Abstract
Low-frequency ultrasound has been studied extensively due to its ability to enhance skin permeability. In spite of this effort, improvements in enhancing the efficacy of transdermal ultrasound treatments have been limited. Currently, when greater skin permeability is desired at a given frequency, one is limited to increasing the intensity or the duration of the ultrasound treatment, which carries the risk of thermal side effects. Therefore, the ability to increase skin permeability without increasing ultrasound intensity or treatment time would represent a significant and desirable outcome. Here, we hypothesize that the simultaneous application of two distinct ultrasound frequencies, in the range of 20 kHz to 3 MHz, can enhance the efficacy of ultrasound exposure. Aluminum foil pitting experiments showed a significant increase in cavitational activity when two frequencies were applied instead of just one low frequency. Additionally, in vitro tests with porcine skin indicated that the permeability and resulting formation of localized transport regions are greatly enhanced when two frequencies (low and high) are used simultaneously. These results were corroborated with glucose (180 Da) and inulin (5000 Da) transdermal flux experiments, which showed greater permeant delivery both into and through the dual-frequency pre-treated skin.
Keywords: Cavitation, Permeability, Skin, Diffusion, Transdermal Drug Delivery, Ultrasound
1. Introduction
Ultrasound-assisted transdermal drug delivery (TDD) has been studied extensively for decades. Work in this area originally focused mainly on using therapeutic and high-frequency ultrasound (US) (>0.7 MHz) to enhance drug delivery through the skin[1]. It was only in the late-1980s and early 1990s that the use of low-frequency US (<100kHz) began to be more widely investigated for drug-delivery applications[2]. This switch occurred primarily because it was recognized that US-enhanced skin permeability results from transient cavitation near the skin surface, which is inversely correlated with the US frequency[3]. Since these discoveries were made, extensive research has been conducted into the mechanisms and applications of low-frequency sonophoresis (LFS)[3].
In addition to low-frequency US treatment, chemical penetration enhancers (CPEs), most often surfactants, have also been studied extensively for their permeability-enhancing abilities. Interestingly, CPEs have been found to act synergistically with low-frequency US in enhancing skin permeability[4]. Upon the application of US and a CPE, two distinct regions of skin are observed, known as localized transport regions (LTRs) and non-localized transport regions (non-LTRs). LTRs are US and CPE-induced regions in the skin in which a high degree of perturbation of the stratum corneum (SC) has taken place[5]. As a result, transport through the LTRs is greatly enhanced relative to that through native skin. It has been found that LTRs are up to 80-fold more permeable than non-LTRs and that non-LTRs also exhibit enhanced transport relative to native skin when a CPE is present during LFS treatment[1], [6]. Within the LTRs, the SC no longer presents a significant barrier to permeant transport, as the dermis becomes the primary diffusional barrier[6]. In non-LTRs, however, the SC still possesses considerable barrier function[2], [6]. Enhancement within the non-LTRs has been shown to be due to increased penetration of CPEs via acoustic streaming, resulting in a slight disruption of the SC[3], [7]. In LTRs, however, increased enhancement results from the synergistic effects of the simultaneous application of US and CPEs. By causing transient cavitation events near the skin surface, US is able to physically disrupt the skin. These same cavitation events also actively deliver the CPEs into the skin, leading to higher concentrations of CPEs in the LTRs relative to the non-LTRs, which contributes to causing greater skin permeability[3], [5], [6], [8].
LTR formation as a result of US and CPE treatment is an unpredictable and stochastic process. Additionally, even if treatment times in excess of 10 minutes are utilized, typically only 5–10% of the treated skin surface area may result in LTRs[4], [9]. If a larger portion of the treated skin area could reliably and reproducibly form LTRs, greater amounts of drugs could be delivered to, and through, the skin. Furthermore, this would allow for smaller skin areas to be treated to achieve the same amount of drug delivery, requiring less US power and potentially shorter treatment times.
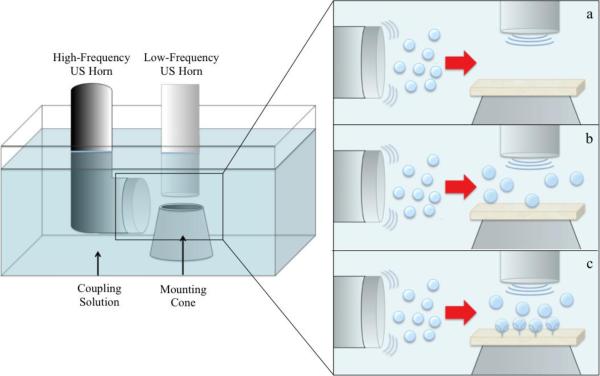
Building upon the proven methods of US-mediated skin permeability enhancement, a potential method for increasing LTR formation and decreasing treatment times would involve generating more bubbles in the acoustic field adjacent to the skin. These additional bubbles could be nucleated using a second US horn. Specifically, higher frequencies of US (>0.7 MHz) are capable of nucleating a relatively large number of small cavitation bubbles, relative to low-frequency US. Along these lines, recent reports have shown that by combining US horns operating at frequencies in the range of 20 kHz to 3 MHz, acoustic cavitation activity is enhanced compared to that of single US frequencies operating alone[5], [10], [11]. Here, we hypothesize that the addition of a second, high-frequency, US horn oriented parallel to the surface of the skin will nucleate a greater number of bubbles in the vicinity of the skin (see Figure 1a). These additional bubbles can then cavitate and collapse in response to acoustic waves generated from a low-frequency US horn oriented perpendicular to the skin surface (see Figures 1b and 1c), as is done with typical LFS treatments.
Figure 1.
Schematic diagram of the setup used showing the coupling solution bath and the mounting cone over which the skin is mounted. For the aluminum foil experiments, the diffusion cell is placed within the rubber cone. It is hypothesized that the high-frequency US horn nucleates small bubbles, which are then pushed below the low-frequency horn by streaming (a). The additional bubbles nucleated by the high-frequency horn grow by rectified diffusion under the influence of the low-frequency horn (b). Once the bubbles reach the threshold size, they collapse against the skin (c).
2. Experimental Section
2.1. Materials
Phosphate buffered saline (PBS) and sodium lauryl sulfate (SLS) were obtained from Sigma-Aldrich Company (St. Louis, MO). Allura red was obtained from TCI America (Portland, OR). All chemicals were used as received. 3H-labeled glucose (20 Ci/mmol activity) and 14C-labeled 5000 Da molecular weight inulin (2 mCi/g activity) were obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO). The tissue solubilizer Soluene-350 and scintillation cocktail Hionic-Fluor were obtained from Perkin-Elmer (Waltham, MA).
2.2. Aluminum Foil Pitting Experiments
In order to gauge the number and radius of acoustic cavitation microjets generated by the applied single- or dual-frequency US treatments, a series of aluminum foil (Reynolds, Richmond, VA) pitting experiments were carried out following previously published protocols[12]. Experiments were conducted in a custom-fabricated 2.2 L tank (see Figure 1). The tank was constructed in order to allow for two frequencies of US to be applied simultaneously to aluminum foil and skin samples. Aluminum foil samples were carefully cut into 25-mm by 25-mm squares to avoid wrinkling, and mounted on the receiver compartment of 12 mL Franz diffusion cells (PermeGear, Hellertown, PA). Vacuum grease (Dow Corning, Midland, MI) was utilized to adhere the aluminum foil samples to the receiver compartment of the diffusion cells. The receiver compartments of the diffusion cells were then filled with 12 mL of PBS and placed in the diffusion cell holder in the tank.
Three low-frequency ultrasounds (20, 40, and 60 kHz), in combination with two high-frequency ultrasounds (1 and 3 MHz), were considered in a pairwise fashion (e.g., 20 kHz + 1 MHz, 20 kHz + 3 MHz, etc.). Low-frequency US was generated utilizing three separate US systems (Sonics and Materials, Inc., Newtown, CT), each operating at a different frequency: 20 kHz (VCX 500), 40 kHz (VCX 130), and 60 kHz (custom order). 1 and 3 MHz US was generated utilizing a tunable US probe (Enraf Nonius, Rotterdam, The Netherlands). The low-frequency US horn was positioned perpendicular to the aluminum foil surface, offset by 1 cm, with the high-frequency US probe positioned parallel to the aluminum foil surface, approximately 1 cm from the leading edge of the diffusion cell (see Figure 1 for a schematic of the experimental setup). The intensities of the low- and high-frequency ultrasounds utilized were 8.0 W/cm2 and 1.5 W/cm2, respectively, and were calibrated by calorimetry. The respective intensities were held constant for all the US horns used, and were chosen for consistency with previously published reports of single-frequency US-enhanced transdermal drug delivery[2], [3]. The coupling solution between the US horns and the aluminum foil sample contained either PBS or 1 wt% SLS in PBS. Single-frequency experiments were conducted with the low-frequency US horn operating at the conditions described above and the high-frequency US set to zero intensity (sham ultrasound). Dual-frequency experiments were conducted by first turning on the high-frequency US horn, and then running the low-frequency US horn for 5 s in continuous or pulsed (1s ON: 1s OFF) mode. For each combination of frequencies (3 low × 2 high), coupling solution formulation (PBS or 1% SLS in PBS), and wave pulsing (continuous or pulsed), five replicates were tested for statistical analysis. All the single-frequency samples were repeated 4–5 times.
2.3. Collection and Analysis of Aluminum Foil Samples
Following US treatment, samples were removed from the receiver compartment of the diffusion cell, trimmed to the treated area, mounted on heavy stock paper, and then laminated. Samples were then scanned using a Canon CanoScan 8800F at 1200 dpi in grayscale mode and saved in the BMP file format. The pits in each sample were then manually counted and sized using custom software that was developed in house for this purpose (available at: http://web.mit.edu/dbgroup/current_research.shtml). This software allows the user to specify pit centers and radii (assuming hemispherical pits) through direct interaction with the scanned images using any pointing device (e.g., mouse, stylus), thereby facilitating rapid and accurate quantification of the pit population distribution. Pit radii are stored in units of pixels, and then converted into centimeters based on the scan resolution. Pit count and size data collected using this software was then imported into Microsoft Excel (Redmond, WA) for statistical analysis.
