Abstract
Spontaneous regression of renal cell carcinoma (RCC) is a well-recognized and interesting phenomenon that is poorly understood and rarely documented. There are very few reported cases of spontaneously regressed primary RCC. We present a 63-year-old male with a biopsy-proven RCC that regressed with complete resolution of symptoms.
Introduction
We report a case of spontaneous regression observed in primary renal cell carcinoma (RCC). A biopsy demonstrated papillary type 1 RCC. This case is unique for two reasons: (1) Spontaneous regression of primary RCC is extremely uncommon and (2) Most cases include spontaneous regression of metastatic RCC (particularly pulmonary metastases).1–3 Up to 2002, 95 documented cases of spontaneous regression in RCC metastases have been reported; only 22% of these were histologically demonstrated.1 Histologically proven regression of a primary RCC is exceedingly rare.
Case report
In June 2005, a 63-year-old male with a long smoking history presented with episodes of gross hematuria. Renal ultrasound confirmed a 1.7-cm exophytic, circumscribed, cystic and solid structure in the lower pole of the right kidney, thought to be a complex cystic renal mass at risk of being RCC. Hematuria workup was otherwise negative. The patient declined definitive therapy of this lesion and was enrolled in a small renal mass trial,4 that included negative metastatic workup, bi-annual computed tomography (CT) follow-ups and laboratory evaluation and physical examination.
CT-guided biopsy of this complex solid and cystic lesion in March 2006 revealed a 2.2-cm papillary type 1 RCC Fuhrman Grade I out of IV (Fig. 1, Fig. 2). In September 2006, a CT scan found the tumour at 1.5 × 1.0 cm. Infused CT in March 2007 demonstrated it at 1.1 × 0.7 cm in size with no signs of metastatic disease. On September 2007, infused CT revealed the lesion to be 0.8 × 0.6 cm with no metastases.
Fig. 1.
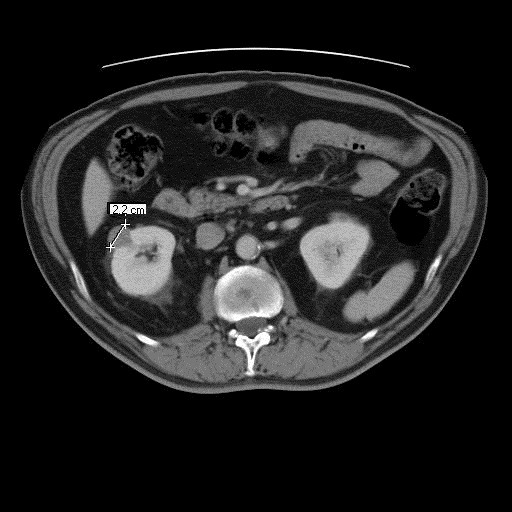
Computed tomography scan, March 2006.
Fig. 2.
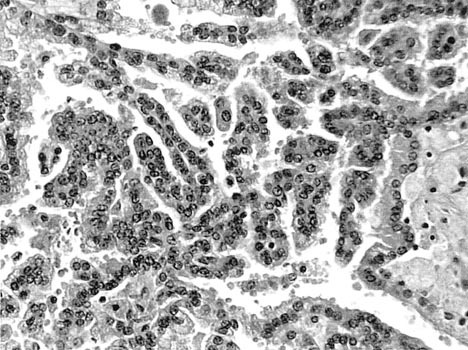
Type 1 papillary renal cell carcinoma.
In April 2008, the mass was 0.5 × 0.8 cm (Fig. 3) – now described as “scar-like” and linear rather than mass-like. The renal vein and inferior vena cava remained patent; there was no lymphadenopathy, and the left kidney, adrenals and liver were unremarkable. He had no constitutional symptoms, no gross hematuria, weight loss, flank pain or fever.
Fig. 3.
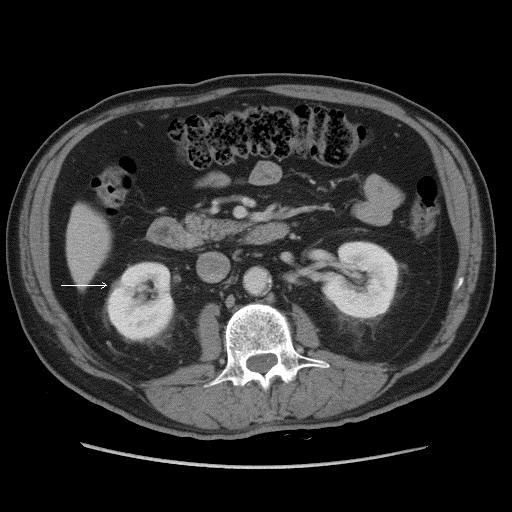
Computed tomography scan, April 2008.
