Abstract
While neuritic plaques and neurofibrillary tangles in older adults are correlated with cognitive impairment and severity of dementia, it has long been recognized that the relationship is imperfect as some people exhibit normal cognition despite high levels of AD pathology. We compared the cellular, synaptic and biochemical composition of midfrontal cortices in female subjects from the Religious Orders Study who were stratified into three subgroups: 1) pathological AD with normal cognition (“AD-Resilient”), 2) pathological AD with AD-typical dementia (“AD-Dementia)” and 3) pathologically normal with normal cognition (“Normal Comparison”). The AD-Resilient group exhibited preserved densities of synaptophysin-labeled presynaptic terminals and synaptopodin-labeled dendritic spines compared to the AD-Dementia group, and increased densities of GFAP astrocytes compared to both the AD-Dementia and Normal Comparison group. Further, in a discovery antibody microarray protein analysis we identified a number of candidate protein abnormalities that were associated with diagnostic group. These data characterize cellular and synaptic features and identify novel biochemical targets that may be associated with resilient cognitive brain aging in the setting of pathological AD.
Keywords: cognitive reserve, synapse, synaptophysin, synaptopodin, glial fibrillary acidic protein, antibody microarray
1. Introduction
Amyloid-β plaques and hyperphosphorylated tau neurofibrillary tangles are common age-related lesions that are presumed to play primary pathophysiological roles in the dementia of Alzheimer’s disease (AD)(Jellinger, 2009). However, it is also recognized that the correlation between these neuropathological lesions and cognition is modest and accounts for a limited amount of the variance of cognition among older adults. Normal cognition despite pathological AD was recognized over 80 years ago and has been described in case reports and series (Terry, et al., 1991,Tomlinson, et al., 1968). More recently, there has been increasing interest in cognitive “resilience” or “reserve” in large-scale epidemiological studies. For example, the Nun Study has shown discordance of cognition with pathology as well as the important effects of education, diet, linguistic ability, and childhood positive emotion on cognitive function in late life (Iacono, et al., 2009,Tyas, et al., 2007). The Baltimore Longitudinal Study of Aging (O’Brien, et al., 2009), the Honolulu-Asia Aging Study (White, 2009), and the MRC-CFAS (Savva, et al., 2009) have similarly reported dissociations between plaques, tangles or other pathological lesions and cognition. This relatively poor correlation may become even more pronounced in the oldest-old (Ewbank and Arnold, 2009,Haroutunian, et al., 2008).
In the Religious Orders Study (ROS) and its companion study, the Rush Memory and Aging Project (MAP)(Bennett, 2006), we have found that a third of older people with normal cognition have densities of plaques and tangles that meet NIA-Reagan criteria for intermediate or even high likelihood of AD, as well as infarctions and Lewy bodies (Bennett, et al., 2006b,Schneider, et al., 2007). While such cases may be the minority, it is evident that healthy cognition occurs amidst a spectrum of brain pathology - from those who remain cognitively intact and whose brains are relatively free of neurodegenerative disease or other pathological lesions to those who remain resilient even with significant accumulations of pathology.
Aside from noting such discordance in clinicopathological studies, there has been little pursuant postmortem investigation of neurobiological factors that might confer or be associated with resilient brain aging. Here, we describe neuropathological, cellular, synaptic and molecular features associated with resilient cognition in the face of AD pathology in participants from the well-characterized ROS cohort.
2. Methods
2.1 Case Materials
2.1.1 Clinical Characterization
The ROS cohort consists of >1,100 older Catholic nuns, priests, and brothers who agreed to annual clinical evaluations and signed an informed consent and an Anatomic Gift Act donating their brains for research at the time of death (Wilson, et al., 2004). The ROS is conducted by the Rush Alzheimer’s Disease Center in Chicago with approval of the Institutional Review Board at Rush University. Participants were seen annually and had up to 18 clinical evaluations documenting level of cognition and clinical diagnoses of mild cognitive impairment (MCI), AD, and other types of dementia (Bennett, et al., 2006a), with clinical diagnoses conforming to common, established criteria (AmericanPsychiatricAssociation, 2000, McKhann, et al., 1984, Petersen and Negash, 2008). Annual evaluations included a uniform structured interview and examination consisting of medical history, neurological examination and cognitive testing by trained personnel who are blind to previous evaluations, as previously described (Bennett, et al., 2002). Twenty-one cognitive tests assessed a broad range of abilities commonly impaired in older persons with and without dementia. Neuropsychological indices of cognition were summarized as a global measure based on the average z-score of all tests, using the mean and standard deviation from the measures of the baseline assessments, encompassing measures of episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. Follow-up evaluations, identical in all essential details, were performed annually by examiners blinded to previously collected data. Detailed information on the individual tests and on the derivation and correlates of composite measures is contained in previous publications (Wilson, et al., 2004,Wilson, et al., 2002).
2.1.2 Neuropathological Processing and Diagnosis
Brain autopsies were conducted in a standardized fashion as previously described (Bennett, et al., 2004,Mufson, et al., 1999). Briefly, after macroscopic inspection and photodocumentation, precise 1 cm coronal slabs were alternately fixed in 4% paraformaldehyde for 24–48 hours or frozen. The hemisphere used for diagnostic and frozen blocks alternated randomly between cases. Diagnostic blocks were dissected from fixed slabs and included midfrontal, superior or middle temporal, inferior parietal cortex, entorhinal cortex, hippocampus, anterior basal ganglia, anterior thalamus, and midbrain. These were embedded in paraffin, cut into 6 μm sections, and mounted on glass slides. Bielschowsky silver staining was used to visualize neuritic plaques, diffuse plaques, and neurofibrillary tangles in the frontal, temporal, parietal, entorhinal cortex, and the hippocampus. Immunohistochemistry for α-synuclein was used to identify any Lewy bodies (Schneider, et al., 2007). Macroscopic infarctions identified at the time of brain dissection were verified by histopathology and microinfarcts were documented upon inspection of hemotoxylin and eosin stained tissue sections (Arvanitakis, et al., 2011). Neuropathologic diagnoses of AD were established by a board-certified neuropathologist blinded to age and all clinical data according to NIA-Reagan (Hyman and Trojanowski, 1997), Braak (Braak and Braak, 1995), and CERAD (Mirra, et al., 1991) classifications.
2.1.3 Stratification for Final Case Selection
To contrast neuropathological AD with and without dementia with semi-quantitative neurohistological and proteomic analyses, we applied an a priori stepwise stratification strategy and selected 10 “AD-Resilient” cases, 10 AD-Dementia cases and 10 cognitively and pathologically “Normal Comparison” (NC) cases. This yielded well-matched subgroups of cases that highlighted contrasts of cognition and pathology while minimizing demographic and other residual factors. We first limited our selection to female participants in the ROS cohort, thus minimizing sex and major lifestyle differences (given relatively similar lifestyles activities, diet, habits and healthcare of Catholic female clergy). We excluded any cases with clinical or pathological diagnoses other than normal, MCI or AD that potentially contributed to cognitive impairment (e.g., abundant Lewy body pathology, infarcts, hippocampal sclerosis, etc.). Cases with coincidental minor pathologies that were not deemed to contribute to cognition were allowed. We next stratified according to composite cognition scores from the last assessment before death and global pathology (average plaque and tangle burdens in diagnostic blocks). Cases in the highest quartile for cognition and highest quartile for pathology were classified as “AD-Resilient”, cases in the lowest quartile for cognition but highest quartile for pathology were classified as “AD-Dementia”, and those with high cognition and low pathology were classified as NC. We then filtered 10 cases from each group that were most closely matched for age and postmortem interval. Finally, we inspected the selected cases for other clinical data that might skew the relationship between pathology and cognition, i.e., unusual medical or psychiatric conditions, medicines, or causes of death, and substituted alternative cases as necessary. All cases selected for the AD-Dementia group had exhibited an insidious onset and slowly progressive decline in memory and other domains of cognition and function and met established criteria for a clinical diagnosis of dementia of the Alzheimer’s type (AmericanPsychiatricAssociation, 2000, McKhann, et al., 1984) and autopsy established a neuropathological diagnosis of definite AD (Hyman and Trojanowski, 1997,McKhann, et al., 1984).
2.2 Immunohistochemistry of AD Lesions, Neurons, Glia, Synapses and Spines
The midfrontal gyrus cortex (corresponding predominantly to Brodmann area 46) was chosen for semi-quantitative image analyses of AD pathology, cellular and synaptic markers because of its important role in complex cognition and its common neuropathological involvement in the spectrum of AD (Arnold, et al., 1991). Six micron thick sections from paraffin blocks were immunolabeled with antibody AT8 recognizing PHFtau phosphorylated at serine 202 and threonine 205 (1:800, Innogenex [San Ramon, CA, USA]) for neurofibrillary tangles and an antibody directed at the N-terminus of amyloid-β (Clone 6F/3D, MO0872, 1:100, Dako [Carpenteria, CA, USA]) for plaques, using the avidin–biotin complex (ABC, Vectastain, Vector Laboratories, Inc. Burlingame, CA) method as reported elsewhere (Schneider, et al., 2007). Neurons were immunolabeled with mouse anti-NeuN (MAB377, 1:4000; Chemicon/Millipore, Billerica, MA, USA) with labeling enhanced by tyramide signal amplification (TSA Biotin System kit; Perkin Elmer, Waltham, MA, USA) and developed using nickel-enhanced diaminobenzidine (DAB) chromogen (Soetanto, et al., 2010). Astrocytes were immunolabeled with rabbit anti-GFAP (ZO334, 1:20,000; DAKO, Carpenteria, CA, USA) and developed using ABC with nickel-enhanced DAB chromogen. The presynaptic vesicle protein synaptophysin was immunolabeled with mouse anti-synaptophysin (MO776, 1:50; DAKO) using the ABC method with DAB as the chromogen. The postsynaptic spine apparatus protein synaptopodin was immunolabeled with mouse anti-synaptopodin (Q44590M, 1:2000, Biodesign, Saco, ME, USA) using TSA and nickel-DAB. Data was collected in single sections that were all stained in single runs with uniform development times.
2.3 Image Acquisition and Analysis
All image acquisition and analyses were conducted masked to case information. Measurements of amyloid-β plaque and PHFtau neurofibrillary tangle lesion densities were conducted using computer-based image acquisition and analysis methods previously described (Bennett, et al., 2004,Mitchell, et al., 2000). Density measures of NeuN neurons, GFAP astrocytes, synaptophysin expression, and synaptopodin spines were estimated in layers II – VI using methods previously described (Soetanto, et al., 2010). Briefly, under uniform lighting conditions, gray-scale photomicrographs covering the entire gray matter region of interest in each section were captured and software was used to create composite images and then quantify relevant features of the images. Contiguous images were captured at 100X magnification for NeuN neuron counts, GFAP astrocyte counts, and SY38 synaptophysin presynaptic terminal optical density measurements. Because of data file size limitations for the higher magnification images needed to resolve discrete synaptopodin-labeled spine puncta for counting, we used random, systematic sampling to capture separate 1000X images (oil objective lens) throughout the region of interest. Quantitative analyses of cellular elements were conducted with algorithmic image filtering using Image-Pro Plus software (Media Cybernetics Inc., Silver Springs, MD, USA). For automatic counting of discrete object profiles, i.e., NeuN neurons, GFAP astrocytes and synaptopodin-spine puncta (>0.05 μm), we applied optical density threshold binarization, pixel contiguity, size, and shape segmentation filters to automatically delineate the objects of interest. These were counted to determine the number of cells or spines per unit area. Synaptophysin immunohistochemistry produces a diffuse, non-homogenous labeling in cortical gray matter. Under uniform lighting conditions, this was quantified by measuring the average pixel optical density across the entire cortical region of interest minus the optical density of subjacent white matter.
2.4 Antibody Microarray
The Clontech. Antibody Microarray 500 (Clontech, Mountainview, CA) was used to profile relative expression levels of 507 proteins. The Clontech antibody array has been used in a variety of settings since 2003 (Anderson, et al., 2003,Hatjiharissi, et al., 2007). Independent publications report very high validity of differential protein expression, verified with Western blotting, and our preliminary study findings were consistent with this.
Approximately 100 mg of fresh frozen gray matter from mid-frontal cortex was dissected from ipsilateral slabs adjacent to the blocks in the histological experiments. Arrays only validly measure the expression level of protein in one sample relative to another, so we prepared a large quantity of pooled homogenate from an independent set of 5 NC brains from the University of Pennsylvania Alzheimer’s Disease Center collection to serve as a reference standard in all experiments. These cases were without cognitive impairment by history and diagnostic neuropathological examination findings were normal, with Braak stages 0–2. Thus data for each protein on the array for each individual (“Sample A”) was expressed as an internally normalized ratio (INR) relative to this reference standard (“Sample B”), allowing analysis of all cases as a single dataset.
Extraction, labeling and analysis followed manufacturer’s instructions. Briefly, tissue was homogenized directly in the extraction/labeling buffer, centrifuged and protein concentration of the supernatant was measured using bicinchoninic acid. Each tissue extract was diluted to 1.1 mg/ml and then divided into two equal portions. One portion of “Sample A” was labeled with Cy3 and the other with Cy5 (Amersham/GE Healthcare, