in the treatment of urodynamic stress incontinence. J Urol. 2006;175:1788–92. doi: 10.1016/S0022-5347(05)01023-2. discussion 93. [DOI] [PubMed] [Google Scholar]
- 62.Cerruto MA, Artibani W. Transobturator versus retropubic synthetic slings: comparative efficacy and safety. Curr Opin Urol. 2011;21:275–80. doi: 10.1097/MOU.0b013e3283476edb. [DOI] [PubMed] [Google Scholar]
- 63.Lapitan MC, Cody JD, Grant A. Open retropubic colposuspension for urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn. 2009;28:472–80. doi: 10.1002/nau.20780. [DOI] [PubMed] [Google Scholar]
- 64.Centemero A, Rigatti L, Giraudo D, et al. Preoperative pelvic floor muscle exercise for early continence after radical prostatectomy: a randomised controlled study. Eur Urol. 2010;57:1039–43. doi: 10.1016/j.eururo.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 65.Burgio KL, Goode PS, Urban DA, et al. Preoperative biofeedback assisted behavioral training to decrease post-prostatectomy incontinence: a randomized, controlled trial. J Urol. 2006;175:196–201. doi: 10.1016/S0022-5347(05)00047-9. [DOI] [PubMed] [Google Scholar]
- 66.Goode PS, Burgio KL, Johnson TM, 2nd, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: a randomized controlled trial. JAMA. 2011;305:151–9. doi: 10.1001/jama.2010.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welk BK, Herschorn S. Are male slings for post-prostatectomy incontinence a valid option? Curr Opin Urol. 2010;20:465–70. doi: 10.1097/MOU.0b013e32833ecd09. [DOI] [PubMed] [Google Scholar]
- 68.Saussine C, Azzouzi AR, Ballereau C, et al. [Management of male urinary incontinence after radical prostatectomy (CTMH AFU 2006-3/5): place of periurethral balloon and suburethral tape] Prog Urol. 2008;18:23–8. doi: 10.1016/j.purol.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Khullar V, Cardozo L, Dmochowski R. Mixed incontinence: current evidence and future perspectives. Neurourol Urodyn. 2010;29:618–22. doi: 10.1002/nau.20907. [DOI] [PubMed] [Google Scholar]
- 70.Sajadi KP, Vasavada SP. Overactive bladder after sling surgery. Curr Urol Rep. 2010;11:366–71. doi: 10.1007/s11934-010-0136-2. [DOI] [PubMed] [Google Scholar]
- 71.Murray S, Lemack GE. Overactive bladder and mixed incontinence. Curr Urol Rep. 2010;11:385–92. doi: 10.1007/s11934-010-0146-0. [DOI] [PubMed] [Google Scholar]
- 72.Katsumi HK, Rutman MP. Can we predict if overactive bladder symptoms will resolve after sling surgery in women with mixed urinary incontinence? Curr Urol Rep. 2010;11:328–37. doi: 10.1007/s11934-010-0133-5. [DOI] [PubMed] [Google Scholar]
- 73.Fonda D, Abrams P. Cure sometimes, help always--a “continence paradigm” for all ages and conditions. Neurourol Urodyn. 2006;25:290–2. doi: 10.1002/nau.20187. [DOI] [PubMed] [Google Scholar]
- 74.Campeau L, Tu LM, Lemieux MC, et al. A multicenter, prospective, randomized clinical trial comparing tension-free vaginal tape surgery and no treatment for the management of stress urinary incontinence in elderly women. Neurourol Urodyn. 2007;26:990–4. doi: 10.1002/nau.20440. [DOI] [PubMed] [Google Scholar]