Abstract
Renal cell carcinoma (RCC) has a high metastatic potential due to its hematogen and vascular features. It metastasizes frequently to the lungs, the bones, the liver, the lymph nodes and the brain. Metastasis of RCC to the head and neck region is quite rare. In this case report, two RCC patients with head and neck metastases are presented: one occurring after 5 years and the other occurring 17 years after diagnosis.
Introduction
About a third of patients with renal cell carcinoma (RCC) manifest as metastatic disease, whereas 40% to 50 % become metastatic after diagnosis.1 Head and neck metastases are not common, only 8% to 15 % of the overall head and neck metastasis cases are associated with RCC.2 The nose, the tongue, the thyroid gland and the parotid glands are included in the reported cases.3 Sometimes it is difficult to differentiate RCC with its histopathological morphology. In this case, the immunohistochemical positivity of various markers, such as CD-10, vimentin and epithelial membrane antigen (EMA), support the diagnosis. Surgical resection of metastasis to the head and neck region, especially in isolated thyroid metastasis, is crucial for survival. However, surgery for the metastasis at other sites is generally only for palliative purposes. Though RCC is known as a radioresistant tumour, radiotherapy may have a role in providing local control with regard to pressure symptoms and pain.
Case 1
A 60-year-old male patient underwent a right nephrectomy in 2004, due to a mass detected at the right kidney region. The pathologic examination was consistent with papillary type RCC (T3N2M0). In May 2009, he was admitted to our clinic with complaints of dyspnea and hoarseness, which had been occurring for about 2 months. The ultrasonography examination of the neck revealed irregular restrictions in the superior right lobe of the thyroid, a 35 × 57-mm diameter of heterogeneous hypoechoic solid nodule with calcifications leading to lobulation of the glandular contour, and a 20-mm diameter nodule at the left lobe. Thyroid function tests of the patient were within normal serum levels. A mass lesion infiltrating the right side of the laringeal cartilage, the right aryepiglottic fold and the right fold were observed in the computed tomography (CT) of the neck. In addition, the right piriform sinus was obliterated by the mass lesion and a manifested subglottic expansion was also observed in the CT (Fig. 1). The biopsy was taken from the right piriformis of the larynx, and revealed tumour cells positive for CD-10 (neprilysin, neutral endopeptidase), vimentin, and EMA and negative for cytokeratin 7. Therefore, by the help of these immunohistochemical findings, the diagnosis of RCC was established (Fig. 2, Fig. 3).
Fig. 1.
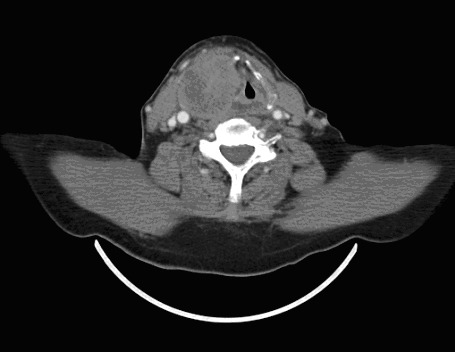
Computed tomography image of the mass.
Fig. 2.
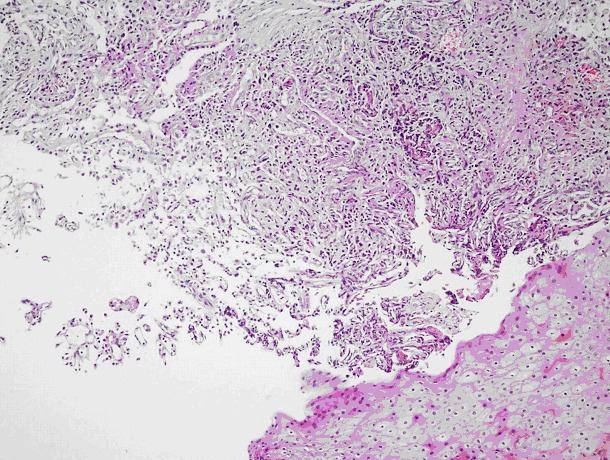
A tumour manifesting an alveolar pattern separated by long, thin vascular structures formed by uniform, round cell nuclei with granular cytoplasm under regular, multi-layered squamous epithelium (hematoxylin and eosin stain ×240).
Fig. 3.