In October 2008, a linear hypoattenuating scar-like density around 0.5 cm in size remained. The radiology report stated that the lesion would unlikely be considered a mass lesion. He had no symptomatology.
Discussion
RCC characteristically presents with a late onset of symptoms, attributable to its known indolent growth phase.5 It is likely that RCC has a pre-invasive period lasting greater than 13 to 20 years with symptoms presenting in the third and eighth decade of life.6,7 In the era prior to the extensive use of imaging studies, non-urological symptoms were generally the earliest manifestations of RCC (i.e., weight loss, weakness and anemia).6 Further, the typical triad of gross hematuria, flank pain and abdominal mass is found in 10% to 15% of patients, whereas 65% present with at least one of these three symptoms.6
The availability of radiologic imaging techniques has allowed us to see more RCC, particularly “incidental” masses (diagnosed in absence of signs or symptoms) and “small renal masses” (defined as solid or cystic lesions less than 4 cm in diameter).8 Small renal masses now account for 48% to 64% of all RCC diagnoses and, of those, 79% to 84% are detected before the onset of genitourinary symptoms.8 Small cell RCC is classified into 3 main cellular types: (1) clear cell carcinoma (78%), (2) papillary carcinoma (15.3%), and (3) chromophobe carcinoma (7%).8 Very few other renal cancer histologies present as small renal masses. Oncocytomas and lipid poor angiomyolipomas are benign lesions that often appear suspicious on imaging but do not pose oncologic risk to patients. Given the relatively indolent growth patterns of small renal cancers coupled with the diagnostic uncertainty, a period of thoughtful active surveillance has been proposed for older patients and those with significant comorbidities. Oncologic safety of this schema was the focus of a recent multicentre Canadian clinical trial that includes renal biopsy.4
Papillary carcinoma has been further divided into two morphologic subtypes based on histological properties and clinical course; Type 1 and Type 2.9 Type 1 papillary carcinoma cells contain scanty pale cytoplasm arranged in a single layer on the basement membrane, whereas type 2 cells have pseudo-stratified nuclei with voluminous eosinophilic cytoplasm.9 Type 1 papillary carcinoma has a better prognosis than type 2.10
Everson defines spontaneous regression as “the partial or complete disappearance of a malignant tumour in the absence of all treatment, or in the presence of therapy which is considered inadequate.”11 A recent study reviewed 1078 cases of RCC, and found only two cases with well-documented total regression of metastases.1 The same study found 95 cases before 2002 with spontaneous regression of RCC metastases, with the first documented case by Bumpus in 1928.1,12 Regression of metastatic papillary RCC was first published in 2009, making any cases of regression of primary papillary RCC unlikely.13
A literature search revealed three published articles involving spontaneous regression of primary RCC. Choi and colleagues in 1986 did not find any case of primary RCC regression in the literature.14 CT findings provided evidence of regression, but the tumour was incompletely regressed at the time of nephrectomy. Also, non-specific tumour characteristics, such as hyalinization, calcification and necrotic tissue, found upon microscopic examination were used to confirm that it was a carcinoma.14 Although the excised mass had some of the characteristics suggesting carcinoma, it was not biopsy-proven prior to surgery.
