Abstract
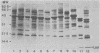
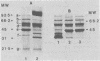
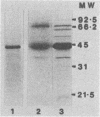
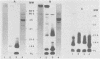
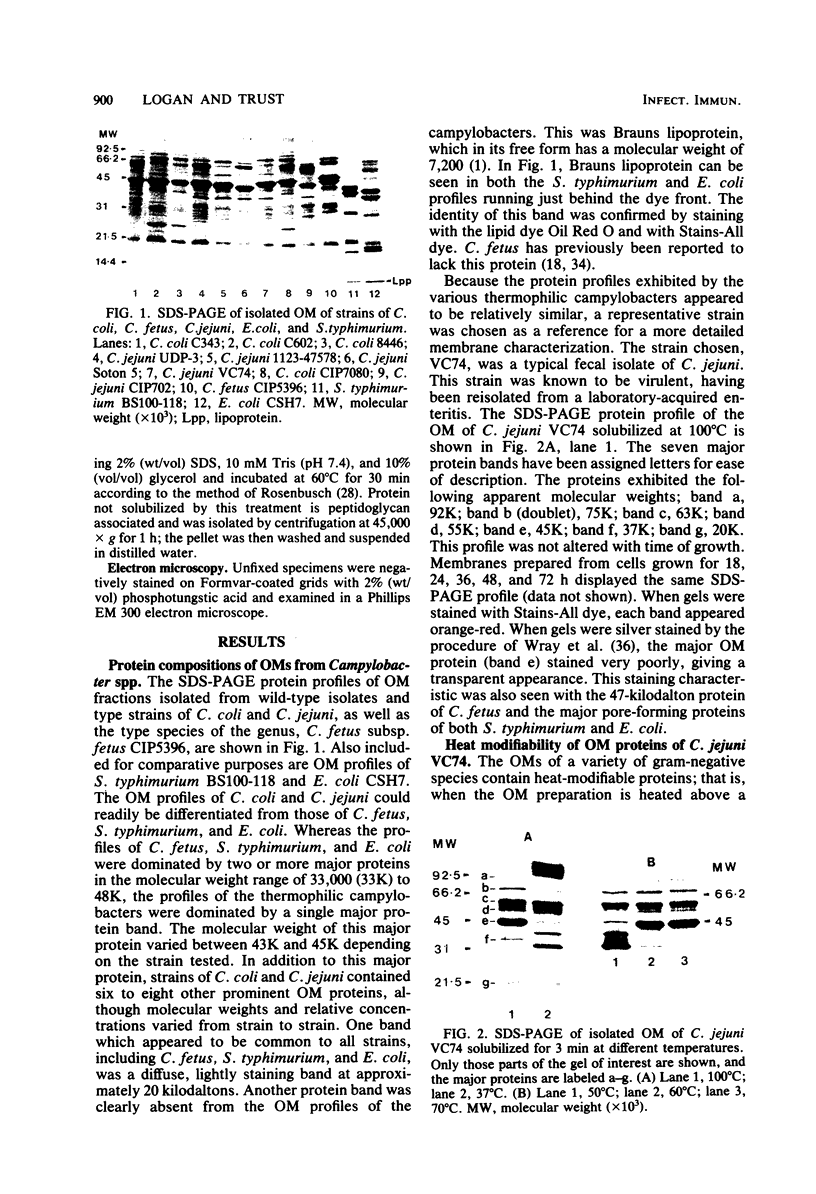
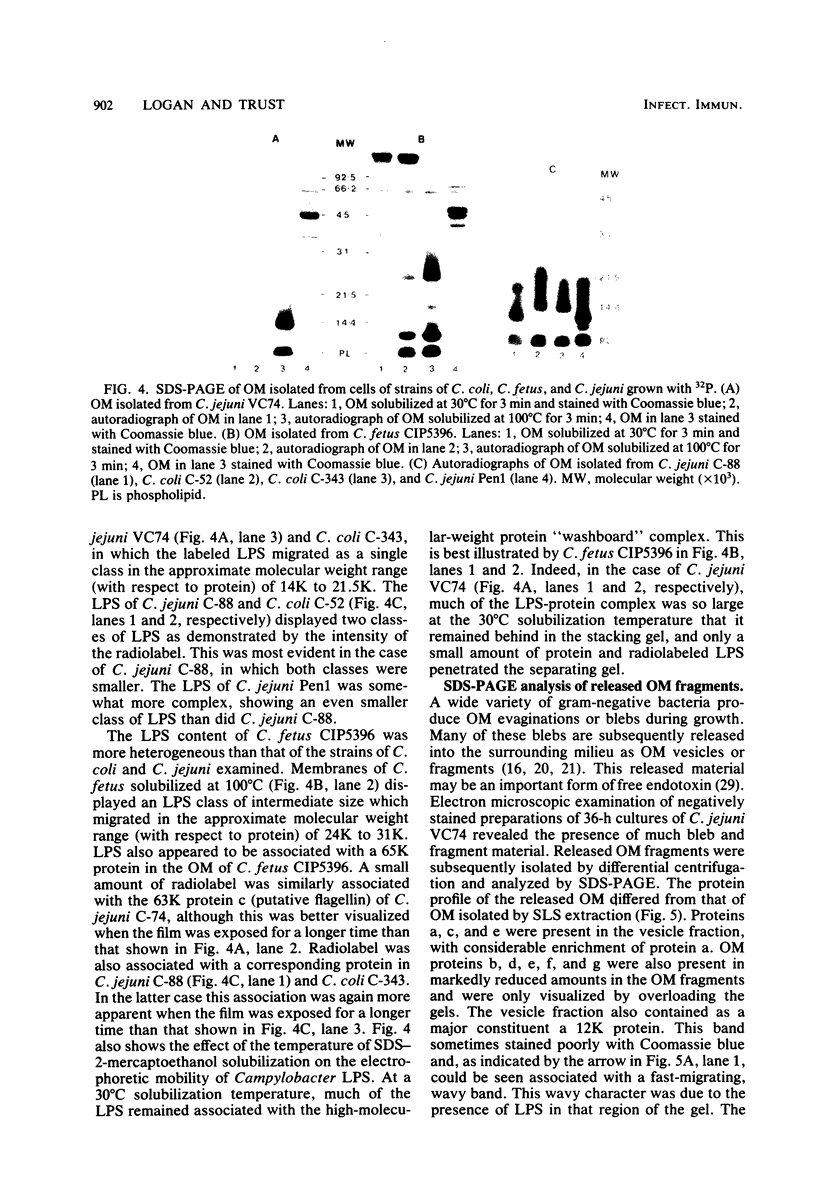
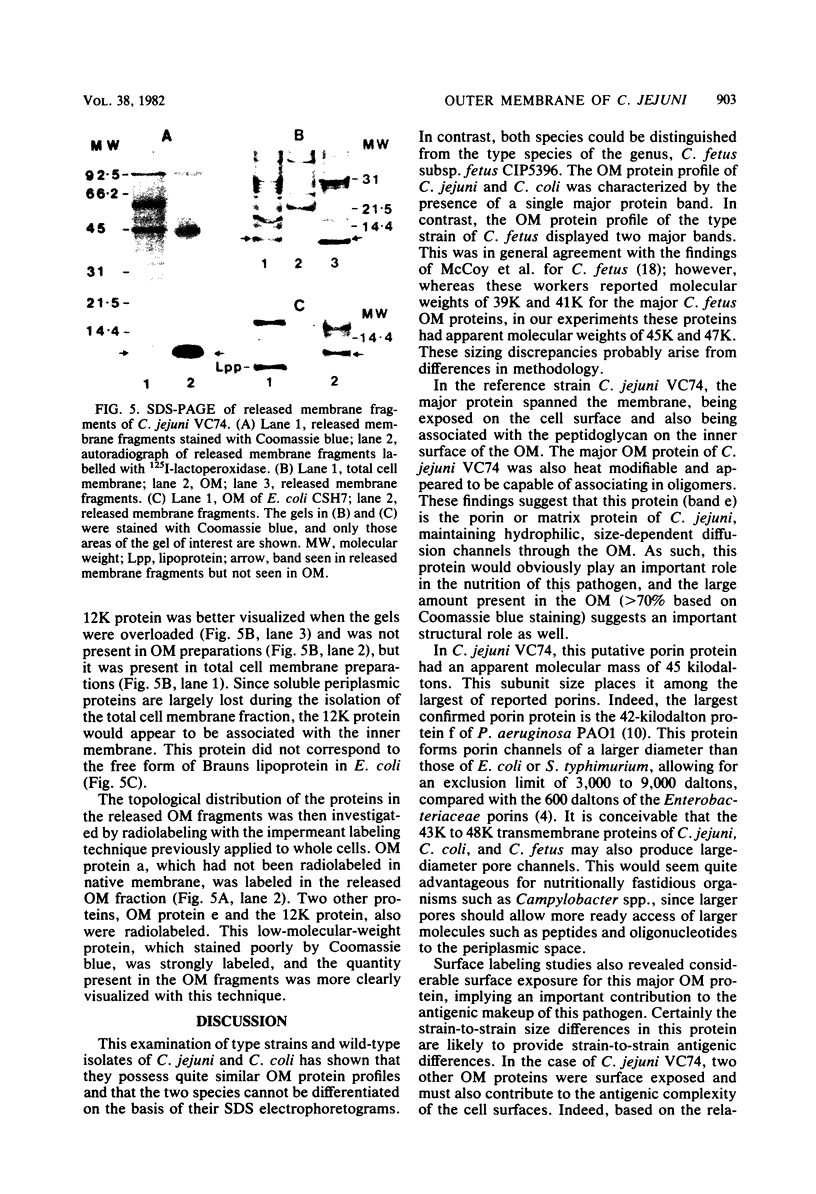
Outer membranes were isolated from type strains and wild-type isolates of Campylobacter jejuni and Campylobacter coli by sodium lauryl sarcosinate extraction, and the polypeptide complement and lipopolysaccharide (LPS) content were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein profiles exhibited by membranes from both species were quite similar, but could be distinguished from the type strain of the genus, C. fetus subsp. fetus CIP5396. The sodium dodecyl sulfate electrophoretograms of C. jejuni and C. coli were dominated by a major polypeptide band. In the reference strain C. jejuni VC74, this polypeptide had an apparent molecular weight of 45,000, was heat modifiable, and was shown to be transmembrane by virtue of its peptidoglycan association and surface exposure. Two other proteins with approximate molecular weights of 37,000 and 73,000 were also surface exposed on C. jejuni VC74 and represented potential surface antigens. The LPS of C. jejuni and C. coli was of low molecular weight, suggesting that serotypic differences due to LPS were based on different carbohydrate compositions of core LPS. In contrast, the LPS of C. fetus CIP5396 exhibited O antigen polysaccharide chains of intermediate chain length. Fragments of outer membranes released during growth of C. jejuni VC74 displayed a polypeptide profile which differed from that of sarcosinate-extracted outer membranes. Radiolabeling demonstrated that the proteins exposed on the surface of this released membrane differed from those exposed on the cell surface and would likely contribute to the antigenic complexity of C. jejuni.