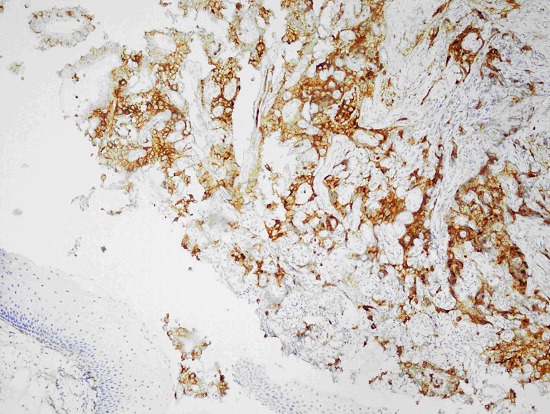
Immunohistochemically membranous CD-10 positivity of the tumour cells.
Palliative radiotherapy was given to the patient due to his inoperable mass causing pressure symptoms, although no visceral metastasis was present. Subsequently, interferon alpha-2a (s.c., 3 MU/3 times/week) was started. Since the RCC continued to progress in the second month of treatment, 50 mg doses of sunitinib (total of 4 weeks at 2-week intervals) were initiated. The patient is currently in the tenth month of this treatment. Minimal regression has been observed in the neck mass, and no other metastasis has developed.
Case 2
A 64-year-old male who underwent a right nephrectomy 17 years ago due to RCC was admitted to our clinic in September 2009. He was complaining of symptoms, such as neck edema and dyspnea. In the neck ultrasonographic examination, enlargement of both lobes (evident at the right lobe), numerous nodules in the parenchyma, an indefinite border between the solid nodule at the bottom right and the jugular vein, and thrombus in the jugular vein were detected. Thyroid hormones and autoantibodies were normal. In the CT of the neck, we observed a mass at the right lobe of the thyroid gland which became more evident at the isthmus. The mass also covered the left lobe and was causing an evident heterogeneous increase in volume. The mass fully surrounded the trachea and was causing annular moderate stenosis (Fig. 4). After a detailed workup, including abdomen, pelvis and chest CT scan which were negative for metastatic disease, the patient underwent total tyroidectomy and bilateral neck dissection in November 2009. In the pathologic examination, a 4.5 × 3 × 2-cm sized solid tumour tissue in the left thyroid and a 6-cm tumour tissue in the right thyroid lobe were detected. Immunohistochemical staining was negative for TTF-1 (thyroid transcription factor) and thyroglobulin; it was positive for vimentin, EMA and CD-10. These were consistent with RCC metastasis (Fig. 5, Fig. 6).
Fig. 4.
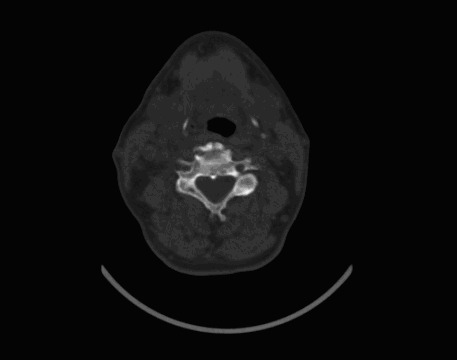
Computed tomography image of the mass, positioned in both lobes of the thyroid, which is pressing onto the trachea.
Fig. 5.
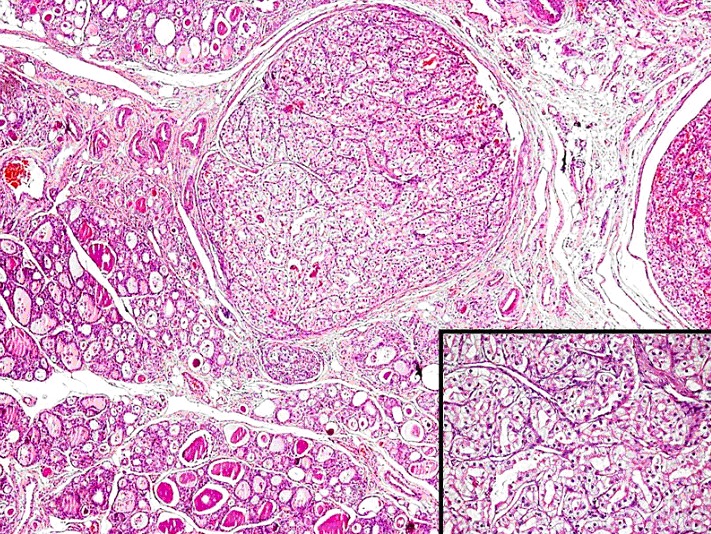
Tumour tissue with luminous cell morphology inside the thyroid tissue.
