Abstract
Cysteinyl leukotrienes (cys-LTs) can mediate Th2 immunity to the house dust mite, Dermatophagoides farinae (Df), via the type 1 receptor CysLT1R on dendritic cells (DCs). However, the role of the homologous type 2 receptor CysLT2R in Th2 immunity is unknown. Df sensitization and challenge of CysLT2R-deficient mice showed a marked augmentation of eosinophilic pulmonary inflammation, serum IgE, and Th2 cytokines. Wild-type (WT) mice sensitized by adoptive transfer of Df-pulsed CysLT2R-deficient bone marrow-derived DCs (BMDCs) also had a marked increase in Df-elicited eosinophilic lung inflammation and Th2 cytokines in restimulated hilar nodes. This response was absent in mice sensitized with Df-pulsed BMDCs lacking leukotriene C4 synthase (LTC4S), CysLT1R, or both CysLT2R/LTC4S, suggesting that CysLT2R negatively regulates LTC4S- and CysLT1R-dependent DC-mediated sensitization. CysLT2R-deficient BMDCs had increased CysLT1R-dependent LTD4-induced ERK phosphorylation, whereas N-methyl LTC4 activation of CysLT2R on WT BMDCs reduced such signaling. Activation of endogenously expressed CysLT1R and CysLT2R occurred over an equimolar range of LTD4 and N-methyl LTC4, respectively. Although the baseline expression of cell surface CysLT1R was not increased on CysLT2R-deficient BMDCs, it was upregulated at 24 h by a pulse of Df, as compared to WT or CysLT2R/LTC4S-deficient BMDCs. Importantly, treatment with N-methyl LTC4 reduced Df-induced CysLT1R expression on WT BMDCs. Thus, CysLT2R negatively regulates the development of cys-LT-dependent Th2 pulmonary inflammation by inhibiting both CysLT1R signaling and Df-induced LTC4S-dependent cell surface expression of CysLT1R on DCs. Furthermore, these studies highlight how the biologic activity of cys-LTs can be tightly regulated by competition between these endogenously expressed receptors.
Keywords: Knockout mouse, Lipid mediator, G protein-coupled receptor, Asthma, Lung
INTRODUCTION
The cysteinyl leukotrienes (cys-LTs), leukotriene C4 (LTC4), LTD4, and LTE4, are generated from arachidonic acid through the 5-lipoxygenase and LTC4 synthase (LTC4S) pathway (1, 2). They have been implicated in the pathobiology of bronchial asthma on the basis of their potent bronchoconstrictive activity at the type 1 cys-LT receptor, CysLT1R (3). However, CysLT1R, the high-affinity receptor for LTD4, is expressed not only on human smooth muscle cells of the airway and microvasculature but also on hematopoietic cells, such as mast cells, macrophages, eosinophils, basophils, and dendritic cells (DCs), that participate in innate and adaptive immune responses (4–6). Indeed, an influence of cys-LTs in Th2 immunity has been observed in clinical trials where CysLT1R antagonists lower serum total and antigen-specific IgE in children with asthma or food allergy (7, 8).
In mouse models of allergic pulmonary inflammation induced by OVA or by extracts from the clinically relevant house dust mite, Dermatophagoides farinae (Df), pharmacologic antagonism of CysLT1R or genetic deletion of LTC4S or CysLT1R attenuates Th2 pulmonary inflammation (9–12). In vitro Df stimulation of bone marrow-derived DCs (BMDCs) triggers the robust generation of cys-LTs, and the adoptive transfer of Df-pulsed BMDCs from LTC4S-deficient (Ltc4s−/−) or CysLT1R-deficient (Cysltr1−/−) mice to sensitize naïve wild-type (WT) recipients has shown that each protein is needed to prime for Th2 pulmonary inflammation after Df challenge (12). These studies highlight the importance of the cys-LT/CysLT1R axis in DCs at the initiation phase of a Th2 immune response.
CysLT2R is a G protein-coupled receptor with 37–38% amino acid homology to CysLT1R. CysLT2R binds both LTC4 and LTD4 with ~10-fold less affinity than that of CysLT1R for LTD4 in transfected cells (13–15). Evidence of a role for CysLT2R in the pathobiology of human asthma has been limited by the lack of a specific receptor antagonist. However, a polymorphism in the CysLT2R gene that produces a mutant CysLT2R with decreased responsiveness to LTD4 has been associated with increased atopy, suggesting that CysLT2R may play a regulatory role in asthma immunobiology (16). CysLT2R is expressed on human innate immune cells such as mast cells, macrophages, and DCs (5, 17, 18). An in vitro study showed that CysLT2R could negatively regulate CysLT1R expression and LTD4-elicited mitogenic response in human mast cells (17). Whether CysLT2R can negatively regulate Th2 pulmonary inflammation in vivo has not been addressed.
We previously generated CysLT2R-deficient (Cysltr2−/−) mice (19), and now provide CysLT2R/LTC4S-deficient (Cysltr2/Ltc4s−/−) mice to address whether this receptor can regulate cys-LT-dependent immune responses. We show that Cysltr2−/− mice have markedly increased eosinophilic pulmonary inflammation and Th2 cytokines in response to intranasal Df sensitization and challenge. Analysis of the sensitization function by adoptive transfer of Df-pulsed BMDCs showed that Cysltr2−/− BMDCs generated markedly enhanced responses to Df challenge, while the lack of CysLT1R or LTC4S or the double deficiency of CysLT2R and LTC4S significantly suppressed the response well below that of WT BMDCs. We considered that the enhanced responses from Cysltr2−/− BMDCs and reduced responses from Cysltr2/Ltc4s−/− BMDCs may reflect enhanced or reduced CysLT1R function, respectively, and assessed CysLT1R-dependent ERK phosphorylation and CysLT1R expression in BMDCs from relevant deficient strains. We found that pharmacologic inhibition or genetic deficiency of CysLT2R on BMDCs increased LTD4-initiated CysLT1R-dependent ERK phosphorylation, whereas N-methyl LTC4 activation of CysLT2R on WT BMDCs was inhibitory. Although there was no increase in baseline CysLT1R cell surface expression on Cysltr2−/− BMDCs, they had upregulated Df-induced CysLT1R expression as compared to WT BMDCs. N-methyl LTC4 activation of CysLT2R on WT BMDCs reduced CysLT1R induction. Thus, CysLT2R negatively regulates both CysLT1R activation and cell surface expression. The opposing functions of CysLT2R and CysLT1R on DCs at the level of the allergen sensitization reveal a critical balance of two receptors likely influenced by the local generation of their ligands.
MATERALS AND METHODS
Generation of Cysltr2/Ltc4s−/− mice
Cysltr1−/− and Cysltr2−/− mice generated from C57BL/6 embryonic stem cells (19, 20), were maintained by breeding with C57BL/6 mice (Charles River Laboratories), and N15 and N5 or N6 generations, respectively, were used. Ltc4s−/− mice were established from 129-derived embryonic stem cells (21), were backcrossed onto the C57BL/6 background, and N12 generations were used. To generate Cysltr2/Ltc4s−/− mice, Cysltr2−/− males and Ltc4s−/− females were bred to obtain Cysltr2+/−/Ltc4s+/− males and females. The Cysltr2+/−/Ltc4s+/− mice were further intercrossed to obtain Cysltr2/Ltc4s−/− mice. The Cysltr2/Ltc4s−/− mice were viable and had no apparent abnormalities up to at least 8 mo of age. WT littermates from breeding for Cysltr1−/−, Cysltr2−/−, Ltc4s−/−, and Cysltr2/Ltc4s−/− strains were used. Both mutant and WT mice were 8–12-wk-old when studied. All animal studies were approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute.