2.4. Preparation and Treatment of Skin Samples
This procedure has been approved by the MIT Committee on Animal Care. Tissue was procured by Research 87 (Boylston, MA). Previously published protocols were utilized for the storage and preparation of skin samples[13]. Briefly, skin was harvested from the back and flank of female Yorkshire pigs within one hour of sacrificing the animal. Subcutaneous fat was removed from the dermis with a razor blade, and then sectioned into 25-mm strips prior to storage at −85°C for up to six months. Skin was thawed for 20 minutes in PBS, prior to use in experiments, and then excess hair was trimmed using surgical scissors. Skin was then dermatomed to 700 μm using an electric reciprocating dermatome (Zimmer Orthopedic Surgical Products, Dover, Ohio), and cut into 25 × 25-mm samples. The skin samples were then mounted in the bath as shown in Figure 1.
Skin samples were treated similarly to the procedure described in Section 2.2, with the exception that only 20 kHz US was investigated and the low-frequency US horn was positioned 3 mm from the surface of the skin for consistency with prior LFS research [13–15]. The tank was filled with a solution of 1% SLS in PBS until the high-frequency US horn was fully submerged. Skin samples were then suspended over a rubber ring directly below the 20 kHz probe using metal pins. The intensities of the low- and high-frequency US horns were identical to those described in Section 2.2. Note that only 1MHz was tested for the high-frequency US because experiments with aluminum foil showed that the resulting pitted area is larger when using 1 MHz than when using 3 MHz (see Section 3.2). The 20 kHz probe was set to 50% duty cycle (1s on, 1s off). Treatment time ranged between two and six minutes, which are typical treatment time ranges established from prior LFS research[6], [7], [13].
2.5. Quantification of LTR Area in Ultrasound-Treated Skin
Immediately after US treatment, the skin was removed from the tank and washed thoroughly with PBS to remove any residual SLS. The skin samples were then mounted into 15-mm inner-diameter diffusion cells (PermeGear, Hellertown, PA) using the following protocol. High vacuum grease (Dow Corning, Midland, MI) was applied to the inner flange of the donor compartment in order to provide a water-tight seal between the skin and the diffusion cell. The receiver chamber was then filled with PBS and the skin sample was positioned over the receiver chamber with the treated area centered in the diffusion cell. The donor compartment was then placed on top of the skin and the diffusion cell was clamped tightly. The donor chamber was filled with the dye allura red at a concentration of 0.04 wt% in PBS, and was allowed to sit for 1.5 hours.
After the skin was stained, the allura red solution was removed from the donor compartment, and the skin sample was washed thoroughly with PBS. The skin sample was then gently blotted dry to prepare it for imaging. The skin surface was imaged using a digital camera (Panasonic DMCZS7, 12.1 megapixels, shutter speed 1/2000s) positioned approximately 20 cm above the skin.
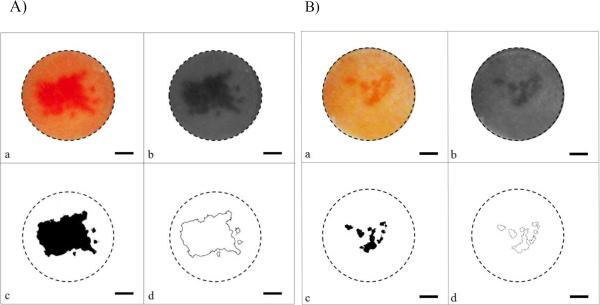
The images were processed using Adobe Photoshop CS5.1 using the following protocol. First, the blue-channel of the image was isolated because this provided the best contrast for measuring the red dyed portion of the skin (see Figure 6) [16]. The image was then cropped such that only the portion of skin exposed to allura red was captured (1.5 cm × 1.5 cm). The threshold of the image was then adjusted such that only the dyed portion of the skin was captured (see Figure 6c). Once an appropriate threshold level was found, all samples were processed using the same threshold value for consistency. The adjusted image was then opened in ImageJ (National Institutes of Health, Bethesda, MD), and the area was quantified with the “Analyze Particles” option using the default size and circularity options. The reported values were then converted from units of pixels to mm by utilizing standards of known dimensions. All samples below a minimum resolution value of 0.01% LTR area were reported to have an LTR area of 0.
Figure 6.
Representative images of skin treated with both low- and high-frequency US (A) for six minutes, and of skin treated with only low-frequency US for six minutes (B). Native Image (a). Isolated blue channel (b). Threshold-adjusted image (c). Area measured by ImageJ and outlined (d). The circular dotted line indicates the area of the skin that was treated with US and exposed to the dye allura red. The scale bar in the images represents 3mm.
2.6. Quantification of Glucose and Inulin Penetration into Treated Skin
After pretreatment of the skin with US, 1 mL of glucose (concentration of 1 mg/mL spiked with 2 μCi/mL tritiated glucose, or 1.8×10−3 % labeled glucose by mass) was added to the donor compartment of the diffusion cell and allowed to permeate for two hours. At the end of the experiment, the glucose solution was removed, and the donor compartment was washed thoroughly with PBS to remove excess glucose that did not penetrate into the skin. The receiver compartment was collected and the diffusion cell disassembled. The area of the skin exposed to the glucose solution was carefully excised using a razor blade and collected. 10 mL of Soluene 350 was added to each skin sample, and the sample was heated in a water bath between 70–85 °C to facilitate solubilization of the tissue. Upon solubilization, 5 mL was aliquoted for radiometric analysis. 2 mL aliquots of the receiver solutions from each experiment were also sampled and analyzed. 15 mL of Hionic Fluor Scintillation Cocktail was added to all tissue and receiver solution aliquots and allowed to rest for 40 minutes for the fluorescence signal to equilibrate. The samples were then run on a Tri-Carb Liquid Scintillation Counter (Perkin Elmer) to determine the decompositions per minute (DPM). The resulting DPM values were used to calculate the total mass of glucose delivered to, and through, the skin for each sample using the ratio of labeled to unlabeled glucose and the known activity of the 3H glucose.