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzler J. P., Dekeyser P., Detrain M., Dehaen F. Related vibrio in stools. J Pediatr. 1973 Mar;82(3):493–495. doi: 10.1016/s0022-3476(73)80131-3. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gankema H., Wensink J., Guinée P. A., Jansen W. H., Witholt B. Some characteristics of the outer membrane material released by growing enterotoxigenic Escherichia coli. Infect Immun. 1980 Aug;29(2):704–713. doi: 10.1128/iai.29.2.704-713.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V., Peacock A. C. Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem. 1973 Nov;56(1):43–51. doi: 10.1016/0003-2697(73)90167-x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Buckley J. T., Ishiguro E. E., Phipps B. M., Monette J. P., Trust T. J. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida. J Bacteriol. 1981 Sep;147(3):1077–1084. doi: 10.1128/jb.147.3.1077-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. T., Parker C. D. Identification and preliminary characterization of Vibrio cholerae outer membrane proteins. J Bacteriol. 1981 Feb;145(2):1018–1024. doi: 10.1128/jb.145.2.1018-1024.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Lior H., Woodward D. L., Edgar J. A., LaRoche L. J. Serotyping by slide agglutination of Campylobacter jejuni and epidemiology. Lancet. 1981 Nov 14;2(8255):1103–1104. doi: 10.1016/s0140-6736(81)91293-9. [DOI] [PubMed] [Google Scholar]
- MacIntyre S., Trust T. J., Buckley J. T. Identification and characterization of outer membrane fragments released by Aeromonas sp. Can J Biochem. 1980 Oct;58(10):1018–1025. doi: 10.1139/o80-138. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McCoy E. C., Wiltberger H. A., Winter J. Major outer membrane protein of Campylobacter fetus: physical and immunological characterization. Infect Immun. 