Active sensitization and challenge with repeated intranasal injections of Df
Mice received either 1 µg of Df (Greer Laboratories, Lenoir, NC) or saline intranasally twice per week for 3 wks as described (11) (Figs. 1 & 2) or 0.5 µg of Df on day 0 and 4 and 0.1 µg on day 15 and day 18 (Fig. S1). 2 d after the last injection, mice were killed by i.p. injection of pentobarbital. The latter protocol was adjusted for a comparable eosinophil response in the bronchoalveolar lavage (BAL) fluid of WT mice with a different batch of Df. The LPS levels in batches of Df varied by 3.5 fold (from 6.1 EU/µg protein - 21.3 EU/µg protein).
FIGURE 1.
CysLT2R deficiency increases Df-induced pulmonary inflammation. A. Inflammatory cell counts in BAL fluid. For active sensitization and challenge, WT, Cysltr1−/−, Cysltr2−/−, and Ltc4s−/− mice received 1 µg of Df (filled columns) or PBS (open columns) by intranasal injection twice per wk for 3 weeks, and BAL was performed 2 days after the last injection. Total and differential cell counts for monocytes/macrophages (MΦs), neutrophils, eosinophils, and lymphocytes are shown. Values are the means ± SEM (n = 8–10) combined from 3 independent experiments. *P < 0.05 vs. Df-challenged WT. B. Histologic analyses of the lung. After BAL, lung tissues were fixed with paraformaldehyde and stained with H&E, Congo red, or periodic acid-Schiff (PAS). b, bronchi, v, vessels. Eosinophils are indicated by arrows in Congo red staining, and mucus is stained in purple in PAS. Scale bars = 100 µm for HE and PAS, 50 µm for Congo red.
FIGURE 2.
CysLT2R deficiency increases Df-induced Th2 immunity. A. Total IgE and Df-specific IgG1 in serum of PBS-injected (open columns) or Df-challenged (filled columns) are shown. Values are the means ± SEM (n = 8–10) combined from 3 independent experiments. *P < 0.05 vs. Df-challenged WT. B. Relative expression of mRNA for IL-4, IL-5, IL-13 to GAPDH in lungs of PBS-injected (open columns) or Df-challenged (filled columns) mice from 2 experiments was assessed by quantitative RT-PCR. Values are the means ± SEM (n = 5–7). *P < 0.05 vs. Df-challenged WT. C. Peribronchial LN cells were harvested, counted, and restimulated with 20 µg/ml Df for 72 h. The concentrations of IL-5, IL-17A, and IFN-γ in the culture supernatants are shown. Values are the means ± SEM (n = 8–10) combined from the 3 independent experiments depicted in Fig. 1. *P < 0.05 vs. Df-challenged WT.
BAL fluid cell analysis
2 d after the last intranasal injection, the trachea was cannulated and BAL fluid was obtained by three repeated lavages with 0.75 ml of Ca2+- and Mg2+-free PBS with 1 mM EDTA. The BAL fluid was centrifuged at 500 × g for 5 min. Cells were resuspended in 0.2 ml of PBS with 1% BSA, and the total cells were counted manually with a hemocytometer. For the differential cell counts of macrophages, neutrophils, eosinophils, and lymphocytes, the cells were cytospun onto a glass slide and stained with Diff-Quik, and cell types in a total of 200 cells were identified by morphologic criteria.
Histology
The lung tissues were excised, and the left lung was fixed and stained as described previously (10). For general morphology, tissue sections were stained with hematoxylin and eosin (H&E). The extent of cellular infiltration in the bronchovascular bundles was assessed in a blinded manner. Congo red staining was used to identify eosinophils, and periodic acid-Schiff staining was used to assess mucus and goblet cells. The slides were analyzed with a Leica DM LB2 microscope (Leica Microsystems, Germany). The pictures were taken by a Nikon digital camera DXM 1200 with Nikon ACT-1 (version 2.70) image acquisition software.
Measurement of total IgE and Df-specific IgG1
Sera were collected by cardiac puncture 2 d after the last intranasal injection. Total IgE was determined with an ELISA kit (BD Biosciences, San Jose, CA). Df-specific IgG1 was measured as described (22). Briefly, 96-well plates were coated with a 5 µg/ml solution of Df and incubated with diluted serum followed by alkaline phosphatase-conjugated anti-mouse IgG1 (SouthernBiotech, Birmingham, AL) and p-nitrophenyl phosphate substrate (Sigma-Aldrich, St. Louis, MO).
Measurement of cytokine mRNA expression in the lung
Total RNA was isolated from the right lungs with TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Quantities of mRNA for IL-4, IL-5, IL-13, IL-17A, and IFN-γ were measured relative to GAPDH using the Mx3005P Real-Time PCR System (Agilent Technologies, Santa Clara, CA) with gene-specific primers.
Cytokine production by peribronchial lymph node (LN) cells after ex vivo restimulation with Df
2 d after the last intranasal injection, three peribronchial LNs were excised from each mouse and homogenized. The cell suspensions were filtered through a 70-µm cell strainer, centrifuged at 300 × g for 5 min at room temperature, and resuspended in RPMI1640 medium containing heat-inactivated 10% FBS. After the total number of cells was counted for each mouse, cells were cultured at 4 × 106 cells/ml (100 µl) in the presence of 20 µg/ml Df in a 96-well plate for 72 h. The concentrations of IL-4, IL-5, IL-13, IL-17A, and IFN-γ in the supernatants were measured with ELISA kits (eBiosciences, San Diego, CA).
Transfer of Df-pulsed BMDCs into mice and Df challenge
Adoptive transfer of Df-pulsed BMDCs into naïve mice was carried out as described (11, 12). Bone marrow cells were harvested from femurs and tibiae of each mouse and cultured in RPMI medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine, 50 µM 2-ME, and recombinant mouse GM-CSF as described (23). Floating cells were harvested on day 7 and pulsed with either PBS or 50 µg/ml of Df at a concentration of 1 × 106 cells/ml in a 35-mm culture dish (Sumilon Celltight X, Sumitomo Bakelite, Japan) for 24 h. In some experiments, BMDCs were preincubated with N-methyl LTC4 (Cayman Chemical, Ann Arbor, MI) for 10 min before Df stimulation. The next day, the BMDCs were washed twice with PBS and resuspended in PBS. 1 × 104 cells in 25 µl were transferred intranasally to recipients. The cells were routinely greater than 85% CD11c+. At days 10 and 14 after DC transfer, recipient mice were challenged with 3 µg Df intranasally. 2 d after the last challenge, mice were killed by i.p. injection of pentobarbital. BAL fluid analysis, lung histology, and assessment of cytokine production in the lungs were performed as described above.
Flow cytometry
Day 7 BMDCs were pulsed with either PBS or 50 µg/ml of Df for 24 h. Cells were harvested, washed, blocked in PBS containing 1 mM EDTA and 1% mouse IgG (Jackson Immunoresearch, West Grove, PA), and stained with either rat anti-mouse CD11c-PECy7, MHC class II-PE, CD80-PE, CD86-PE, CD40-PE, OX40L-PE, or isotype controls (BD Biosciences). For CysLT1R cell surface expression, BMDCs were fixed with 100% ice cold methanol at −20°C for 5 min. Cells were washed with PBS, blocked with 10% donkey serum, and incubated serially with 10 µg/ml RB34, a custom generated polyclonal rabbit anti-CysLT1R IgG against a peptide in the 3rd extracellular loop of CysLT1R (Orbigen) (17) and allophycocyanin-conjugated donkey anti-rabbit IgG. Nonspecific rabbit IgG (Jackson Immunoresearch) was used as a control. For total cell CysLT1R expression, BMDCs were fixed with 100% ice cold methanol at −20°C for 5 min, permeabilized with saponin (eBiosciences), and stained as above. Analyses were performed on a FACSCanto II flow cytometer (BD Biosciences), and data were analyzed with the FlowJo 7.5.
Cys-LT and cytokine measurement
Cys-LTs in the supernatants of stimulated cells were measured by enzyme immunoassay according to the manufacturer’s protocol (Cayman Chemical). TNF-α, IL-6, and IL-10 were measured by ELISA (eBiosciences).