Separate skin samples pretreated with US had 1 mL of inulin (concentration of 1 mg/mL spiked with 2 μCi/mL 14C-labeled inulin, or 100 % labeled inulin by mass) added to the donor compartment, and were also allowed to permeate for two hours. Quantification of inulin delivery followed an identical procedure as that performed for glucose.
2.7. Statistical Significance
We have characterized statistical significance using t-tests. F-tests were performed to analyze variances. Statistical significance is defined throughout as p < 0.05. All calculations were performed using Microsoft Excel (Redmond, Washington).
3. Results and Discussion
3.1. Aluminum Foil Pitting
Each aluminum foil pitting experiment was performed in quintuplicate. These experiments aimed to quantify the effect of four different sets of variables on pit size (radius), number of pits, and total pitted area. These variables include: i) the coupling solution composition (with or without SLS), ii) the duty cycle of the low-frequency US horn (100% or 50%), iii) the frequency of the low-frequency US horn (20, 40, or 60kHz), and iv) the use and frequency of the high-frequency US horn (not used, 1, or 3 MHz).
3.1.1. Coupling Solution
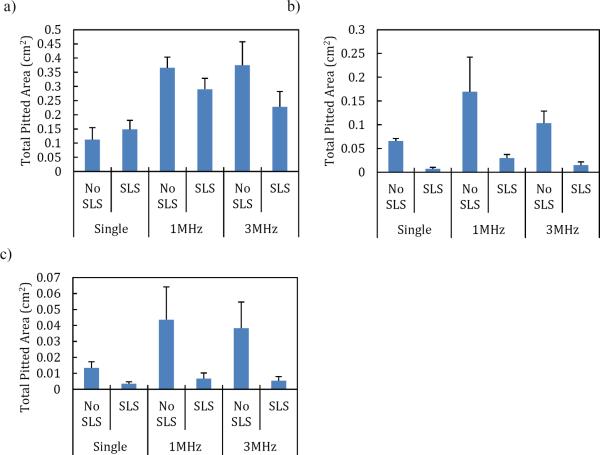
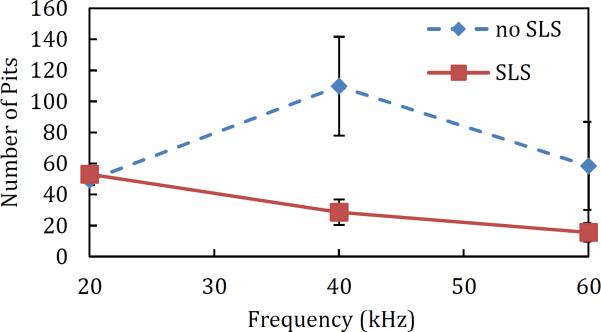
The effect of SLS in the coupling solution was visible in the number of pits, and therefore, in the total pitted area observed on the aluminum foil. The pit radius was found to be unaffected by the presence of SLS (the pit radius was only affected by the low-frequency US horn used (Figure 2), as expected based on previously published reports on the relationship between cavitation bubble size and US frequency[3]). With regards to the total pitted area, SLS had a greater effect on 40 kHz and 60 kHz compared to 20 kHz, as shown in Figure 3. SLS in the coupling solution acted to decrease the number of pits present on the aluminum foil, thereby decreasing the total pitted area when 40 and 60kHz was used.
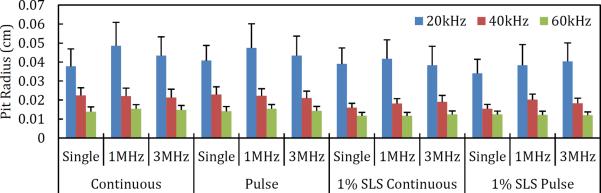
Figure 2.
Average pit radius due to a variety of treatment conditions. Continuous denotes continuous operation of the low-frequency US horn. Pulse denotes 50% duty cycle of the low-frequency US horn (1s on, 1s off). Error bars represent one standard deviation.
Figure 3.
The effect of SLS in the coupling solution on the total pitted area for various combinations of low-frequency and high-frequency US: (a) 20 kHz, (b) 40 kHz, and (c) 60 kHz. Error bars represent one standard deviation.
This is consistent with previously published results[4], and can be attributed to the amphiphilic nature of SLS. Briefly, when bubbles are formed, surfactants like SLS preferentially organize at the bubble-liquid interface, thus reducing the bubble surface tension[17]. While this reduction in surface tension does lead to greater bubble growth rates, bubble collapse intensities are decreased[18]. If a charged surfactant like SLS is used, then SLS adsorption onto the bubble-liquid interface also leads to electrostatic repulsions between the charged bubbles, which decreases bubble coalescence. This, in turn, results in fewer bubbles above the threshold size needed to grow via rectified diffusion, when compared to the number of bubbles without SLS. As a result, these smaller bubbles produced at 40–60 kHz simply dissolve back into the solution due to the Laplace Pressure[19]. Also, as the bubble collapse intensities are decreased in the presence of SLS, it is likely that some bubbles do not collapse with sufficient energy to cause observable pitting of the foil and are therefore not accounted for in our analysis. This explains the decreased number of pits observed when SLS is added to the coupling solution.