1976 Apr;13(4):1258–1265. doi: 10.1128/iai.13.4.1258-1265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Metal ion dependence of a heat-modifiable protein from the outer membrane of Escherichia coli upon sodium dodecyl sulfate-gel electrophoresis. J Bacteriol. 1977 Oct;132(1):314–320. doi: 10.1128/jb.132.1.314-320.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J. M., Witholt B. K88-mediated binding of Escherichia coli outer membrane fragments to porcine intestinal epithelial cell brush borders. Infect Immun. 1981 Jan;31(1):42–51. doi: 10.1128/iai.31.1.42-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mug-Opstelten D., Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim Biophys Acta. 1978 Apr 4;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepersack F., Prigogyne T., Butzler J. P., Yourassowsky E. Campylobacter jejuni post-transfusional septicaemia. Lancet. 1979 Oct 27;2(8148):911–911. doi: 10.1016/s0140-6736(79)92738-7. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Nikaido H. Outer membrane of gram-negative bacteria. XVIII. Electron microscopic studies on porin insertion sites and growth of cell surface of Salmonella typhimurium. J Bacteriol. 1978 Aug;135(2):687–702. doi: 10.1128/jb.135.2.687-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink J., Witholt B. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur J Biochem. 1981 May 15;116(2):331–335. doi: 10.1111/j.1432-1033.1981.tb05338.x. [DOI] [PubMed] [Google Scholar]
- Winter A. J., McCoy E. C., Fullmer C. S., Burda K., Bier P. J. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect Immun. 1978 Dec;22(3):963–971. doi: 10.1128/iai.22.3.963-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Wright E. P. Meningism associated with Campylobacter jejuni enteritis. Lancet. 1979 May 19;1(8125):1092–1092. doi: 10.1016/s0140-6736(79)92996-9. [DOI] [PubMed] [Google Scholar]