Measurement of CysLT1R transcript in BMDCs
Total RNA was isolated from day 7 BMDCs with TRIzol reagent (Invitrogen), according to the manufacturer’s protocol. Quantities of mRNA for CysLT1R transcript were measured relative to GAPDH using the Mx3005P Real-Time PCR System (Agilent Technologies) with gene-specific primers.
Quantification of phospho ERK in response to LTD4
Day 8–9 BMDCs from WT, Cysltr1−/−, and Cysltr2−/− mice were harvested, washed in PBS and resuspended and plated in Hanks’ balanced salt solution at 2.5 × 104 cells in a 96-well plate provided with InstantOneELISA™ (eBiosciences). Cells were incubated at 37°C for 1–2 h prior to the addition of ligand to allow for equilibration. Cells were then incubated for 5 min with various concentrations of LTD4 in ethanol or for 0, 2, 5, 10 min with 300 nM of LTD4. Cells were then lysed in the 96-well plate by adding lysis buffer provided in the InstantOne ELISA™ kit and shaking at ~300 rpm for 10 min at room temperature. Phospho ERK1/2 was quantified with InstantOne ELISA™ according to the manufacturer’s protocol. In some experiments, cells were preincubated with N-methyl LTC4 or HAMI3379 (Cayman Chemical) for 10 min before LTD4 stimulation.
For ERK analysis by Western blot, day 7–8 BMDCs were harvested, washed in PBS, and seeded at 5 × 105 cells per 100 µl of serum free media in 1.5-ml Eppendorf tubes. The cells were stimulated with vehicle (ethanol) or LTD4 at 300 nM in 100 µl of serum free medium for 0, 2, 5, and 10 min at 37 °C. The cells were then placed on ice, centrifuged, and lysed in 0.1 ml of 50 mM Tris-HCl (pH 8.0) buffer containing 0.15 M NaCl, 0.5% Triton X-100, 0.05% Tween 20, a protease inhibitor mixture (Roche), 10 mM NaF, and 1 mM Na3VO4 on ice for 20 min. The samples were centrifuged for 5 min at 14,000 × g at 4°C and the supernatants were transferred to new Eppendorf tubes. 4 × SDS/PAGE sample buffer with 10 mM DTT was added to the supernatants and the samples were boiled for 5 min. Twenty microliters (about 8 × 104 cells) per sample were resolved by SDS/PAGE on a NuPAGE Novex 10% Bis-Tris gel (Invitrogen) with MOPS running buffer under reducing conditions and transferred to a polyvinylidene fluoride membrane (Bio-Rad). The membranes were incubated with a 1:1,000 dilution of rabbit polyclonal anti-phospho p44/p42 ERK1/2 Ab or polyclonal anti-total p44/p42 ERK1/2 Ab (Cell Signaling) and HRP-conjugated donkey anti-rabbit IgG (Pierce) and developed by ECL (SuperSignal Pico, Pierce) according to the manufacturer’s instructions.
Statistical analysis
Results were expressed as means ± SEM. Student’s unpaired, two-tailed t test was used for the statistical analysis in cases in which the variance was homogeneous, and Welch’s test was used when the variance was heterogeneous. To compare between multiple genotypes, one-way ANOVAs were used, and to compare multiple genotypes over doses or time, two-way ANOVAs were used with Bonferroni posttests. A value of p < 0.05 was considered significant.
RESULTS
CysLT2R negatively regulates Df-induced CysLT1R-dependent pulmonary inflammation, serum levels of total IgE and Df-specific IgG1, and Th2 cytokine expression
To examine the role of the CysLT2R in in vivo Th2 immune function, we assessed Df-elicited pulmonary inflammation in C57BL/6 WT, Cysltr1−/−, Cysltr2−/−, and Ltc4s−/− mice. Mice received intranasal injections of 1 µg of Df or PBS twice per week for 3 wks and were killed for assays 48 h after the last injection. Df-challenged WT mice had a significantly increased total cellular infiltration in BAL fluid (p < 0.01) composed of monocytes/macrophages, neutrophils, eosinophils, and lymphocytes as compared to PBS-challenged WT mice (Fig. 1A). Df-challenged Cysltr1−/− and Ltc4s−/− mice showed significantly reduced total cellular infiltration in BAL fluid, as compared to Df-challenged WT mice. In contrast, Df-challenged Cysltr2−/− mice had significant increases in BAL fluid cell numbers of neutrophils, eosinophils, and lymphocytes as compared to Df-challenged WT mice.
Histologic analysis of the bronchovascular bundles in the lung showed a modest cellular infiltration in WT mice that was largely absent in Cysltr1−/− and Ltc4s−/− mice, but was marked in Cysltr2−/− mice (Fig. 1B, H&E). Congo red staining revealed that the eosinophilic infiltration present in WT mice was more prominent in Cysltr2−/− mice and virtually absent in Cysltr1−/− or Ltc4s−/− mice (Fig. 1B, Congo Red). Goblet cell metaplasia with mucus production was particularly prominent in Df-challenged Cysltr2−/− mice, compared to similarly challenged WT mice, as assessed by periodic acid-Schiff staining, and was absent in Df-challenged Cysltr1−/− and Ltc4s−/− mice (Fig. 1B, PAS). Thus, Df-induced pulmonary inflammation is dependent on the integrity of LTC4S and CysLT1R and negatively regulated by CysLT2R.
After sensitization and challenge with Df, WT mice had significant increases in serum total IgE and Df-specific IgG1 as compared to PBS-challenged WT mice (p < 0.01) (Fig. 2A). Cysltr1−/− and Ltc4s−/− mice had significantly less total serum IgE and no increase in Df-specific IgG1 as compared to Df-challenged WT mice. In contrast, Df-challenged Cysltr2−/− mice had significantly increased levels of total IgE (~2-fold) and of Df-specific IgG1 (~2.5-fold) as compared to Df-challenged WT mice. PBS-challenged Cysltr2−/− mice also had a significantly increased total IgE level at baseline as compared to PBS-challenged WT mice (p < 0.05). Thus CysLT2R negatively regulates Th2-mediated Ig responses at baseline as well as with sensitization and challenge.
To assess the T cell cytokine expression profile in the lungs after sensitization and challenge with Df, total RNA was isolated from the right lungs of WT, Cysltr1−/−, Cysltr2−/−, and Ltc4s−/− mice, and quantitative RT-PCR for IL-4, IL-5, IL-13, IL-17A, and IFN-γ was performed. In WT mice, sensitization and challenge with Df significantly increased the expression of mRNAs for IL-4, IL-5, and IL-13, but not of mRNAs for IL-17A or IFN-γ, as compared to PBS-challenged WT mice (p < 0.01) (Fig. 2B and data not shown). Df-challenged Cysltr1−/− and Ltc4s−/− mice had significantly reduced mRNAs for IL-4, IL-5, and IL-13 as compared to Df-challenged WT mice. In contrast, Df-challenged Cysltr2−/− mice had significant further increases in expression of IL-4, IL-5, and IL-13 (Fig. 2B), but not IL-17A and IFN-γ (data not shown), as compared to Df-challenged WT mice.
To determine whether the profile of T cell cytokine production was similar in the thoracic draining LNs, the LN cells were dissociated, counted, and restimulated with Df for 72 h, and cytokine concentrations in the supernatants were measured with ELISAs. The total number of LN cells was similar among Df-challenged WT, Cysltr1−/−, and Ltc4s−/− mice (Fig. 2C). In contrast, the total number of LN cells from Df-challenged Cysltr2−/− mice was significantly increased by 3-fold as compared to Df-challenged WT mice. LN cells from Df sensitized and challenged WT mice generated IL-5, IL-17A, and IFN-γ (Fig. 2C), which were undetectable in mice treated with PBS (data not shown). The amounts of IL-5 and IFN-γ were significantly reduced in Df-challenged Cysltr1−/− and Ltc4s−/− mice, while the amounts of IL-17A were not changed, as compared to Df-challenged WT controls. In contrast, the amounts of IL-5, IL-17A, and IFN-γ were significantly increased in Df-challenged Cysltr2−/− mice, as compared to Df-challenged WT controls. These results suggest that CysLT2R negatively regulates both Df-elicited LN hypertrophy and nodal immune responses.