3.1.2. Low-Frequency Ultrasound
The low-frequency US used had a consistent effect on pit radius, as shown in Figure 2. 20 kHz created the largest pits, followed by 40 kHz, with 60 kHz creating the smallest pits. This trend was independent of the three other variables (SLS, high frequency applied, pulsing). The number of pits observed on the aluminum foil was also found to depend greatly on the low-frequency US used. Without SLS, 40 kHz US consistently produced the largest number of pits (see the blue curve in Figure 4). The number of pits generated using 20 and 60 kHz were found to be comparable. Upon addition of SLS, however, the number of pits was found to be inversely correlated with frequency (see the red curve in Figure 4), with the number of pits observed using 20 kHz US remaining the same (as discussed in Section 3.1.1). The effect of SLS on the number of pits observed is consistent with the discussion presented in Section 3.1.1. Specifically, because there is an inverse relationship between cavitation bubble size and applied US frequency, the bubbles generated by 40 and 60 kHz are more susceptible to dissolving back into solution during the OFF pulse phase of the low-frequency horn, thereby resulting in fewer pits at the aluminum foil surface. The duty cycle of the low-frequency US horn was found to have no significant impact on pit radius, number of pits, or total pitted area (data not shown, p > 0.05).
Figure 4.
Number of pits observed in aluminum foil pitting experiments when treated using 1 MHz US and low-frequency ultrasound (20, 40, and 60 kHz) with and without 1% SLS present in the coupling solution. The red and blue lines are drawn to facilitate observation of trends. Error bars represent one standard deviation.
3.1.3. High-Frequency Ultrasound Application
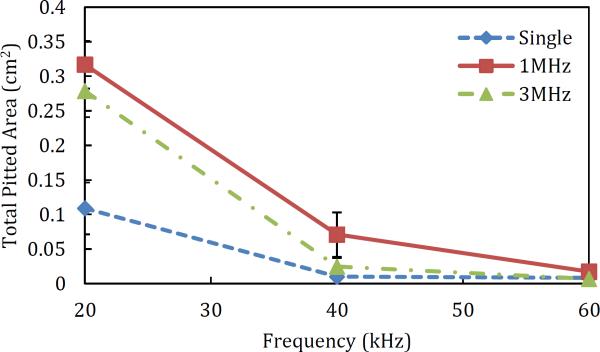
The pit radius was found to be independent of the high-frequency US used (see Figure 2). The greatest effect of the high-frequency US used was observed on the number of pits, as expected. Simultaneous use of the high-frequency US horn was found to always produce more pits on the aluminum foil than found in samples treated without using the high-frequency US. While the 1 MHz frequency generally produced more pits than the 3 MHz frequency (and therefore, a larger pitted area), the effect was typically not significant (see the red and green curves in the plot of total pitted area in Figure 5). Both 1 MHz and 3 MHz US frequencies produced significantly more pits when compared to using no high-frequency US (except for 60 kHz, p = 0.11 and 0.62 for use of 1 and 3 MHz respectively) (data not shown). In the case of 20 and 40 kHz, the high-frequency US horn was observed to nucleate additional bubbles, which could then cavitate and collapse under the influence of the low-frequency US horn. This significant increase in the number of pits leads to a significant increase in the total pitted area, as shown in Figure 5. Figure 5 reveals that the use of 20 kHz always produces the largest pitted area when compared to using 40 or 60 kHz (p < 2.2×10−5 for 20 kHz and 1 MHz compared to 40 or 60 kHz and 1 MHz).
Figure 5.
Variation of the total pitted area in the aluminum foil pitting experiments as a function of the low- and high-frequency US used. All the experiments were carried out using a coupling solution of 1% SLS, and the low-frequency US was pulsed (1s on, 1s off). The red, green, and blue lines are drawn to facilitate observation of trends. Error bars represent one standard deviation.
The slight decrease in the number of pits observed when using 3 MHz compared to 1 MHz US is thought to be due to the difference in the size of the bubbles nucleated by both frequencies. It is known that the average size of nucleated bubbles is inversely proportional to the applied US frequency[3]. The pits are created by bubbles that collapse under the influence of the low-frequency horn. Not all bubbles, however, can couple to the signal of the low-frequency horn and cavitate. Only bubbles within a specific size range (unique to each US frequency) can couple to the low-frequency US. The 3 MHz horn could be nucleating fewer bubbles with sizes within the range necessary to couple to the low-frequency US horn. In addition, the observed slight decrease in the number of pits could be due to the coupling efficiency of the 3 MHz horn, resulting in a decrease in the number of bubbles that are nucleated. The first explanation suggests that additional US frequencies below 1 MHz may also yield increased pitting. Additionally, there may be an optimal US frequency for use as the high-frequency horn, with respect to the number of pits induced. This interesting possibility may be investigated further using custom-fabricated US horns operating in the range of 100 kHz to 1 MHz.