Fig. 6.
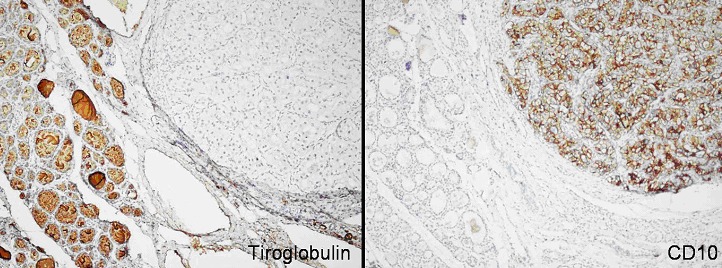
Differing from the surrounding thyroid tissue, tumour tissue dyed negative with thyroglobulin and positive with CD-10.
Furthermore, RCC was detected in the Delphian nodule and the right jugular vein in the right omohyoid muscle, which was taken during the neck dissection. Interferon alpha-2a (s.c., 3 MU/3 times/week) was started and discontinued after 12 months due to the absence of relapse in control tests.
Discussion
RCC comprises 2% to 3% of adult tumours and 85% of primary kidney tumours.4 This type of carcinoma is frequently seen in older adults and rarely in adults under 40.5 Subtypes of RCC, such as clear cell, chromophilic (papillary), chromophobic, oncocytic and those originating from collective blood, are the most prevalent. The clear cell subtype is seen in 75% to 85% of RCC patients.6
RCC had a high potential of metastasis to the lungs, bones, liver, lymph nodes, pancreas, adrenal glands, plevra, brain and skin.7,8 Metastatic disease can be detected at the time of RCC diagnosis in 25% of cases. However, in other cases, metastasis can develop years after the diagnosis. This usually occurs where primary stage metastases are observed more frequently.9,10 Metastasis to the thyroid gland is generally rare in all malignancies, but it has been detected in 1.9% to 22.4% of the cancer cases in an autopsy series.11 Most of these metastases include malignant melanomas and breast cancer. Metastasis of RCC to the head and neck region is much less prevalent (8% to 14%), with the most frequently observed metastasis belonging to the thyroid.12 Solitary metastasis can develop in 1% to 4% of RCC cases, with 1% of these to the thyroid.13,14 Because the prognosis is good in these cases at the postoperative stage, surgery is recommended.13,15,16 Secondary larynx tumours generally comprise 0.09% to 0.40% of all larynx tumours. Cutaneous malignant melanoma, lung and colon adenocarcinoma and renal carcinoma are included in tumours with a potential for metastasis.17 Metastasis of RCC to the larynx and the hypopharynx is much less prevalent compared with metastasis to the thyroid. A small number of isolated metastases which developed 6 to 7 years after nephrectomy have been reported.18–20
RCC manifests various immunohistochemical features in addition to its direct microscopical appeareance; these features differ according to the types of the carcinoma. The use of cell surface dyes with CD-10 antibodies and vimentin supports the diagnosis of RCC. Moreover, it is known that the chromophobe cell type points to parvalbumin and c-kit positivity, while the papillary type points to CK7 and AMACR positivity.21,22 The diagnosis of RCC is set immunochemically in both patients by CD-10, vimentin and a positive EMA result.
We presented two cases: the first patient developed metastasis to the larynx 5 years after nephrectomy and the second patient developed isolated thyroid metastasis 17 years after diagnosis. The first patient did not have surgery due to the invasion of adjacent tissues; the second patient underwent total thyroidectomy and neck dissection. Though RCC metastasis to the head and neck region, especially to the thyroid, has been previously reported, jugular vein invasion without metastasis after 17 years is rare.