CysLT2R on BMDCs negatively regulates Df-induced LTC4S-dependent sensitization of WT recipients
We have previously demonstrated that the presence of LTC4S and of CysLT1R on Df-pulsed BMDCs was critical for their ability to sensitize WT recipients for subsequent Df elicited pulmonary inflammation (12). To determine whether the exaggerated Th2 responses observed in actively sensitized and challenged Cysltr2−/− mice (Figs. 1 & 2) involved CysLT2R regulation of LTC4S-dependent DC sensitization, we generated a Cysltr2/Ltc4s−/− mouse strain. We then adoptively transferred 1 × 104 Df-pulsed BMDCs from this strain and four other genotypes (WT, Cysltr1−/−, Cysltr2−/−, Ltc4s−/−) to WT recipients, challenged them with 3 µg of Df at days 10 and 14, and killed them for assessment at day 16. WT mice sensitized with Df-pulsed WT BMDCs generated a significant (p < 0.05) increase in total BAL fluid cells, composed of neutrophils, lymphocytes, and eosinophils, as compared to WT mice sensitized with saline-pulsed WT BMDCs (Fig. 3A). WT mice sensitized with Df-pulsed Ltc4s−/− or Cysltr1−/− BMDCs had significantly decreased BAL fluid neutrophils and lymphocytes and a trend to reduced eosinophils that was not significant, as compared to mice sensitized with Df-pulsed WT BMDCs. In contrast, mice sensitized with Df-pulsed Cysltr2−/− BMDCs responded to challenge with a further significant increase in monocytes/macrophages and eosinophils but not other cell types, as compared to mice sensitized with Df-pulsed WT BMDCs. This augmented response after adoptive transfer and challenge was abolished in WT mice sensitized with Df-pulsed Cysltr2/Ltc4s−/− BMDCs, suggesting that the CysLT2R effect was dependent on LTC4S.
FIGURE 3.
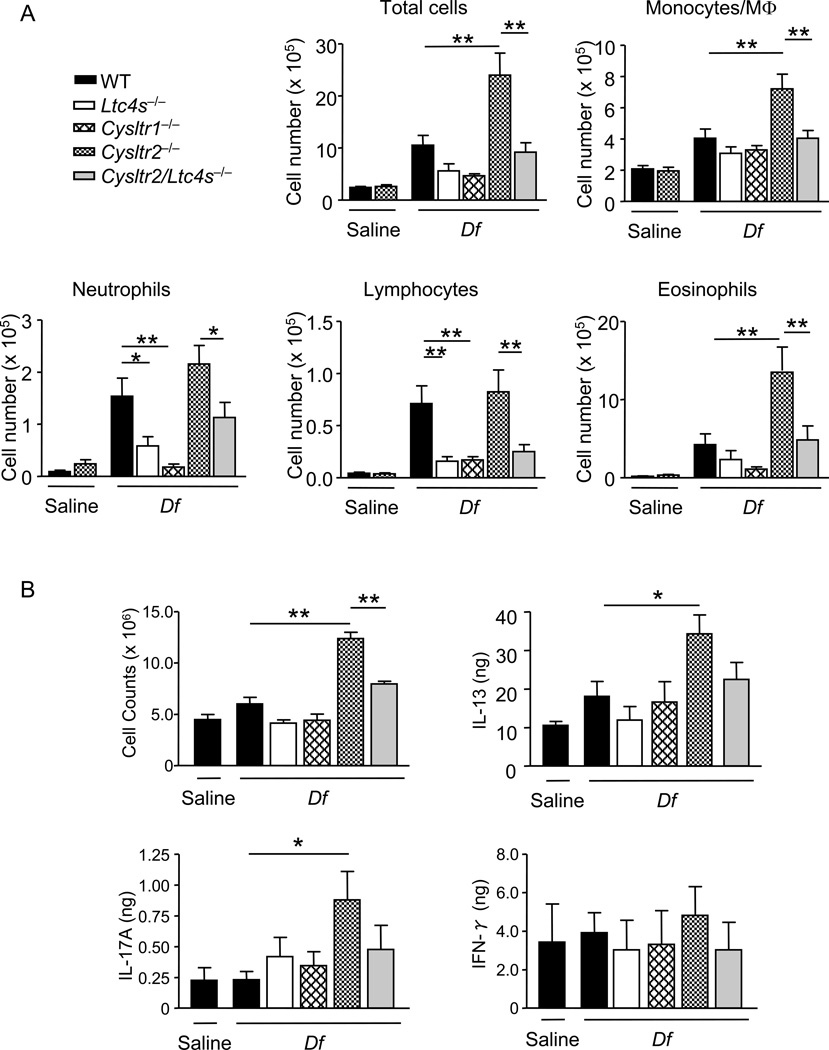
CysLT2R-deficient BMDCs augment Df-induced LTC4S-dependent sensitization of WT recipients. BMDCs from WT, Ltc4s−/−, Cysltr1−/−, Cysltr2−/−, and Cysltr2/Ltc4s −/− mice were pulsed with saline or Df at 50 µg/ml for 24 h, and 104 cells were administered intranasally to sensitize WT recipients. Recipients were challenged with 3 µg of Df intranasally at days 10 and 14 and were killed at day 16 for analyses. A. Inflammatory cell counts in BAL fluid. Total and differential cell counts for BAL fluid monocytes/macrophages (MΦs), neutrophils, eosinophils, and lymphocytes are shown. Values are the means ± SEM (n = 7–15 per group) combined from 3 independent experiments. *P < 0.05, **P < 0.01. B. Peribronchial LN cells were harvested, counted, and stimulated with 20 µg/ml Df for 72 h. Cytokines in the supernatant were measured by ELISA. Values are the means ± SEM (n = 5 for saline and n = 10–15 per group for Df) combined from 3 independent experiments. *P < 0.05, **P < 0.01.
To determine whether the increase in BAL fluid inflammatory cells in mice sensitized with Df-pulsed Cysltr2−/− BMDCs included augmented immune responses in the LNs, we assessed total cell numbers of the draining LN and their potential cytokine production by restimulation with Df. As compared to mice sensitized with saline-pulsed WT BMDCs, there was no significant increase in the total number of LN cells from mice sensitized with Df-pulsed WT, Ltc4s−/−, or Cysltr1−/− BMDCs (Fig. 3B). However, mice sensitized with Df-pulsed Cysltr2−/− BMDCs had significantly increased total LN cell numbers relative to mice receiving Df-pulsed WT BMDCs and these numbers were significantly reduced in mice that received Df-pulsed Cysltr2/Ltc4s−/− BMDCs. These findings suggest the involvement of DC LTC4S in driving the LN hypertrophy in mice receiving Df-pulsed Cysltr2−/− DCs. The restimulated LN cells from mice receiving Df-pulsed WT BMDCs generated IL-13, IL-17A, and IFN-γ (Fig. 3B). The amounts of IL-13 and IL-17A, but not IFN-γ, were significantly increased in WT mice sensitized with Df-pulsed Cysltr2−/− BMDCs, as compared to WT mice sensitized with Df-pulsed WT BMDCs, and these increased responses were abolished in WT mice sensitized with Df-pulsed Cysltr2/Ltc4s−/− BMDCs. These results suggest that BMDC CysLT2R negatively regulates both Df-elicited LN hypertrophy and associated Th2 and Th17 immune responses.
