Abstract
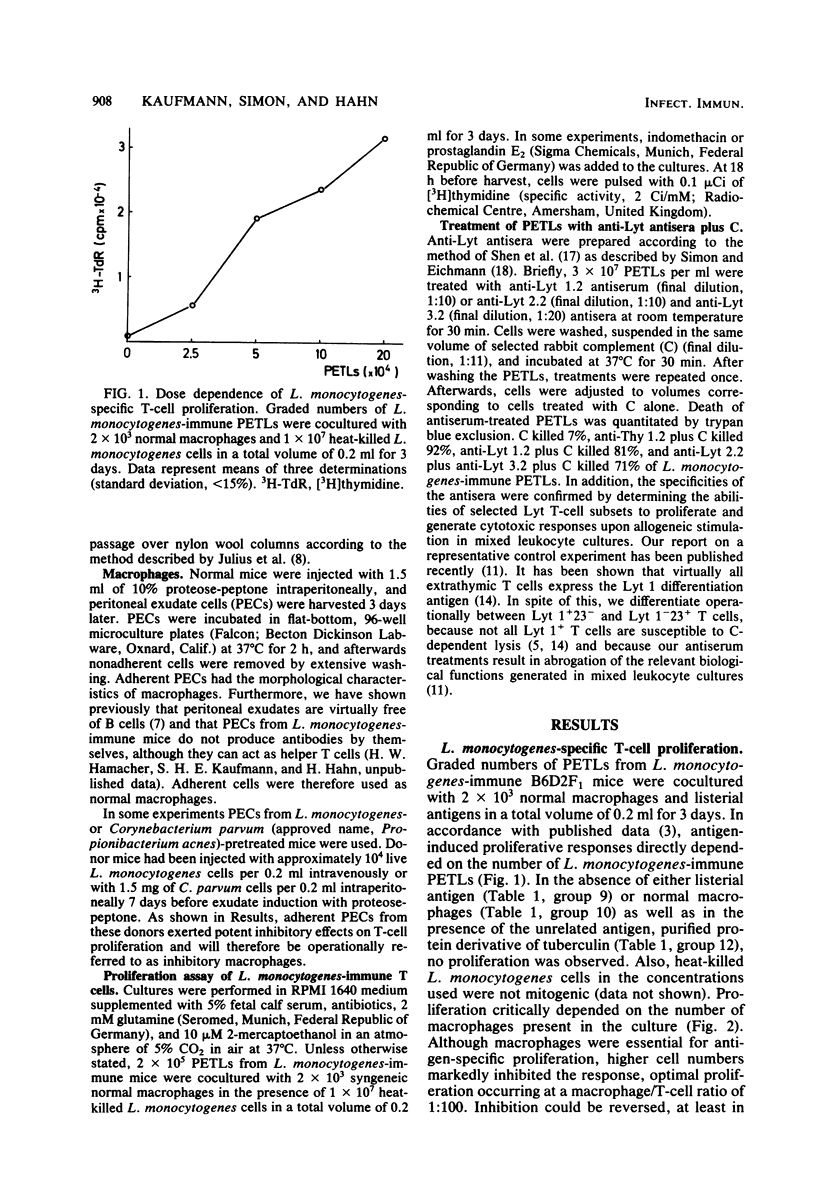
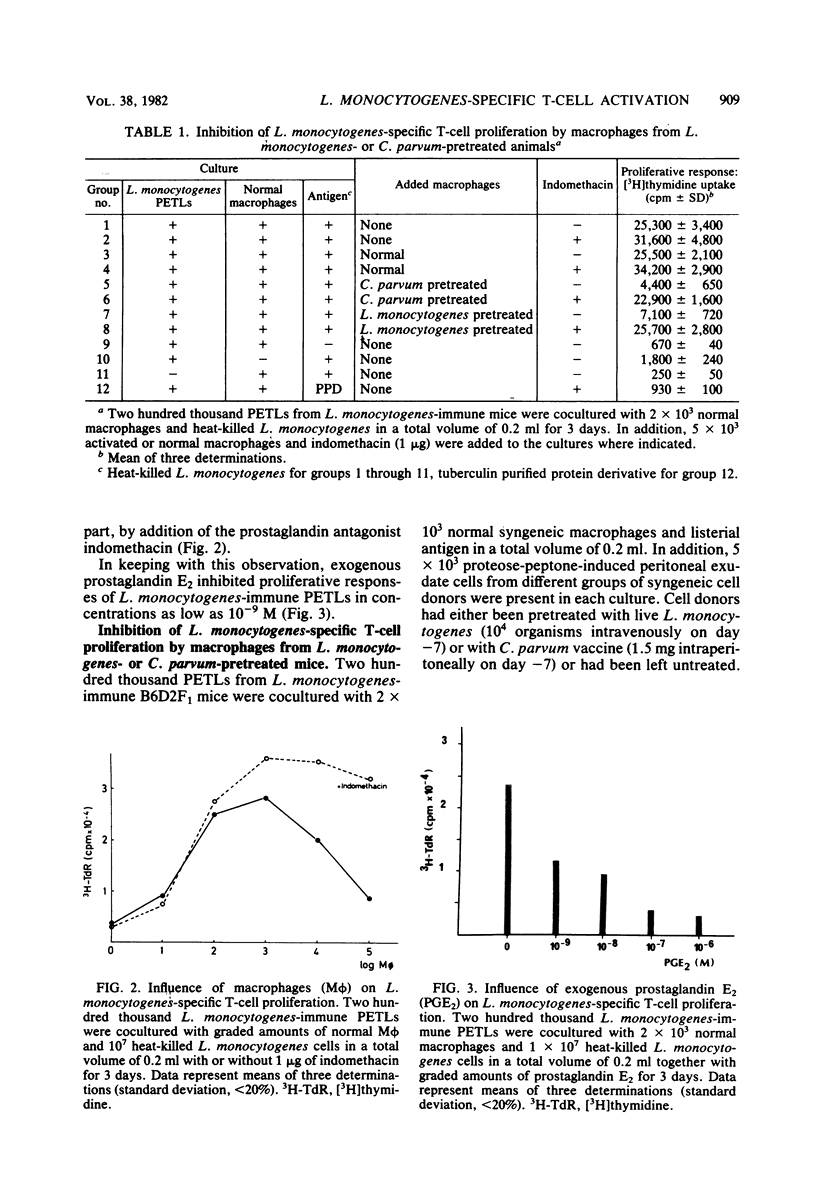
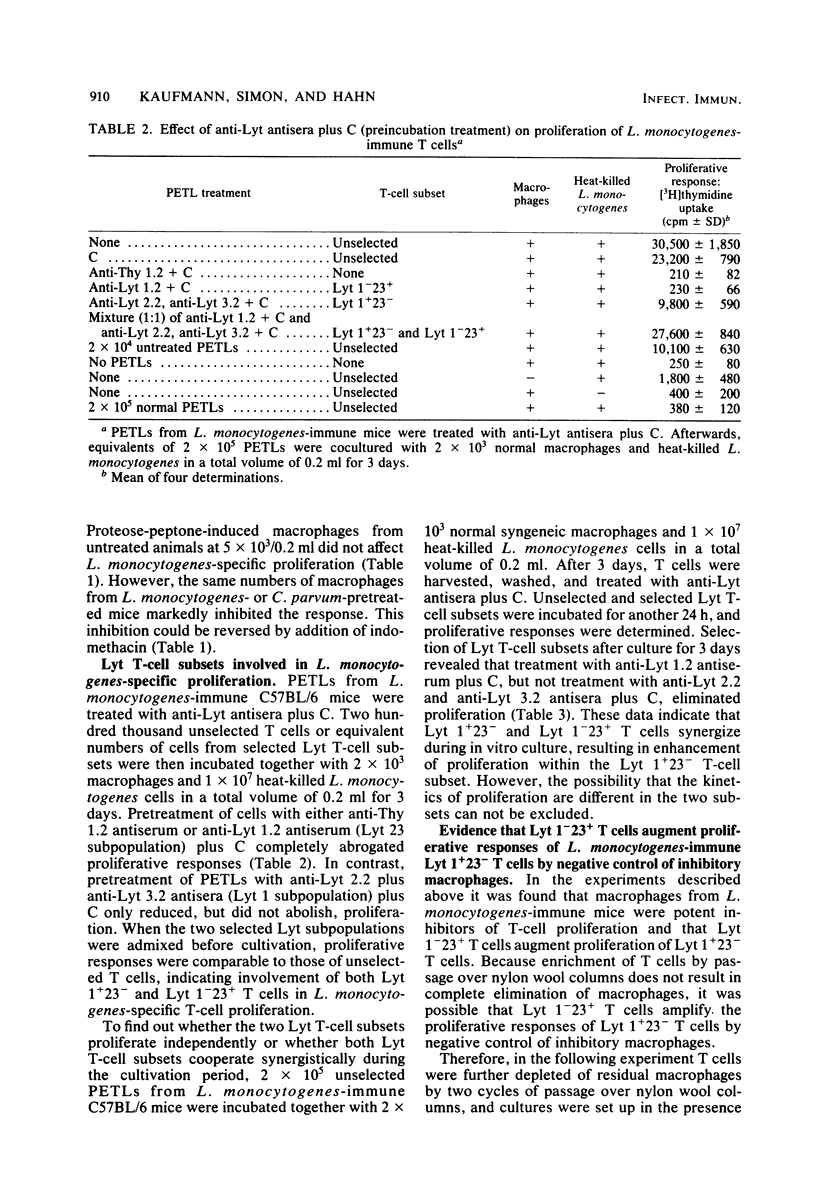
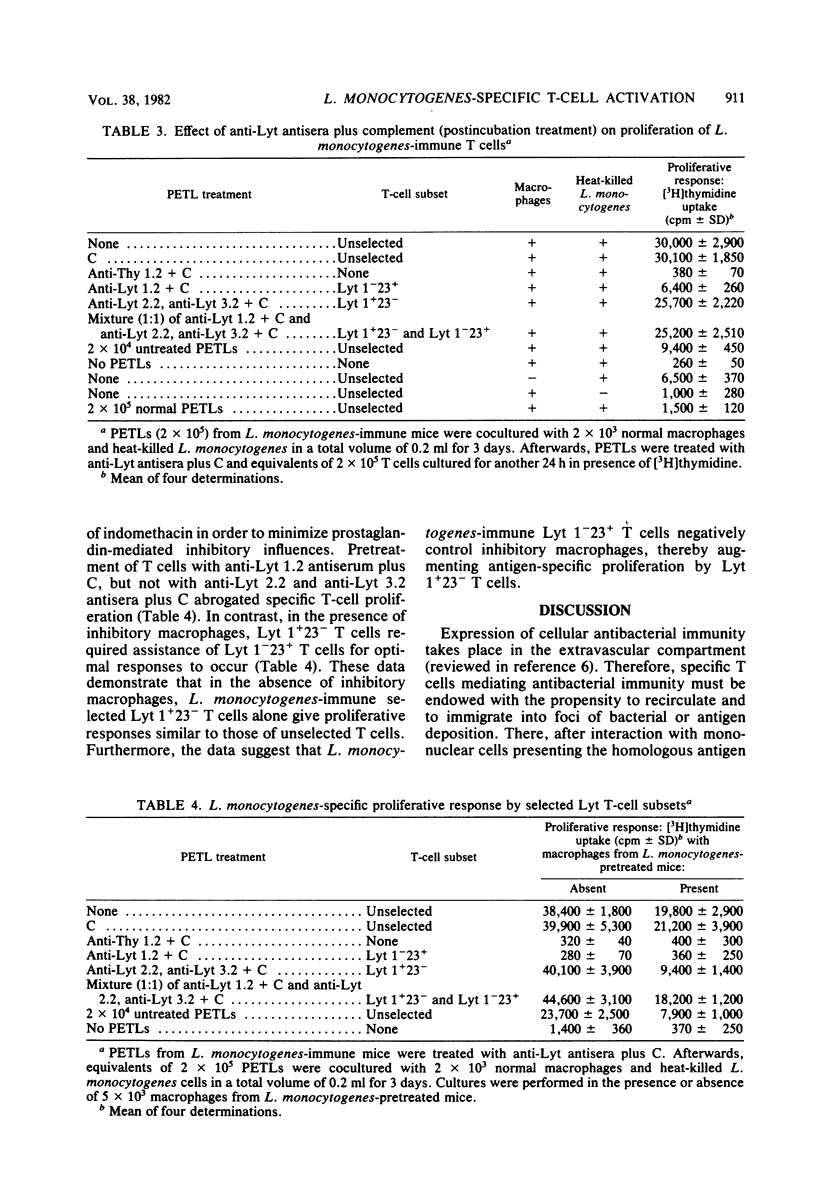
Peritoneal exudate T lymphocytes from Listeria monocytogenes-immune mice in the presence of the homologous antigen (heat-killed L. monocytogenes) and normal macrophages showed L. monocytogenes-specific proliferative responses. Proliferation was inhibited by macrophages from L. monocytogenes- or Corynebacterium parvum-pretreated mice as well as by exogenous prostaglandin E2. Macrophage-dependent inhibition of T-cell proliferation—at least in part—could be reversed by addition of indomethacin. When selected L. monocytogenes-immune Lyt T-cell subsets were cultured in the presence of inhibitory macrophages, pretreatment with anti-Lyt 1 antiserum plus complement completely abrogated proliferation and pretreatment with anti-Lyt 2 and anti-Lyt 3 antisera plus complement markedly reduced proliferation. However, a mixture (1:1) of the two preselected Lyt T-cell subsets resulted in complete reconstitution of proliferative responses. In contrast, when L. monocytogenes-immune peritoneal exudate T lymphocytes were treated with anti-Lyt antisera plus complement after culture, only treatment with anti-Lyt 1 antiserum plus complement affected proliferation, suggesting regulatory interactions between Lyt 1+23− and Lyt 1−23+ T cells during in vitro culture which result in proliferation within the Lyt 1+23− T-cell subset. After rigorous depletion of residual macrophages and in the presence of indomethacin, pretreatment with anti-Lyt 1 antiserum plus complement, but not with anti-Lyt 2 and 3 antisera plus complement, eliminated proliferation. The data presented indicate that interactions between macrophages and Lyt T-cell subsets regulate L. monocytogenes-specific T-cell activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantor H., Gershon R. K. Immunological circuits: cellular composition. Fed Proc. 1979 Jun;38(7):2058–2064. [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- Goronzy J., Schaefer U., Eichmann K., Simon M. M. Quantitative studies on T cell diversity. II. Determination of the frequencies and Lyt phenotypes of two types of precursor cells for alloreactive cytotoxic T cells in polyclonally and specifically activated splenic T cells. J Exp Med. 1981 Apr 1;153(4):857–870. doi: 10.1084/jem.153.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H., Falkenberg F., Chahinin M., Horn W. Peritoneal exudate T lymphocytes with specificity to sheep red blood cells. II. Inflammatory helper T cells and effector T cells in mice with delayed-type hypersensitivity and in suppressed mice. Immunology. 1979 Sep;38(1):51–55. [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H., Simon M. M., Röllinghoff M., Wagner H. Interleukin 2 induction in Lyt 1+ 23- T cells from Listeria monocytogenes-immune mice. Infect Immun. 1982 Sep;37(3):1292–1294. doi: 10.1128/iai.37.3.1292-1294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung J. T., Brooks S. B., Jakway J. P., Leonard L. L., Talmage D. W. Suppression of in vitro cytotoxic response by macrophages due to induced arginase. J Exp Med. 1977 Sep 1;146(3):665–672. doi: 10.1084/jem.146.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Ellner J. J., Kearse L. A., Jr, Kazura J. W., Mahmoud A. A. Role of arginase in killing of schistosomula of Schistosoma mansoni. J Exp Med. 1980 Jun 1;151(6):1557–1562. doi: 10.1084/jem.151.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. M., Eichmann K. T cell subsets participating in the generation of cytotoxic T cells. Springer Semin Immunopathol. 1980 May;3(1):39–62. doi: 10.1007/BF00199925. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Adler B., Blanden R. V., Davidson W. F., Kees U., Dunlop M. B., Shreffler D. C. H-2 restriction of cell-mediated immunity to an intracellular bacterium: effector T cells are specific for Listeria antigen in association with H-21 region-coded self-markers. J Exp Med. 1977 May 1;145(5):1353–1367. doi: 10.1084/jem.145.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]