Conclusion
RCC may have the potential of atypical metastasis that can develop years after diagnosis. Metastasis of RCC to thyroid is not common; metastasis to the larynx is much less common compared with metastasis to the thyroid.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Golimbu M, Joshi P, Sperber A, et al. Renal cell carcinoma: survival and prognostic factors. Urology. 1986;27:291–301. doi: 10.1016/0090-4295(86)90300-6. [DOI] [PubMed] [Google Scholar]
- 2.Pritchyk KM, Schiff BA, Newkirk KA, et al. Metastatic renal cell carcinoma to the head and neck. Laryngoscope. 2002;112:1598–601. doi: 10.1097/00005537-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Torres-Carranza E, Garcia-Perla A, Infante-Cossio P, et al. Airway obstruction due to metastatic renal cell carcinoma to the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E76–8. doi: 10.1016/j.tripleo.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Suh JH, Oak T, Ro JY, et al. Clinicopathologic features of renal cell carcinoma in young adults: a comparison study with renal cell carcinoma in older patients. Int J Clin Exp Pathol. 2009;2:489–93. [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson RH, Ordonez MA, Iasonos A, et al. Renal cell carcinoma in young and old patients--is there a difference? J Urol. 2008;180:1262–6. doi: 10.1016/j.juro.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storkel S, van den Berg E. Morphological classification of renal cancer. World J Urol. 1995;13:153–8. doi: 10.1007/BF00184870. [DOI] [PubMed] [Google Scholar]
- 7.deKernion JB, Ramming KP, Smith RB. The natural history of metastatic renal cell carcinoma: a computer analysis. J Urol. 1978;120:148–52. doi: 10.1016/s0022-5347(17)57082-2. [DOI] [PubMed] [Google Scholar]
- 8.Takashi M, Takagi Y, Sakata T, et al. Surgical treatment of renal cell carcinoma metastases: a prognostic significance. Int Urol Nephrol. 1995;27:1–8. doi: 10.1007/BF02575213. [DOI] [PubMed] [Google Scholar]
- 9.McNicholas DW, Segura JW, DeWeerd JH. Renal cell carcinoma: long-term survival and late recurrence. J Urol. 1981;126:17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie AW, Chisholm GD. The natural history of renal carcinoma. Semin Oncol. 1983;10:390–400. [PubMed] [Google Scholar]
- 11.Willis RA. Metastatic tumors in the thyroid gland. Am J Pathol. 1931;7:187–208. [PMC free article] [PubMed] [Google Scholar]
- 12.Skyes TCF, Patel A, Archer D, et al. Parotid metastasis from renal carcinoma. Br J Urol. 1995;76:398–9. doi: 10.1111/j.1464-410X.1995.tb07724.x. [DOI] [PubMed] [Google Scholar]
- 13.Kierney PC, van Heerden JA, Segura JW, et al. Surgeon’s role in the management of solitary renal cell carcinoma metastases occurring subsequent to initial curative nephrectomy: an institutional review. Ann Surg Oncol. 1994;1:345–52. doi: 10.1007/BF02303572. [DOI] [PubMed] [Google Scholar]
- 14.Tolia BM, Whitmore WF., Jr Solitary metastasis from renal cell carcinoma. J Urol. 1975;114:836–8. doi: 10.1016/s0022-5347(17)67155-6. [DOI] [PubMed] [Google Scholar]
- 15.Weerdenburg JP, Jurgens PJ. Late metastases of a hypernephroma to the thyroid and the pancreas. Diagn Imaging Clin Med. 1984;53:269–72. [PubMed] [Google Scholar]
- 16.Rubin P. Comment: are metastases curable? JAMA. 1968;204:612–3. doi: 10.1001/jama.1968.03140200052016. [DOI] [PubMed] [Google Scholar]
- 17.Nicolai P, Puxeddu R, Cappiello J, et al. Metastatic neoplasms to the larynx: report of three cases. Laryngoscope. 1996;106:851–5. doi: 10.1097/00005537-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Dee SL, Eshghi M, Otto CS. Laryngeal metastasis 7 years after radical nephrectomy. Arch Pathol Lab Med. 2000;124:1833–4. doi: 10.5858/2000-124-1833-LMYARN. [DOI] [PubMed] [Google Scholar]
- 19.Hittel JP, Born IA. Unusual metastatic site of a kidney carcinoma: a case report with review of the literature. Laryngorhinootologie. 1995;74:642–4. doi: 10.1055/s-2007-997817. [DOI] [PubMed] [Google Scholar]
- 20.Issing PR, Heermann R, Ernst A, et al. Distant metastasis of renal cell carcinomas to the head-neck region. Laryngootologie. 1996;75:171–4. doi: 10.1055/s-2007-997557. [DOI] [PubMed] [Google Scholar]
- 21.Avery AK, Beckstead J, Renshaw AA, et al. Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasms. Am J Surg Pathol. 2000;24:203–10. doi: 10.1097/00000478-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Skinnider BF, Amin MB. An immunohistochemical approach to the differential diagnosis of renal tumors. Semin Diagn Pathol. 2005;22:51–68. doi: 10.1053/j.semdp.2005.11.004. [DOI] [PubMed] [Google Scholar]