The other case was published in 2002. Diagnostic modalities were used to report complete regression of primary RCC and inferior vena caval tumour thrombus – all of which were not histologically confirmed.15 Edwards and colleagues highlight a case of primary RCC and pleural metastases regression proven by CT-guided fine needle aspiration.16 However, this case did not indicate resolution of symptomatology or complete regression of the primary tumour.16
We present a unique case displaying spontaneous regression of a primary RCC. The biopsy provided evidence of a type 1 papillary RCC with relatively complete radiographic resolution. To our knowledge, this has yet to be reported. We have no explanation for the processes involved in this tumour regression. We can hypothesize that biopsy prompted regression through insult to tumour vasculature, specifically through the disruption of factors that are important in tumour angiogenesis and in maintaining integrity of mature vessels.17,18 Eradication of vasculature with subsequent tumour regression has been seen with VEGF blockade.17 We can also hypothesize spontaneous regression via yet undescribed anti-cancer autoimmunity that could have been promoted by tumour disruption and subsequent tumour antigen presentation with recruitment of both cellular and cytotoxic immune responses. Studies have shown cellular immunity specifically by T cells, natural killer (NK) cells, and NK T cells under the control of interleukin-2.19 The latter of these explanations provides further support for continued research into anti-tumoural immunity.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Thoroddsen A, Gudbjartsson T, Geirsson G, et al. Spontaneous regression of pleural metastases after nephrectomy for renal cell carcinoma--a histologically verified case with nine-year follow-up. Scand J Urol Nephrol. 2002;36:396–8. doi: 10.1080/003655902320783971. [DOI] [PubMed] [Google Scholar]
- 2.Elhilali MM, Gleave M, Fradet Y, et al. Placebo-associated remissions in a multicentre, randomized, double-blind trial of interferon gamma-1b for the treatment of metastatic renal cell carcinoma. The Canadian Urologic Oncology Group. BJU Int. 2000;86:613–8. doi: 10.1046/j.1464-410x.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- 3.Fairlamb DJ. Spontaneous regression of metastases of renal cancer: A report of two cases including the first recorded regression following irradiation of a dominant metastasis and review of the world literature. Cancer. 1981;47:2102–6. doi: 10.1002/1097-0142(19810415)47:8<2102::AID-CNCR2820470833>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Mattar K, Basiuk J, Finelli A, et al. Active surveillance of small renal masses: a prospective multi-centre Canadian trial. Eur J Urol. 2008;7(Suppl3):309. doi: 10.1016/S1569-9056(08)60948-3. [DOI] [Google Scholar]
- 5.Hamid Y, Poller DN. Spontaneous regression of renal cell carcinoma: a pitfall in diagnosis of renal lesions. J Clin Pathol. 1998;51:334–6. doi: 10.1136/jcp.51.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland JM. Natural history and staging of renal cell carcinoma. CA Cancer J Clin. 1975;25:121–33. doi: 10.3322/canjclin.25.3.121. [DOI] [PubMed] [Google Scholar]
- 7.Vikram R, Ng CS, Tamboli P, et al. Papillary renal cell carcinoma: radiologic-pathologic correlation and spectrum of disease. Radiographics. 2009;29:741–54. doi: 10.1148/rg.293085190. discussion 755–7. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Martin FM, Millan-Rodriguez F, Urdaneta-Pignalosa G, et al. Small renal masses: incidental diagnosis, clinical symptoms, and prognostic factors. Adv Urol. 2008 doi: 10.1155/2008/310694. 310694 Epub 2009 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonelli A, Tardanico R, Balzarini P, et al. Cytogenetic features, clinical significance and prognostic impact of type 1 and type 2 papillary renal cell carcinoma. Cancer Genet Cytogenet. 2010;199:128–33. doi: 10.1016/j.cancergencyto.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Jiang F, Richter J, Schraml P, et al. Chromosomal imbalances in papillary renal cell carcinoma: genetic differences between histological subtypes. Am J Pathol. 1998;153:1467–73. doi: 10.1016/S0002-9440(10)65734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everson TC. Spontaneous regression of cancer. Ann NY Acad Sci. 1964;114:721–35. doi: 10.1111/j.1749-6632.1964.tb40991.x. [DOI] [PubMed] [Google Scholar]
- 12.Bumpus HC., Jr The apparent disappearance of pulmonary metastasis in a case of hypernephroma following nephrectomy. J Urol. 1928;20:185. [Google Scholar]
- 13.Lim R, Tan PH, Cheng C, et al. A unique case of spontaneous regression of metastatic papillary renal cell carcinoma: a case report. Cases J. 2009;2:7769. doi: 10.4076/1757-1626-2-7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi SK, Chang SK, Lee JM, et al. Spontaneous regression of primary renal cell carcinoma--a case report. Yonsei Med J. 1986;27:314–7. doi: 10.3349/ymj.1986.27.4.314. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Sato T, Sunaoshi K, T, et al. Spontaneous regression of primary renal cell carcinoma with inferior vena caval tumor thrombus. J Urol. 2002;167:242–3. doi: 10.1016/S0022-5347(05)65424-9. [DOI] [PubMed] [Google Scholar]
- 16.Edwards MJ, Anderson JA, Angel JR, et al. Spontaneous regression of primary and metastatic renal cell carcinoma. J Urol. 1996;155:1385. doi: 10.1016/S0022-5347(01)66275-X. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Frischer JS, Serur A, et al. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci U S A. 2003;100:7785–90. doi: 10.1073/pnas.1432908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender JG, Cooney EM, Kandel JJ, et al. Vascular remodeling and clinical resistance to antiangiogenic cancer therapy. Drug Resist Updat. 2004;7:289–300. doi: 10.1016/j.drup.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Itsumi M, Tatsugami K. Immunotherapy for Renal Cell Carcinoma. Clin Dev Immunol. 2010 doi: 10.1155/2010/284581. 284581. Epub 2011 Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]