3.2. Skin Permeability
While significant trends were observed in the aluminum foil pitting experiments, it still remained to be seen whether similar trends would be observed when treating skin. Based on the results of the aluminum foil pitting experiments, it was logical to choose 20 kHz as the low-frequency US for the skin studies, since this frequency consistently yielded samples with the largest total pitted area (see Figure 5). Additionally, 1 MHz was chosen as the simultaneous high-frequency US. While the use of 1 MHz US did not produce significantly better results than the use of 3 MHz, the average pitted area using 1MHz was found to be larger than that using 3MHz (see the green and red curves in Figure 5). 1% SLS was utilized in the coupling solution because this was shown to have a minimal effect on the pitting results at 20kHz, as discussed in Section 3.1.1, and has previously been shown to enhance the efficacy of US pretreatment of the skin[20]. Finally, low-frequency US horn was operated at 50% duty cycle to minimize thermal effects, which is consistent with previously published literature reports[3], [6]. Indeed, even at the longest treatment time of six minutes, the temperature of the water bath was found to increase only by 0.33 °C. Because work by our group and others has previously shown that a temperature increase of at least 10 °C is required to increase the skin permeability significantly, it is safe to assume that thermal effects in this case are negligible[2].
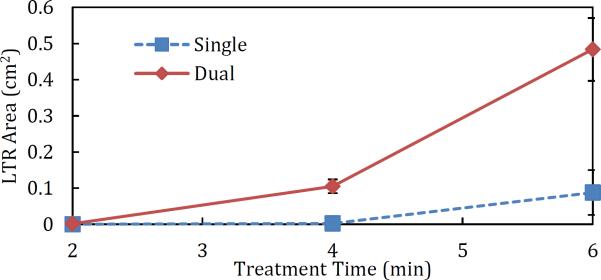
Skin was treated using the above conditions for treatment times ranging from two to six minutes. The resulting LTR area vs. treatment time curve is shown in Figure 7. Six minute treatments resulted in LTR formation in excess of 27% of the treated skin area in the Dual samples (see Table 1). Coverage was less than 5% in the single-frequency samples, in agreement with other published results[9]. For comparison, the Dual samples treated for only four minutes had comparable LTR coverage to single-frequency samples treated for six minutes. This demonstrates that the use of simultaneous low- and high-frequency US decreases the required treatment time to achieve the same size LTR's.
Figure 7.
Resulting LTR area vs. treatment time for the single- (Single) and dual- frequency (Dual) US treatments. The red and blue lines are drawn to facilitate observation of trends. Error bars represent one standard deviation.
Table 1.
Percent LTR coverage as a result of US pretreatment time for the single-frequency and Dual samples. Enhancement is defined as the Dual value divided by the single-frequency value.
| Treatment Time (min) | % LTR Coverage | ||
|---|---|---|---|
| Single | Dual | Enhancement | |
| 2 | 0 | 0.108 | 300 |
| 4 | 0.143 | 5.95 | 41.5 |
| 6 | 4.98 | 27.4 | 5.49 |
Additionally, when high-frequency US was used, the LTR's formed one contiguous area, contrary to the non-contiguous patterns that typically form using low-frequency US alone. This observation is highlighted in Figure 6, which shows skin samples treated with both dual- and single-frequency US for six minutes. The LTR in Figure 6A is localized in the center of the skin sample, and is generally contiguous. LTR's exhibiting this type of localization and contiguity are not typically observed in skin samples treated with only low-frequency ultrasound and CPEs (see Figure 6B). This phenomenon was observed at all treatment times (no LTR's were observed upon visual inspection in the single-frequency samples treated for two minutes). This suggests that the use of dual-frequency ultrasound also helps to centralize LTR's due to increased cavitational activity focused in the region where the low- and high-frequency ultrasound fields overlap.
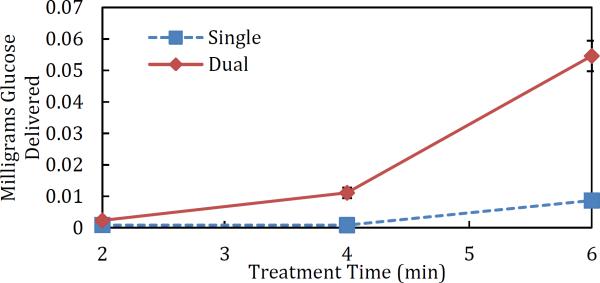
The enhancement in skin permeability as a result of the application of simultaneous low- and high-frequency US was then quantified by glucose transport. The cumulative mass of glucose delivered passively to, and through, skin pretreated simultaneously with both frequencies was found to be 2.7×, 13.6×, and 6.3× greater than that delivered to the single-frequency samples treated for two, four, and six minutes, respectively (see Figure 8 for a plot of glucose delivered vs. treatment time, and Table 2 for the actual values). Similar to the resulting LTR sizes, glucose delivery to single-frequency samples pretreated with US for six minutes was comparable to that seen in the Dual samples pretreated for only four minutes. This substantiates the claim that US pretreatment times can be reduced when using dual-frequency ultrasound without any decrease in the amount of compound delivered. Table 2 shows that glucose delivery to single-frequency samples treated for four minutes was slightly less than glucose delivery to single-frequency samples treated for two minutes. This was not statistically significant (p = 0.42), however, which suggests that there is a minimum treatment time required to achieve increases in delivery when using low-frequency ultrasound alone. The onset of this threshold is reduced with dual-frequency treatment, and less than the treatment time of two minutes. Dual samples treated for four minutes had 4.8× the amount of glucose delivery than Dual samples treated for two minutes (see Glucose Delivery in Table 2), which was statistically significant (p = 0.013).