To determine whether the protection seen in WT mice sensitized with Cysltr2/Ltc4s−/− BMDCs could be extended to a direct sensitization model, WT, Ltc4s−/−, Cysltr2−/−, Cysltr2/Ltc4s−/− were injected intranasally with 0.5 µg Df on days 0 and 4, challenged with 0.1 µg Df on days 15 and 18, and sacrificed on day 20. The increase in BAL fluid inflammatory cells (Fig. S1A) and the increase in draining LN cell counts (Fig. S1B) seen in Cysltr2−/− mice were absent in Cysltr2/Ltc4s−/− mice. LN cells from Df-sensitized and challenged Cysltr2−/− mice had robust generation of IL-13 and IL-17A, which was significantly reduced in Cysltr2/Ltc4s−/− mice. The impaired response in Cysltr2/Ltc4s−/− mice was similar to that in Ltc4s−/− mice. The findings suggest that CysLT2R negatively regulates Df-induced, LTC4S-dependent pulmonary inflammation in mice with either active or adoptive sensitization.
Genetic and pharmacologic approaches demonstrate that CysLT2R regulates LTD4-initiated CysLT1R-dependent ERK phosphorylation in BMDCs
To seek a basis for the augmented in vivo function of Df-pulsed Cysltr2−/− BMDCs, we assessed for upregulation of co-stimulatory molecules, Df-induced cys-LT and cytokine generation, and LTD4/CysLT1R-dependent ERK phosphorylation. There was no difference in the numbers of bone marrow-derived CD11c+ cells generated from the different genotypes and the numbers used for sensitization after a Df pulse were the same. There was also no difference in expression levels of CD80, CD86, CD40, OX40L, and MHC class II by flow cytometric analysis of CD11c+ BMDCs from WT and Cysltr2−/− mice with either PBS or Df pulse (Fig. S2A). Df-elicited cys-LT production by Cysltr2−/− BMDCs was comparable to that of WT and Cysltr1−/− BMDCs. There was no cys-LT production by BMDCs from Ltc4s−/− and Cysltr2/Ltc4s−/− mice, as expected (Fig. S2B). There was no difference in Df-elicited TNF-α, IL-6, or IL-10 production among BMDCs of any genotype (Fig. S2B). There was no IL-4, TGF-β, IL-23p19, or IL-12p70 detected in this response (data not shown).
We next assessed ERK phosphorylation in response to CysLT1R activation by LTD4 as described by Jiang et al (17) by comparing WT and Cysltr2−/− BMDCs and using Cysltr1−/− BMDCs as a control for specificity. After stimulation with 300 nM of LTD4, WT BMDCs showed ERK phosphorylation that was detectable at 2 min, peaked at 5 min, and returned to baseline by 10 min, while Cysltr1−/− BMDCs did not show a response (Fig. 4A, left). ERK phosphorylation in Cysltr2−/− BMDCs was significantly increased at each of these time points, as compared to WT BMDCs. Assessment of ERK phosphorylation by Western blot at each time point (Fig. S3) confirmed an increased LTD4-elicited ERK phosphorylation in Cysltr2−/− BMDCs, as compared to WT BMDCs, that was absent in Cysltr1−/− BMDCs. In a dose-response comparison from 10–300 nM LTD4 at 5 min, the Cysltr2−/− BMDCs had significantly enhanced ERK phosphorylation at 100 and 300 nM, as compared to WT BMDCs (Fig 4A, right).
FIGURE 4.
CysLT2R regulates CysLT1R-dependent ERK phosphorylation in BMDCs. A. WT, Cysltr1−/−, and Cysltr2−/− BMDCs (2.5 × 104 cells) were stimulated with 300 nM of LTD4 for 0, 2, 5, and 10 min (left) or with 10–300 nM of LTD4 for 5 minutes (right). Cells were then lysed, and phospho-ERK1/2 was quantified by ELISA. Values are the means ± SEM (n = 6 for WT and Cysltr2−/− and n = 4 for Cysltr1−/−) combined from 4 independent experiments. *P < 0.05 and **P < 0.01 vs. WT BMDCs. B. 2.5 × 104 WT BMDCs (left) or Cysltr2−/− (right) were preincubated with 300 nM N-methyl LTC4 or vehicle control for 10 min and then stimulated with increasing doses of LTD4 for 5 min. Cells were lysed, and phospho-ERK1/2 was quantified by ELISA. Values are the means ± SEM (n = 6 for WT, n = 4 for Cysltr2−/−) combined from 3 independent experiments. **P < 0.01 vs. vehicle-treated BMDCs. C. 2.5 × 104 WT BMDCs (left) or Cysltr2−/− (right) were preincubated with 300 nM HAMI3379 or vehicle control for 10 min and then stimulated with increasing doses of LTD4 for 5 min. Cells were lysed, and phospho-ERK1/2 was quantified by ELISA. Values are the means ± SEM (n = 6 for WT, n = 4 for Cysltr2−/−) combined from 3 independent experiments. *P < 0.05, **P < 0.01 vs. vehicle-treated BMDCs.
The increased CysLT1R signaling in Cysltr2−/− BMDCs suggested to us that CysLT2R may actively regulate CysLT1R function in WT cells. To more directly examine this, we stimulated WT BMDCs with a newly described selective CysLT2R agonist, N-methyl LTC4 (24), at 300 nM for 10 min and assessed the dose-response to LTD4-induced ERK phosphorylation at 5 min. WT BMDCs treated with N-methyl LTC4 had significantly reduced ERK phosphorylation to 100 and 300 nM LTD4, as compared to cells treated with vehicle alone (Fig. 4B, left). This suppression was specifically mediated through CysLT2R, as there was no reduction in ERK phosphorylation in Cysltr2−/− BMDCs treated with N-methyl LTC4 (Fig. 4B, right). Conversely, pretreatment of WT BMDCs for 10 min with 300 nM HAMI3379, a CysLT2R antagonist (25), significantly increased LTD4-induced ERK phosphorylation at 100 and 300 nM LTD4. This increase was specifically mediated through CysLT2R, as there was no increase in Cysltr2−/− BMDCs. Taken together these studies suggest that CysLT2R activation can negatively regulate CysLT1R signaling in BMDCs.
Finally, to determine whether CysLT2R activation in WT BMDCs could negatively regulate Df-induced sensitization of WT recipients, WT BMDCs were pulsed with Df in the presence or absence of 300 nM N-methyl LTC4 and transferred 24 h later into WT recipients. After Df challenge, mice sensitized with Df-pulsed BMDCs in the presence of N-methyl LTC4 had a significant reduction in BAL fluid eosinophil counts and a significant reduction in LN cellularity, as compared to mice sensitized with Df-pulsed BMDCs treated with vehicle alone (Fig. S4). These results indicate that CysLT2R activation in WT BMDCs can suppress CysLT1R-dependent sensitization of WT recipients to Df.
Genetic and pharmacologic approaches demonstrate that CysLT2R regulates Df-induced CysLT1R expression
To determine whether the enhanced in vivo DC sensitization and enhanced in vitro CysLT1R-dependent ERK phosphorylation observed in Cysltr2−/− BMDCs reflected increased cell surface expression of CysLT1R, we assessed expression by flow cytometry on WT, Cysltr1−/−, and Cysltr2−/− BMDCs. At baseline (not shown) or after saline pulse (Fig. 5A, left column) CysLT1R expression was detectable but not different on WT and Cysltr2−/− BMDCs and absent on Cysltr1−/− BMDCs. After a 24-h Df pulse (Fig. 5A, right column), there was a marginal increase in CysLT1R expression on WT BMDCs, as compared to saline control, whereas Cysltr2−/− BMDCs responded with a significant increase in CysLT1R that was almost six times that of WT BMDCs (Fig. 5B). WT BMDCs treated for 30 min with N-methyl LTC4 and then pulsed with Df had no upregulation of CysLT1R at 24 h (Fig. 5C), while Cysltr2−/− BMDCs treated with this agonist still showed substantial upregulation of CysLT1R, demonstrating that CysLT2R activation in WT BMDCs can regulate CysLT1R cell surface expression in addition to CysLT1R signaling.
FIGURE 5.
Increased cell surface CysLT1R expression on Cysltr2−/− BMDCs after Df-pulsation. WT, Cysltr1−/−, and Cysltr2−/− BMDCs were incubated with PBS or 50 µg/ml Df for 24 h, stained for the cell surface expression of CysLT1R, and analyzed by flow cytometry. A. Representative histograms of CysLT1R expression after PBS (left) or Df (right) pulsation. Isotype control staining (shaded histograms) and CysLT1R (open histograms). B. Mean fluorescence intensities of CysLT1R staining combined from 3 independent experiments. Values are the means ± SEM. *P < 0.04 vs. PBS-pulsed Cysltr2−/− BMDCs. C. WT and Cysltr2−/− BMDCs were preincubated with 300 nM N-methyl LTC4 or saline for 10 min and then stimulated with PBS or 50 µg/ml Df for 24 h, stained for the cell surface expression of CysLT1R, and analyzed by flow cytometry. Mean fluorescence intensities of CysLT1R staining combined from 3 independent experiments. Values are the means ± SEM. **P = 0.01 vs. PBS-pulsed Cysltr2−/− BMDCs.
To determine whether the enhanced expression of CysLT1R on Cysltr2−/− BMDCs was ligand-dependent, we assessed CysLT1R expression on Df-pulsed WT, Cysltr2−/−, and Cysltr2/Ltc4s−/− BMDCs. Whereas Df-pulsed Cysltr2−/− BMDCs responded with a significant increase in CysLT1R expression, Cysltr2/Ltc4s−/− BMDCs showed no increase in CysLT1R, with the level being similar to that observed with saline treatment (Fig. 6A). Thus, LTC4S was required for the Df-induced and CysLT2R-regulated expression of CysLT1R.
FIGURE 6.
Increased cell surface CysLT1R expression on Cysltr2−/− BMDCs is LTC4S-dependent. A. WT, Cysltr2−/−, and Cysltr2/Ltc4s−/− BMDCs were incubated with PBS or 50 µg/ml Df for 24 h, stained for the cell surface expression of CysLT1R, and analyzed by flow cytometry. Mean fluorescence intensities of CysLT1R staining combined from 3 independent experiments. Values are the means ± SEM. *P < 0.05 vs. PBS-pulsed Cysltr2−/− BMDCs. B. Relative expression of mRNA for CysLT1R to GAPDH in Df-pulsed WT or Cysltr2−/− BMDCs. Values are the means ± SEM combined from two experiments. C. WT, Cysltr2−/−, and Cysltr2/Ltc4s−/− BMDCs were incubated with PBS or 50 µg/ml Df for 24 h, fixed and permeabilized, stained for total CysLT1R, and analyzed by flow cytometry. Values are the means ± SEM combined from 3 experiments. D. Representative histograms of CysLT1R expression after Df pulsation in WT, Cysltr1−/−, Cysltr2−/−, and Cysltr2/Ltc4s−/− BMDCs. Isotype control staining (shaded histograms) and CysLT1R (open histograms).
To determine if CysLT2R regulated Df-induced expression of CysLT1R at the level of transcription, we performed quantitative RT-PCR for CysLT1R in WT and Cysltr2−/− BMDCs at 0, 3, 6, 18, and 24 h after the addition of Df. There was no induction of CysLT1R transcript in either WT or Cysltr2−/− BMDCs at any time point (Fig. 6B). Because redistribution to the plasma membrane was another possibility, we assessed expression of CysLT1R protein in Df-pulsed BMDCs after fixation and permeabilization. CysLT1R expression with permeabilization was not greater in Df-pulsed Cysltr2−/− BMDCs as compared to saline-pulsed Cysltr2−/− BMDCs (Fig. 6C). CysLT1R expression was clearly detectable after permeabilization, with staining in Df-pulsed WT, Cysltr2−/−, and Cysltr2/Ltc4s−/− BMDCs that was absent in Cysltr1−/− BMDCs (Fig. 6D). The absence of induced transcript and of an increase in mean fluorescence intensities in permeabilized Cysltr2−/− BMDCs, suggests that CysLT2R regulates Df-induced trafficking of CysLT1R to the cell surface.
DISCUSSION
Despite recent attention, the pathways activated in DCs that program Th2 responses to aeroallergens are poorly understood. We have previously established that the potent sensitizing capacity of house dust mite for mice is mediated, in part, by its ability to trigger Dectin-2-dependent cys-LT generation and CysLT1R activation on DCs (12). In the present study, we initially found that CysLT2R profoundly negatively regulates LTC4S- and CysLT1R-dependent Th2 pulmonary inflammation to dust mite in mice actively sensitized and challenged with Df. We then established negative regulation at the level of DC-mediated sensitization by demonstrating the enhanced capacity of Df-pulsed Cysltr2−/− BMDCs and the reduced capacity of Cysltr2/Ltc4s−/− BMDCs or WT BMDCs activated with a CysLT2R agonist to sensitize WT mice for Df-elicited pulmonary inflammation. Although many inflammatory cells are capable of producing and responding to cys-LTs, IgE/FcεRI-independent Df-induced cys-LT production during sensitization/priming is restricted to Dectin-2-expressing DCs and macrophages (26) (our own observations), highlighting the importance of inflammatory mediator generation from these cells. Notably, while WT mice sensitized with Cysltr2/Ltc4s−/− BMDCs were protected from pulmonary inflammation, the degree of protection in directly sensitized and challenged Cysltr2/Ltc4s−/− mice was greater, suggesting LTC4S present outside the DC compartment also contributes to pathologic inflammation in response to challenge with Df.
CysLT2R recognizes both LTC4, the product of LTC4S, and LTD4, the metabolite of LTC4 generated by the action of γ-glutamyl transpeptidase and/or γ-glutamyl leukotrienase to remove glutamic acid from the glutathione moiety (27, 28). Thus, the generation of sequential ligands for the classical CysLT2R and CysLT1R may contribute to regulation of the biologic consequences of pathway activation. As studies of human monocyte-derived DCs suggest comparable cell surface staining for CysLT1R and CysLT2R (29), it seems possible that LTD4/CysLT1R conditioning of DCs for their sensitizing function may be critically regulated by the ratio of LTC4 to LTD4 present in tissue and the relative affinities of endogenously expressed CysLT2R and CysLT1R for LTC4 and LTD4 in DCs.
To understand whether cys-LTs could influence the balance of endogenous CysLT1R and CysLT2R function, we turned to in vitro studies of receptor function on BMDCs, supplementing genetic approaches with pharmacologic tools. We found that Cysltr2−/− BMDCs had enhanced LTD4-elicited, CysLT1R-mediated ERK phosphorylation, as compared to WT controls, that was significant in both dose- and time-dependent analyses. To our surprise, we found that pretreatment of WT BMDCs, but not Cysltr2−/− BMDCs, with a selective CysLT2R ligand, N-methyl LTC4, significantly reduced ERK phosphorylation to an equimolar dose of LTD4. The potency of N-methyl LTC4 (EC50 46.1 ± 8.7 nM) is similar to that of LTC4 (EC50 38.6 ± 6.1 nM) at heterologously expressed murine CysLT2R in an assay for calcium flux. N-methyl LTC4 also has comparable potency to LTC4 at human CysLT2R for eliciting calcium flux or β-arrestin-2 binding in transfected cells, and for eliciting a vascular leak in transgenic mice overexpressing human CysLT2R (24). Our finding for N-methyl LTC4 suppression of LTD4-induced ERK phosphorylation at equimolar dosing suggests that LTC4 activity at CysLT2R in immune cells may be relevant over a range of concentrations comparable to LTD4 activity at CysLT1R. The dominant products recovered at 30 min from Df-pulsed BMDCs quantified by enzyme immunoassay with fractions separated by HPLC are LTC4 and LTE4 in a 1:3 ratio (data not shown). There was little detectable LTD4 as expected from its rapid conversion to LTE4, as seen during smooth muscle contraction in vitro or during induced vascular leak in vivo (21, 30). Nonetheless, when we treated WT BMDCs with the CysLT2R antagonist HAMI3379, we observed a small but significant increase in LTD4-initiated CysLT1R-dependent ERK phosphorylation, suggesting that LTD4 activity at CysLT2R may also be germane. If each ligand can act at CysLT2R, the timing of LTC4 generation and its conversion to LTD4 may determine the magnitude of the CysLT1R-mediated proinflammatory signal. We observed no change in the cell surface expression of CysLT1R on WT BMDCs during the time period of ERK phosphorylation and conclude that CysLT2R regulation of CysLT1R is at the signaling level.
GPR17 is a seven-transmembrane receptor which does not bind cys-LTs (25, 31, 32), but can inhibit CysLT1R function in transfected cells by interfering with CysLT1R-LTD4 binding without requiring its own ligand/activation (31). Knockdown of GPR17 in BM-derived macrophages resulted in enhanced LTD4-initiated calcium flux and GPR17-deficient mice had augmented CysLT1R-dependent Th2 pulmonary inflammation to Df (11, 31). In contrast to the ligand-independent regulation of CysLT1R by GPR17, we find that negative regulation by CysLT2R in BMDCs is ligand-dependent. Activation of CysLT2R in WT BMDCs by the selective agonist N-methyl LTC4 inhibits CysLT1R-dependent ERK phosphorylation to LTD4, whereas inhibition of CysLT2R by the HAMI3379 antagonist augments LTD4-induced ERK phosphorylation. Heterologous desensitization of CysLT1R has been reported for other G protein-coupled receptors, including P2Y6, BLT1, EP2, and EP4 (33, 34). Our study extends this concept to the regulation of LTD4/CysLT1R activation by its parent compound, LTC4, acting at CysLT2R.
Heterodimerization of CysLT2R with CysLT1R has been demonstrated with human mast cells by fluorescence lifetime imaging microscopy, and knock down of CysLT2R in those cells enhanced their expression of cell surface CysLT1R (17). While we could see augmented cell surface CysLT1R expression on Df-pulsed Cysltr2−/− BMDCs by 24 h, we did not detect basal differences in CysLT1R expression between WT and Cysltr2−/− BMDCs that would account for their differences in LTD4-elicited, CysLT1R-dependent ERK phosphorylation. Furthermore, we found that treatment with the selective CysLT2R agonist N-methyl LTC4 reduced the Df-initiated increase in cell surface CysLT1R expression on WT BMDC at 24 h, suggesting that a biochemical event suppresses both the LTD4 and Df responses.
Our studies suggest that CysLT2R regulates CysLT1R expression at the level of receptor trafficking, as we found upregulation of cell surface CysLT1R with Df-loading, in the absence of augmented transcript or protein in permeabilized Cysltr2−/− BMDCs. The findings for CysLT2R suppression of Df-induced CysLT1R may reflect reduced anterograde trafficking or recycling to the cell surface or enhanced internalization, each of which have been reported to control G protein coupled receptor activation (35–37). Additionally, assessment of Cysltr2/Ltc4s−/− BMDCs with Df-loading showed no upregulation of cell surface CysLT1R, implying that the ligand for CysLT1R or the integrity of LTC4S is required for trafficking of this receptor on the cell membrane.
Opposing roles in the generation of Th2 immunity are found in studies of the human CysLT1R and CysLT2R genes in relation to bronchial asthma. A study of 112 subjects from Tristan da Cunha revealed a CysLT1R mutation at G300S to be associated with atopy and asthma in females (38). This mutation decreases the EC50 for LTD4-elicited calcium flux (from 1.6 nM to 0.5 nM) and for LTD4-elicited inositol phosphate generation (from 46 nM to 5.6 nM) in COS7 transfectants, supporting the notion that enhanced CysLT1R signaling promotes human atopy. Furthermore, a polymorphism in the CysLT2R gene, resulting in a single amino acid substitution M201V, was found in this same population to be associated with atopy (16). In vitro experiments with transient (16) and stable (39) transfectants revealed that the M201V mutant had reduced affinities for LTC4 and LTD4, increased EC50s for calcium flux (from 8.4 to 16 nM for LTC4 and from 17 to 66 nM for LTD4), and reduced inositol phosphate generation in response to LTC4 or LTD4 as compared to the WT transfectant. This suggests that a reduction is CysLT2R function may also promote Th2 immunity in humans, and that the opposing actions of CysLT1R and CysLT2R observed here in the mouse are germane to human disease.
Our studies of the balance between cys-LT receptors highlight their role in regulating both developing Th2 responses and ERK phosphorylation, which has been associated with a Th2-inducing function in DCs. We did not find cys-LT regulation of canonical cytokines associated with Th1, Th2, or Th17 immunity such as TNF-α, IL-12p70, IL-4, IL-10, IL-6, or IL-23. The Toll-like receptor 2 agonist Pam-3-cys induces DCs to instruct the generation of IL-5 and IL-13 producing CD4+ T cells in human in vitro or murine in vivo experiments (40, 41). Activation of murine splenic CD11c+ cells with Pam-3-cys triggers ERK phosphorylation, IL-10 production, and suppression of IL-12p70, which is dramatically reduced in ERK1-deficient DCs or in DCs treated with the MEK1/2 inhibitor UO126 (40). This same pattern of ERK phosphorylation and cytokine production is seen in human DCs stimulated with Shistosoma egg antigen (41) or murine DCs stimulated with lacto-N-fucopentaose, a Th2-inducing glycan derived from Shistosoma egg antigen (42). Selective ERK phosphorylation (in the absence of p38 MAPK or JNK phosphorylation) is also triggered in human monocyte-derived DCs by the peanut allergen Ara h1 (43). While the ERK-dependent function(s) that promotes Th2 immunity is yet to be identified, our data suggest that this signaling can be enhanced or inhibited by CysLT1R or CysLT2R activation, respectively, and that DC-dependent Th2 immune responses to aeroallergens are not merely a default pathway (44), but can be finely regulated by the cys-LT pathway.
Supplementary Material
ACKNOWLEDGMENTS
We thank Juying Lai for technical assistance for histology.
This work is supported by National Institutes of Health Grants T32AI007306 (to K.F.A.), U19AI095219, R01HL090630 (to Y.K.), and K08AI080948 (to N.A.B.).
The abbreviations used in this paper
- BAL
bronchoalveolar lavage
- BMDC
bone marrow-derived dendritic cell
- cys-LT
cysteinyl leukotriene
- CysLT1R and CysLT2R
type 1 and type 2 cys-LT receptors
- DC
dendritic cell
- Df
extracts from Dermatophagoides farinae
- LT
leukotriene
- LTC4S
LTC4 synthase
- LN
lymph node
- WT
wild-type
REFERENCES
- 1.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 2.Austen KF, Maekawa A, Kanaoka Y, Boyce JA. The leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implications. J. Allergy Clin. Immunol. 2009;124:406–414. doi: 10.1016/j.jaci.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning PJ, Watson RM, Margolskee DJ, Williams VC, Schwartz JI, O'Byrne PM. Inhibition of exercise-induced bronchoconstriction by MK-571, a potent leukotriene D4-receptor antagonist. N. Engl. J. Med. 1990;323:1736–1739. doi: 10.1056/NEJM199012203232504. [DOI] [PubMed] [Google Scholar]
- 4.Figueroa DJ, Breyer RM, Defoe SK, Kargman S, Daugherty BL, Waldburger K, Liu Q, Clements M, Zeng Z, O'Neill GP, Jones TR, Lynch KR, Austin CP, Evans JF. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am. J. Respir. Crit. Care Med. 2001;163:226–233. doi: 10.1164/ajrccm.163.1.2003101. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa DJ, Borish L, Baramki D, Philip G, Austin CP, Evans JF. Expression of cysteinyl leukotriene synthetic and signalling proteins in inflammatory cells in active seasonal allergic rhinitis. Clin. Exp. Allergy. 2003;33:1380–1388. doi: 10.1046/j.1365-2222.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 6.Parameswaran K, Liang H, Fanat A, Watson R, Snider DP, O'Byrne PM. Role for cysteinyl leukotrienes in allergen-induced change in circulating dendritic cell number in asthma. J. Allergy Clin. Immunol. 2004;114:73–79. doi: 10.1016/j.jaci.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Stelmach I, Bobrowska-Korzeniowska M, Majak P, Stelmach W, Kuna P. The effect of montelukast and different doses of budesonide on IgE serum levels and clinical parameters in children with newly diagnosed asthma. Pulm. Pharmacol. Ther. 2005;18:374–380. doi: 10.1016/j.pupt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Yamakawa Y, Ohtsuka Y, Ohtani K, Fujii T, Nagata S, Yamashiro Y, Shimizu T. Effects of leukotriene receptor antagonists on peripheral eosinophil counts and serum IgE levels in children with food allergy. Drugs R D. 2010;10:147–154. doi: 10.2165/11586150-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am. J. Respir. Crit. Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 10.Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J. Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 11.Maekawa A, Xing W, Austen KF, Kanaoka Y. GPR17 regulates immune pulmonary inflammation induced by house dust mites. J. Immunol. 2010;185:1846–1854. doi: 10.4049/jimmunol.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, Kanaoka Y. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J. Exp. Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL, Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O'Neill GP, Metters KM, Lynch KR, Evans JF. Characterization of the human cysteinyl leukotriene 2 receptor. J. Biol. Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 14.Hui Y, Yang G, Galczenski H, Figueroa DJ, Austin CP, Copeland NG, Gilbert DJ, Jenkins NA, Funk CD. The murine cysteinyl leukotriene 2 (CysLT2) receptor. cDNA and genomic cloning, alternative splicing, and in vitro characterization. J. Biol. Chem. 2001;276:47489–47495. doi: 10.1074/jbc.M107556200. [DOI] [PubMed] [Google Scholar]
- 15.Ogasawara H, Ishii S, Yokomizo T, Kakinuma T, Komine M, Tamaki K, Shimizu T, Izumi T. Characterization of mouse cysteinyl leukotriene receptors mCysLT1 and mCysLT2: differential pharmacological properties and tissue distribution. J. Biol. Chem. 2002;277:18763–18768. doi: 10.1074/jbc.M109447200. [DOI] [PubMed] [Google Scholar]
- 16.Thompson MD, Storm van's Gravesande K, Galczenski H, Burnham WM, Siminovitch KA, Zamel N, Slutsky A, Drazen JM, George SR, Evans JF, O'Dowd BF. A cysteinyl leukotriene 2 receptor variant is associated with atopy in the population of Tristan da Cunha. Pharmacogenetics. 2003;13:641–649. doi: 10.1097/00008571-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–3270. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thivierge M, Stankova J, Rola-Pleszczynski M. Toll-like receptor agonists differentially regulate cysteinyl-leukotriene receptor 1 expression and function in human dendritic cells. J. Allergy Clin. Immunol. 2006;117:1155–1162. doi: 10.1016/j.jaci.2005.12.1342. [DOI] [PubMed] [Google Scholar]
- 19.Beller TC, Maekawa A, Friend DS, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J. Biol. Chem. 2004;279:46129–46134. doi: 10.1074/jbc.M407057200. [DOI] [PubMed] [Google Scholar]
- 20.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J. Biol. Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 21.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 22.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J. Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 23.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 24.Yan D, Stocco R, Sawyer N, Nesheim ME, Abramovitz M, Funk CD. Differential signaling of cysteinyl leukotrienes and a novel cysteinyl leukotriene receptor 2 (CysLT2) agonist, N-methyl-leukotriene C4, in calcium reporter and beta arrestin assays. Mol. Pharmacol. 2011;79:270–278. doi: 10.1124/mol.110.069054. [DOI] [PubMed] [Google Scholar]
- 25.Wunder F, Tinel H, Kast R, Geerts A, Becker EM, Kolkhof P, Hutter J, Erguden J, Harter M. Pharmacological characterization of the first potent and selective antagonist at the cysteinyl leukotriene 2 (CysLT2) receptor. Br. J. Pharmacol. 2010;160:399–409. doi: 10.1111/j.1476-5381.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PR, Reid DM, Heinsbroek SE, Brown GD, Gordon S, Wong SY. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur. J. Immunol. 2005;35:2163–2174. doi: 10.1002/eji.200425785. [DOI] [PubMed] [Google Scholar]
- 27.Parker CW, Falkenhein SF, Huber MM. Sequential conversion of the glutathionyl side chain of slow reacting substance (SRS) to cysteinyl-glycine and cysteine in rat basophilic leukemia cells stimulated with A-23187. Prostaglandins. 1980;20:863–886. doi: 10.1016/0090-6980(80)90139-2. [DOI] [PubMed] [Google Scholar]
- 28.Anderson ME, Allison RD, Meister A. Interconversion of leukotrienes catalyzed by purified gamma-glutamyl transpeptidase: concomitant formation of leukotriene D4 and gamma-glutamyl amino acids. Proc. Natl. Acad. Sci. USA. 1982;79:1088–1091. doi: 10.1073/pnas.79.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dannull J, Schneider T, Lee WT, de Rosa N, Tyler DS, Pruitt SK. Leukotriene C4 induces migration of human monocyte-derived dendritic cells without loss of immunostimulatory function. Blood. 2012;119:3113–3122. doi: 10.1182/blood-2011-10-385930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krilis S, Lewis RA, Corey EJ, Austen KF. Bioconversion of C-6 sulfidopeptide leukotrienes by the responding guinea pig ileum determines the time course of its contraction. J. Clin. Invest. 1983;71:909–915. doi: 10.1172/JCI110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maekawa A, Balestrieri B, Austen KF, Kanaoka Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc. Natl. Acad. Sci. USA. 2009;106:11685–11690. doi: 10.1073/pnas.0905364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benned-Jensen T, Rosenkilde MM. Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants. Br. J. Pharmacol. 2010;159:1092–1105. doi: 10.1111/j.1476-5381.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capra V, Ravasi S, Accomazzo MR, Citro S, Grimoldi M, Abbracchio MP, Rovati GE. CysLT1 receptor is a target for extracellular nucleotide-induced heterologous desensitization: a possible feedback mechanism in inflammation. J. Cell Sci. 2005;118:5625–5636. doi: 10.1242/jcs.02668. [DOI] [PubMed] [Google Scholar]
- 34.Capra V, Accomazzo MR, Gardoni F, Barbieri S, Rovati GE. A role for inflammatory mediators in heterologous desensitization of CysLT1 receptor in human monocytes. J. Lipid Res. 2009;51:1075–1084. doi: 10.1194/jlr.M003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2004;44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558. [DOI] [PubMed] [Google Scholar]
- 36.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim. Biophys. Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim. Biophys. Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Thompson MD, Capra V, Takasaki J, Maresca G, Rovati GE, Slutsky AS, Lilly C, Zamel N, McIntyre Burnham W, Cole DE, Siminovitch KA. A functional G300S variant of the cysteinyl leukotriene 1 receptor is associated with atopy in a Tristan da Cunha isolate. Pharmacogenet. Genomics. 2007;17:539–549. doi: 10.1097/FPC.0b013e328012d0bf. [DOI] [PubMed] [Google Scholar]
- 39.Brochu-Bourque A, Veronneau S, Rola-Pleszczynski M, Stankova J. Differential signaling defects associated with the M201V polymorphism in the cysteinyl leukotriene type 2 receptor. J. Pharmacol. Exp. Ther. 2011;336:431–439. doi: 10.1124/jpet.110.172411. [DOI] [PubMed] [Google Scholar]
- 40.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 42.Thomas PG, Carter MR, Atochina O, Da'Dara AA, Piskorska D, McGuire E, Harn DA. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J. Immunol. 2003;171:5837–5841. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 43.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J. Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J. Exp. Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.