Abstract
Once established, serum antibody responses against a specific pathogen may last a lifetime. We describe a cohort of four subjects who received smallpox vaccination, and a single subject who received multiple vaccinations, with antibody levels to unrelated antigens monitored for 1–3 years. These immunizations provided the opportunity to determine if infection/vaccination and the resulting toll-like receptor stimulation would alter antigen-specific serological memory to other antigens, including bacterial toxins (tetanus, diphtheria, and pertussis) and viruses (yellow fever virus, measles, mumps, rubella, Epstein-Barr virus, and varicella-zoster virus). Our results indicate that serum IgG levels are remarkably stable and infection or vaccination are unlikely to increase or decrease pre-existing antigen-specific antibody responses.
Keywords: antibody, vaccination, immunological memory
1. Introduction
Maintenance of pathogen-specific serum antibody levels following infection or vaccination is important in establishing protective immunity. Long-term maintenance of T cell-dependent serum antibody responses to viral and vaccine antigens has been previously described[1], but the mechanisms that contribute to maintaining long-term humoral immunity remain unclear and controversial[2–4]. Three primary theories on the maintenance of long-term antibody responses have emerged[2–4]. The polyclonal stimulation model[3] is based on memory B cells (which do not produce immunoglobulins directly) becoming activated continuously or intermittently through toll-like receptor (TLR) engagement or bystander T cell activation to proliferate and differentiate into antibody-secreting daughter cells, which then repopulate a declining antibody-secreting plasma cell pool. The prediction from this model is that pre-existing antibody responses to unrelated antigens will increase following a defined vaccination or infection. The plasma cell displacement theory[4–6] is based on long-lived plasma cells surviving within a niche in the bone marrow, until the plasma cells are forced out following competition with newly arriving plasma cells, resulting in their release into the circulation and eventual decline. This model predicts that pre-existing antibody responses to unrelated antigens will decrease following sequential vaccinations or infections, and that pre-existing antibody responses will demonstrate a uniform rate of decline over time. The third model[2] suggests that plasma cells and memory B cells represent independently regulated populations and that plasma cell lifespan results from imprinting at the time of induction, and not due to repopulation by memory B cells or competition for an immunological niche. The prediction from this model is that pre-existing antibody responses will remain unaltered following repeated infection or vaccination.
To determine if vaccines or microbial infections either increase or decrease pre-existing antibody levels, we followed serum antibody responses against nine or more antigens in two different settings. In one cohort, antibody responses from four individuals were monitored using 40 serum samples collected over the course of a one-year period following smallpox vaccination, representing a defined infection with vaccinia virus. In a second case study, humoral responses in an individual subject receiving multiple vaccinations were monitored using 32 serum samples collected during a 3.4-year period of time.
2. Materials and Methods
2.1 Human subjects
Adult subjects provided informed written consent and completed medical history questionnaires before participation in the study. All human subject research was approved by the Institutional Review Board for OHSU.
2.2 Antigen specific ELISA
Antigen-specific IgG levels and total IgG were measured by ELISA as previously described[7, 8]. Antigens included vaccinia-WR (VV, prepared in-house as previously described)[7], yellow fever 17D (YFV, prepared in-house), measles-Edmonston (MV, Biodesign, Saco, ME), mumps-Enders (Biodesign), rubella-HPV77 (RUBV, Viral Antigens, Inc, Memphis, TN), varicella-zoster virus-rod (VZV, Biodesign), Epstein-Barr virus-gp125 (EBV, Viral Antigens Inc.), tetanus toxin C-fragment and diphtheria toxin (TT and DT, EMD Biosciences, San Diego, CA), and pertussis toxin (PT, List Biological Laboratories, Campbell, CA). For total IgG ELISA, affinity purified goat α-human IgG (minimally cross-reactive to bovine, horse, and mouse serum proteins, JacksonImmuno Research Laboratories, West Grove, PA) was used to coat ELISA plates at 1 μg/ml. For determining the concentration of serum IgG (mg/ml), purified human IgG (ChromePure human IgG, JacksonImmuno Research Laboratories) was included as a standard. Each ELISA plate included an internal standard/positive control sample for normalization between plates and between experiments performed on different days. Serum antibody titers of >120 ELISA units (EU) were considered seropositive.
2.3 Statistical analysis
Statistical analysis was performed by two-tailed Student’s t-test using StatPlus;mac (AnalystSoft Inc.) for Microsoft Excel. Significance thresholds were calculated using both the Bonferroni correction and the Holm procedure[9], based on an experimentwise α of 0.05.
3. Results
3.1 Limited effect of vaccinia virus infection on pre-existing antibody responses
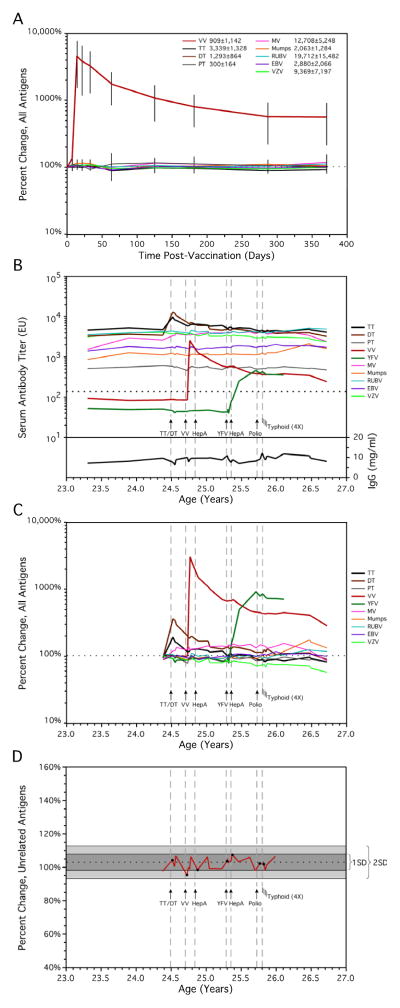
We had previously investigated the stability of serum antibody responses in a longitudinal setting spanning up to 25 years in individual subjects[1]. Our results indicated that antibody responses to specific antigens were well maintained, with some responses persisting indefinitely. To better understand the role that vaccination or infection might play in increasing or decreasing these pre-existing antibody responses to unrelated antigens, we examined a subset subjects that had received a single, defined live vaccination[1]. Four previously vaccinated subjects received a booster vaccination with the live smallpox vaccine (vaccinia virus) and were monitored closely (days 7, 14, 21, 33, 63) as well as long-term (months 4, 6, 9, 12) after booster vaccination (Fig. 1A). We chose to follow immune responses after booster vaccination with vaccinia virus since this stimulates pre-existing T cell help, in addition to causing an acute infection as evidenced by the formation of a Jennerian vesicle on all four subjects. The four vaccinees showed strong vaccine-induced antibody responses, with mean vaccinia-specific IgG titers increasing from 909 EU/mL at baseline to 21,615 EU/mL at the peak of the response 14 days after vaccination (range: 8- to 80-fold increase in antibody titers). Since pre-existing antibody titers vary considerably between individuals and even between different antigens within an individual[1], each antigen-specific antibody response was normalized to 100% at baseline prior to vaccinia booster immunization. This allowed the average percent change in each individual antigen-specific antibody response to be measured and compared among the four subjects regardless of the individual variation in absolute magnitude of the ELISA titer. As shown in Figure 1A, booster vaccination had little influence on pre-existing antibody responses to other non-vaccinia antigens such as tetanus toxoid, diphtheria toxoid, measles, mumps, rubella, Epstein-Barr virus, or varicella-zoster virus. Small fluctuations in serum antibody levels were observed, but these were not statistically significant (Table 1). Based on analysis of 30 individual ELISA titers specific for up to 8 antigens examined at each time point, there was an apparent significant increase in antibody titers at day 14 and day 33 post-vaccination (P= 0.03 at day 14 and 33, unadjusted for multiple testing analysis). However, these are unlikely to be biologically meaningful differences since there was no statistically significant increase or decrease in the levels of pre-existing serum antibody levels at an earlier time point (day 7, P = 0.18), an intermediate time point (day 21, P = 0.07), or at a later time point (day 63, P = 0.75). Indeed, after correction for multiple testing analysis, no significant trends were observed (Table 1). Overall, these observations indicate that robust vaccine-specific immune responses following smallpox vaccination have no consistent or sustained impact on pre-existing antibody levels to other heterologous antigens.
Figure 1. Serum antibody responses demonstrate minimal change following infection or vaccination.
(A) Mean antibody titers for up to nine antigens in four subjects were examined for one year following smallpox booster vaccination. The percent change for each antigen was calculated from preceding baseline levels just prior to smallpox immunization and plotted versus time. Error bars represent ± one standard deviation (SD). Although one subject was seronegative for EBV and one was seronegative for pertussis toxin, in all there were 30 antigen-specific ELISA titers measured at each time point. The mean baseline serum antibody levels (ELISA units per mL) are listed for each antigen in the figure key. (B) Longitudinal analysis of serum antibody titers against ten antigens was performed following administration of multiple live or inactivated vaccines (indicated by arrows) in one subject. The immunization schedule included (in chronological order); combined tetanus and diphtheria booster vaccination, primary smallpox vaccination (Dryvax®, Wyeth), primary hepatitis A vaccination (HAVRIX®, GlaxoSmithKline), primary yellow fever vaccination (YF-VAX®, Aventis Pasteur), booster hepatitis A vaccination, booster polio vaccination (IPOL®, Aventis Pasteur), and live, oral typhoid vaccination (Vivotif®, Berna Biotech). Unlike the injected vaccines, the typhoid vaccine consists of four oral caplets (2–6 × 109 viable colony-forming units and 5–50 × 109 non-viable bacterial cells), with one dose taken every two days, as illustrated with four lines to represent each caplet administered. Serum antibody levels are presented in ELISA units (EU) and the dotted line indicates the cut-off for distinguishing between seronegative and seropositive serum samples. Total serum IgG (mg/ml) at each time point was determined for comparison. Vertical dashed lines are shown to indicate the time point at which each vaccination was received. (C) The percent change for each antigen was calculated from preceding baseline levels (illustrated as a dotted line) just prior to the first immunization (mean of the preceding two time points spanning six months), and plotted versus time. (D) As another approach to visualizing potential vaccine-induced changes to unrelated pre-existing serological memory, the percent change for independent antigens (PT, MV, Mumps, RUBV, EBV, and VZV) was calculated as above. The mean change in antibody titers for these six antigens is illustrated as a horizontal dashed line, with one and two SD regions shown as dark gray and light gray regions, respectively. In three instances, the mean serum antibody titers showed a small decrease following vaccination, whereas in four instances a small increase was observed. In only one case did the change following vaccination exceed one standard deviation (5%), in which the mean antibody response transiently declined 8% below baseline levels at eight days following VV vaccination. List of abbreviations: TT, tetanus toxin C-fragment; DT, diphtheria toxin; PT, pertussis toxin; VV, vaccinia virus; YFV, yellow fever virus; MV, measles virus; RUBV, rubella virus; EBV, Epstein-Barr virus; VZV, varicella-zoster virus; Typhoid, Salmonella typhi Ty21a; HepA, hepatitis A virus.
Table 1.
Statistical analysis of serological responses after smallpox vaccination
| Days after Smallpox Vaccination | Average Percent Changea | Unadjusted P valueb | Significance Thresholdc | Significant? |
|---|---|---|---|---|
| 7 | 6 (−4, 13) | 0.18 | 0.010 | No |
| 14 | 8 (4, 14) | 0.03 | 0.006 | No |
| 21 | 7 (2, 13) | 0.07 | 0.007 | No |
| 33 | 7 (2, 8) | 0.03 | 0.006 | No |
| 63 | −2 (−16, 8) | 0.75 | 0.050 | No |
| 125 | 6 (−3, 11) | 0.18 | 0.008 | No |
| 181 | 4 (−7, 14) | 0.45 | 0.017 | No |
| 287 | 2 (−10, 6) | 0.66 | 0.025 | No |
| 371 | 5 (−3, 5) | 0.26 | 0.013 | No |
The average percent change from baseline values to the indicated time points after smallpox vaccination were determined for up to eight unrelated antibody titers in four subjects. The data are based on 30 individual ELISA measurements performed at each time point and the range in average percent change per individual is shown in parentheses.
Unadjusted P values were calculated by Student’s t-test prior to adjustment for multiple testing.
In these studies, independent analyses were collectively performed to test a single hypothesis (i.e., change in pre-existing antibody level from baseline due to smallpox vaccination) and therefore multiple testing correction is required[9]. Multiple test significance thresholds were calculated using the Holm procedure and were based on an experimentwise α = 0.05 as described previously [1]. Data was ranked by the significance threshold and then placed in the table according to the time after smallpox vaccination. For comparison, a statistically significant value after the Bonferroni correction would be a P value of <0.006.
3.2 Case study results examining multiple replicating or non-replicating vaccine antigens
The data in Fig. 1A is based on humoral immune responses elicited by multiple individuals to multiple antigens following administration of one vaccine. This represents an anamnestic response with only one potential form of polyclonal stimulation (vaccinia virus infection) and we did not observe any significant changes in pre-existing serum antibody levels. It is possible that a single infection or vaccination is not enough of an antigenic or inflammatory insult to induce a measurable increase[10] or decrease[4] in pre-existing antibody responses to other antigens. However, one might predict that multiple vaccinations/infections would augment a potential increase or decrease in pre-existing antibody responses if serological memory is amenable to manipulation by these factors. To further examine this question, we measured the humoral immunity of one subject who received multiple vaccinations over a relatively short period of time, including a tetanus/diphtheria booster vaccination, primary smallpox and yellow fever vaccinations, and vaccinations against hepatitis A (primary and booster vaccination), polio (booster vaccination) and Salmonella typhi (primary vaccination administered as four oral doses given two days apart). These vaccinations were either recommended or required for occupational risk or for international travel. Booster vaccination against tetanus (which includes diphtheria toxoid) was administered in a hospital setting due to an accidental puncture wound. This was an unexpected event that occurred 40 days after a scheduled blood sample had been drawn. For this reason, there was no serum sample obtained immediately prior to booster vaccination and it was 12 days following tetanus/diphtheria vaccination before the next serum sample was drawn (Fig. 1B). Tetanus/diphtheria booster immunization is likely to activate antigen-specific memory T cells and this was previously associated with a transient spike in circulating antibody-secreting cells shortly after vaccination[3]. To determine if tetanus/diphtheria vaccination would result in a measurable and sustained increase or decrease in pre-existing serum antibody levels, we closely monitored antibody responses to ten virus/vaccine antigens (Fig. 1B). Following tetanus/diphtheria vaccination, antibody titers against these two antigens increased by 200–400% at day 12 post-immunization, as expected during an antigen-specific humoral immune response (Fig. 1C). In contrast, there was no clear pattern of increased or decreased antibody responses to the other individual antigens tested and the combined pre-existing antibody responses to six nonspecific antigens (pertussis toxin, measles, mumps, rubella, EBV, and VZV) remained largely unchanged, (<2% difference from baseline, Fig. 1D). Similarly, immunization with the inactivated hepatitis A or polio vaccines had no discernible effect on pre-existing antibody titers to unrelated antigens. This indicates that vaccination with protein antigens capable of stimulating host T cell responses did not impact pre-existing serological memory.
Vaccination with live viruses or bacteria provide a more robust measure of the effects of inflammation and TLR engagement on pre-existing serum antibody responses. For instance, smallpox vaccination (i.e., vaccinia virus infection) elicits strong antibody responses and T cell activation [8, 11] as well as stimulation of innate immunity through TLR2[12]. Vaccinia virus-specific antibody responses peaked by day 22 post-infection with virus-specific antibody levels increasing by ~30-fold (~3,000%, Fig. 1C). This is in sharp contrast to antibody responses against other unrelated antigens, which decreased transiently but remained within 8% of their baseline levels at this time point.
In contrast to vaccinia virus, which causes a localized infection of the skin, the yellow fever vaccine causes a systemic viral infection with viremia typically detected for a few days after vaccination [11]. Following yellow fever vaccination, strong antiviral T cell responses are mounted [11] as well as activation of several TLR including TLR2, TLR7, TLR8, and TLR9[13]. Yellow fever virus-specific antibody levels increased ~9-fold (~900%, Fig. 1C) and peaked within six months after vaccination whereas the average serum antibody response against unrelated antigens remained within 2% of baseline during the acute phase (up to 3 weeks) following YFV vaccination.
Failing to identify significant changes to pre-existing antibody responses following live viral infection, we examined the effects of a live, attenuated bacterial infection. The Salmonella typhi Ty21a vaccine is capable of stimulation through TLR4 (lipopolysaccharide), TLR5 (flagella), and TLR9 (bacterial CpG DNA)[14]. Following vaccination, the subject experienced mild malaise and a temperature of 100.3°F, indicative of a systemic inflammatory response. Nevertheless, at 3, 10, or 17 days after the final dose, the average antibody response to unrelated antigens demonstrated only a slight decrease of 2–5% from baseline, with this change representing less than one standard deviation from the mean (Fig. 1D).
As an additional test of the polyclonal stimulation hypothesis, we also examined total serum IgG levels. In an initial study proposing polyclonal stimulation, a 100-fold increase in total IgG-producing antibody-secreting cells in the circulation was observed[3]. In our studies, we identified only minor variations in total serum IgG levels post-vaccination, with an average change of less than 3% observed during the first 3 weeks following vaccination (Fig. 1B). When plotted as individual percent change (Fig. 1C) or as the average percent change of multiple antigen specificities (Fig. 1D), there was no clear trend resulting in an increase or reduction in pre-existing antibody titers over time, indicating that serum antibody levels are relatively resistant to physiological changes that might be associated with inflammatory vaccine or infection-related events.
4. Discussion
Despite exposure to physiological stimulation via multiple TLR (TLR2, TLR4, TLR5, TLR7, TLR8, and TLR9), potential bystander activation from vaccine-induced T cells, and de novo antigen-specific immune responses, we were unable to demonstrate a significant change in pre-existing antibody responses of unrelated specificities. In some instances following vaccination, minor increases to unrelated antigens were observed, while in other instances minor decreases occurred. One caveat with this methodology is that our serological measurements may lack the sensitivity required to detect small changes in serum antibody responses. In an effort to overcome this issue, we measured serum antibody responses to multiple unrelated antigens with the notion that if a biologically relevant trend in increased or decreased pre-existing antibody responses were to be found, then the resulting effect would be augmented by combining the results from multiple independent tests (i.e., each antigen specificity multiplied by the number of subjects under study). However, despite analysis of multiple subjects following one infection/vaccination (Fig. 1A) or analysis of one subject following multiple infections/vaccinations (Fig. 1B, C, D), we were unable to identify a statistically significant increase or decrease in serological memory (Table 1). Although TLR stimulation can clearly activate B cells under in vitro conditions[3], the results presented here showing no change in serological memory following vaccination or infection are supported by similar clinical studies[15] as well as experiments conducted in mice in which memory B cells are unresponsive to TLR activation in vivo[16, 17]. Additionally, evidence of prolonged antibody maintenance after peripheral B cell depletion in mice[18, 19] in addition to observational studies in human subjects following B cell ablative treatment [20, 21] indicates that polyclonal MBC activation is not a requirement for sustaining plasma cell numbers and long-term antibody responses.
In contrast to polyclonal stimulation, competition from newly formed plasma cells for survival niches has also been provided as an explanation of antibody maintenance patterns[4–6]. One prediction based on this model is that a pre-existing antigen-specific antibody response may wane at a rate of ~0.1% for each new antibody specificity added to the host immune system [4]. Based on this theory, one might expect to identify a uniform decrease of 1% or more in the pre-existing antigen-specific antibody responses of the subject described in Figure 1C. If one takes into account that primary infection with vaccinia virus alone may induce an antibody response to over 40 different antigen specificities[22] and bacteria such as Salmonella may present >2,000 proteins[23] to the host immune system, it is impressive that serological memory is well maintained during these types of antigenic exposures and deviates less than 2 standard deviations from the mean, regardless of the type of infection or vaccination that is encountered (Fig. 1D). This information, along with prior longitudinal studies demonstrating serological antibody half-life estimates of >50 years for certain viral antigens [1]would suggest that competition for survival niches is not a common phenomenon and not likely to play a major role in determining the duration of serum antibody responses [2].
An alternative hypothesis for maintaining serological memory is that plasma cell lifespan is determined at the time of priming and is not regulated by polyclonal stimulation or competition for survival niches[2]. In this model, the duration of an ongoing antibody response is dictated by the combination of antigenic structure and the availability of CD4+ T cell help during the time of antigen-specific B cell activation. This “imprinted lifespan” hypothesis might explain why some antibody responses to non-repetitive protein antigens (e.g., tetanus, diphtheria) are preferentially short-lived whereas antibody responses to highly repetitive antigens such as viruses (e.g., measles, vaccinia) are often long-lived[1]. By gaining a better understanding of the mechanisms involved with inducing or maintaining long-term antibody responses, we hope to improve current vaccine strategies with the goal of producing strong vaccine-mediated serological memory that can last a lifetime.
Acknowledgments
We thank the study subjects for their time and their participation in this research study.
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health grants, R44 AI079898 [to I.J.A. and M.K.S.], UO1 AI082196 U54 [to M.K.S.], and ONPRC grant 8 P51OD 011092-53 [to M.K.S.].
Abbreviations
- DT
diphtheria toxin
- EBV
Epstein-Barr virus
- EU
ELISA units
- HepA
hepatitis A virus
- MV
measles virus
- PT
pertussis toxin
- RUBV
rubella virus
- TLR
toll-like receptor
- TT
tetanus toxin C-fragment
- Typhoid
Salmonella typhi Ty21a
- VV
vaccinia virus
- VZV
varicella-zoster virus
- YFV
yellow fever virus
Footnotes
Conflict of Interest Statement
Oregon Health and Science University (OHSU), I.J.A., E.H. and M.K.S. have a financial interest in Najít Technologies, Inc., a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU and the Integrity Program Oversight Council. M.W.L. declares no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 2.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 4.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 5.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, et al. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 6.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 7.Amanna IJ, Slifka MK. Quantitation of rare memory B cell populations by two independent and complementary approaches. J Immunol Methods. 2006;317:175. doi: 10.1016/j.jim.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 9.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54:343. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 10.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 11.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 15.Di Genova G, Roddick J, McNicholl F, Stevenson FK. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood. 2006;107:2806. doi: 10.1182/blood-2005-08-3255. [DOI] [PubMed] [Google Scholar]
- 16.Benson MJ, Elgueta R, Schpero W, Molloy M, Zhang W, Usherwood E, et al. Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med. 2009;206:2013. doi: 10.1084/jem.20090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richard K, Pierce SK, Song W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo. J Immunol. 2008;181:1746. doi: 10.4049/jimmunol.181.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008;105:4802. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 20.Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, et al. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48:2146. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- 21.Pescovitz MD, Torgerson TR, Ochs HD, Ocheltree E, McGee P, Krause-Steinrauf H, et al. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol. 2011;128:1295. doi: 10.1016/j.jaci.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies DH, Molina DM, Wrammert J, Miller J, Hirst S, Mu Y, et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7:1678. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- 23.Ansong C, Yoon H, Norbeck AD, Gustin JK, McDermott JE, Mottaz HM, et al. Proteomics analysis of the causative agent of typhoid fever. J Proteome Res. 2008;7:546. doi: 10.1021/pr070434u. [DOI] [PubMed] [Google Scholar]