Figure 8.
Resulting passive glucose delivery to, and through, the skin vs. the US pretreatment time. The red and blue lines are drawn to facilitate observation of trends. Error bars represent one standard deviation.
Table 2.
Glucose and inulin delivery as a result of US pretreatment time for the single-frequency and Dual samples. Enhancement is defined as the Dual value divided by the single-frequency value.
| Treatment Time (min) | Glucose Delivery | Inulin Delivery | ||||
|---|---|---|---|---|---|---|
| Single (mg) | Dual (mg) | Enhancement | Single (mg) | Dual (mg) | Enhancement | |
| 2 | 8.59×10−4 | 2.32×10−3 | 2.70 | 6.34×10−4 | 1.42×10−3 | 2.22 |
| 4 | 8.14×10−4 | 1.11×10−2 | 13.6 | 6.06×10−4 | 2.31×10−3 | 3.81 |
| 6 | 8.64×10−3 | 5.46×10−2 | 6.32 | 1.82×10−3 | 3.66×10−3 | 2.01 |
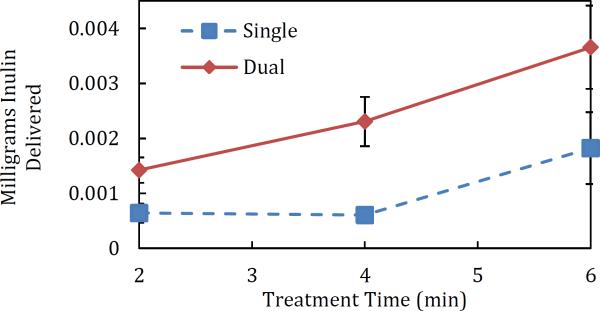
Finally, the phenomenon of enhanced delivery using dual-frequency US was quantified using a larger molecule, specifically, inulin (5000 Da). The mass of inulin delivered to, and through, the skin is reported in Table 2, and a plot is shown in Figure 9. Similar to what was observed with glucose, the four-minute treatment time yielded the greatest enhancement (the enhancements at each time point are reported in Table 2). The hypothesis that there is a minimum treatment time required to achieve significant enhancement when using low-frequency US alone is also supported by the inulin results, since there was no significant difference between delivery after two and four minutes of treatment for the single-frequency samples (p = 0.11) (see the blue curve in Figure 9). This demonstrates the ability of dual-frequency US to enhance delivery of compounds with a wide range of molecular sizes.
Figure 9.
Resulting passive inulin delivery to, and through, the skin vs. the US pretreatment time. The red and blue lines are drawn to facilitate observation of trends. Error bars represent one standard deviation.
Interestingly, we found that the enhancement observed in the resulting LTR area was generally greater than the enhancement observed in the case of molecular permeability. In the context of the aqueous-porous pathway model, LTR's have previously been shown to have a greater density of aqueous channels relative to that corresponding to non-LTR's as a result of the ultrasound treatment[6]. This result is consistent with the hypothesis that the dual-frequency ultrasound treatment generates a greater number of bubble collapse events when compared with the single-frequency ultrasound treatment. Nevertheless, in spite of the increased number of channels, larger molecules, such as inulin, may still only “sample” and diffuse through the largest aqueous pores generated. This is why transport of glucose (180 Da) exhibited greater enhancement as a result of the dual-frequency ultrasound treatment than that experienced by inulin (5000 Da). This finding is also consistent with the literature, where it was shown, in the context of the aqueous-porous pathway model, that the estimated pore size generated by the ultrasound treatment depends on the permeant used to probe transport across the skin[6].
4. Conclusions
The effect of dual low- and high-frequency US on aluminum foil pitting and skin perturbation was investigated. Aluminum foil pitting experiments were carried out to quantify the number of pits, pit radius, and the total pitted area observed in single- and dual-frequency ultrasound experiments. The number of pits was found to depend strongly on whether high-frequency US was used, with significantly more pits found using dual-frequency US. This finding was then tested in porcine skin samples in vitro. Skin samples were treated between two and six minutes. The resulting LTR area was found to be significantly greater in samples treated simultaneously with low- and high-frequency US, compared to samples treated with LFS alone, with the maximum LTR coverage exceeding 27% of the treated area. Passive delivery of both glucose (180 Da) and inulin (5000 Da) to, and through, the US-pretreated skin was also found to be significantly enhanced when dual-frequency US was used. Based on our findings, we conclude that dual-frequency US represents a new and viable method for reducing required US treatment times, for decreasing the lag-time associated with transdermal uptake of permeants following US treatment, and for enhancing overall skin permeability.
Acknowledgements
This work was funded by the National Institutes of Health (Grant# EB-00351). The contents of this manuscript represent solely the views of the authors and do not necessarily reflect the position of the U.S. Government. No official endorsement should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Newman MK, Kill M, Frampton G. Effects of ultrasound alone and combined with hydrocortisone injections by needle or hypo-spray. Am J Phys Med. 1958 Aug;37(4):206–209. [PubMed] [Google Scholar]
- [2].Mitragotri S, Edwards DA, Langer R. A mechanistic study of ultrasonically-enhanced transdermal drug delivery. J Pharm Sci. 1995 Jun;84(6):697–706. doi: 10.1002/jps.2600840607. 4. [DOI] [PubMed] [Google Scholar]
- [3].Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J Control Release. 2011;152(3):330–348. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tezel A, Sens A, Tuchscherer J, Mitragotri S. Synergistic effect of low-frequency ultrasound and surfactants on skin permeability. J Pharm Sci. 2002;91(1):91–100. doi: 10.1002/jps.10000. [DOI] [PubMed] [Google Scholar]
- [5].Kushner J, Kim D, So PTC, Blankschtein D, Langer RS. Dual-channel two-photon microscopy study of transdermal transport in skin treated with low-frequency ultrasound and a chemical enhancer. J Invest Dermatol. 2007;127(12):2832–2846. doi: 10.1038/sj.jid.5700908. [DOI] [PubMed] [Google Scholar]
- [6].Kushner J, Blankschtein D, Langer R. Evaluation of hydrophilic permeant transport parameters in the localized and non-localized transport regions of skin treated simultaneously with low-frequency ultrasound and sodium lauryl sulfate. J Pharm Sci. 2008;97(2):906–918. doi: 10.1002/jps.21028. [DOI] [PubMed] [Google Scholar]
- [7].Polat BE, Deen WM, Langer R, Blankschtein D. A physical mechanism to explain the delivery of chemical penetration enhancers into skin during transdermal sonophoresis - Insight into the observed synergism. J Control Release. 2011 Nov; doi: 10.1016/j.jconrel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Polat BE, Figueroa PL, Blankschtein D, Langer R. Transport Pathways and Enhancement Mechanisms Within Localized and Non-Localized Transport Regions in Skin Treated with Low-Frequency Sonophoresis and Sodium Lauryl Sulfate. J Pharm Sci. 2011;100(2):512–529. doi: 10.1002/jps.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ogura M, Pahwal S, Mitragotri S. Low-frequency sonophoresis: Current status and future prospects. Adv Drug Deliver Rev. 2008;60(10):1218–1223. doi: 10.1016/j.addr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- [10].Liu H-L, Hsieh C-M. Single-transducer dual-frequency ultrasound generation to enhance acoustic cavitation. Ultrason Sonochem. 2009 Mar;16(3):431–438. doi: 10.1016/j.ultsonch.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [11].Hasanzadeh H, Mokhtari-Dizaji M, Bathaie SZ, Hassan ZM, Nilchiani V, Goudarzi H. Enhancement and control of acoustic cavitation yield by low-level dual frequency sonication: A subharmonic analysis. Ultrason Sonochem. 2011;18(1):394–400. doi: 10.1016/j.ultsonch.2010.07.005. [DOI] [PubMed] [Google Scholar]
- [12].Mitragotri S, Ray D, Farrell J, Tang H, Yu B, Kost J, Blankschtein D, Langer R. Synergistic effect of low-frequency ultrasound and sodium lauryl sulfate on transdermal transport. J Pharm Sci. 2000;89(7):892–900. doi: 10.1002/1520-6017(200007)89:7<892::AID-JPS6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- [13].Seto JE, Polat BE, Lopez RFV, Blankschtein D, Langer R. Effects of ultrasound and sodium lauryl sulfate on the transdermal delivery of hydrophilic permeants: Comparative in vitro studies with full-thickness and split-thickness pig and human skin. J Control Release. 2010;145(1):26–32. doi: 10.1016/j.jconrel.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Terahara T, Mitragotri S, Kost J, Langer R. Dependence of low-frequency sonophoresis on ultrasound parameters; distance of the horn and intensity. International Journal of Pharmaceutics. 2002;235:35–42. doi: 10.1016/s0378-5173(01)00981-4. [DOI] [PubMed] [Google Scholar]
- [15].Polat BE, Seto JE, Blankschtein D, Langer R. Application of the Aqueous Porous Pathway Model to Quantify the Effect of Sodium Lauryl Sulfate on Ultrasound-Induced Skin Structural Perturbation. J Pharm Sci. 2011;100(4):1387–1397. doi: 10.1002/jps.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kushner J, Blankschtein D, Langer R. Experimental demonstration of the existence of highly permeable localize transport regions in low-frequency sonophoresis. J Pharm Sci. 2004;93(11):2733–2745. doi: 10.1002/jps.20173. [DOI] [PubMed] [Google Scholar]
- [17].Wu S, Leong T, Kentish S, Ashokkumar M. Frequency Effects during Acoustic Cavitation in Surfactant Solutions. J Phys Chem B. 2009;113(52):16568–16573. doi: 10.1021/jp9083458. [DOI] [PubMed] [Google Scholar]
- [18].Lee J, Kentish S, Ashokkumar M. Effect of surfactants on the rate of growth of an air bubble by rectified diffusion. J Phys Chem B. 2005;109(30):14595–14598. doi: 10.1021/jp051758d. [DOI] [PubMed] [Google Scholar]
- [19].Lee J, Kentish S, Matula T, Ashokkumar M. Effect of surfactants on inertial cavitation activity in a pulsed acoustic field. J Phys Chem B. 2005;109(35):16860–16865. doi: 10.1021/jp0533271. [DOI] [PubMed] [Google Scholar]
- [20].Lavon I, Grossman N, Kost J. The nature of ultrasound-SLS synergism during enhanced transdermal transport. J Control Release. 2005;107(3):484–494. doi: 10.1016/j.jconrel.2005.06.011. [DOI] [PubMed] [Google Scholar]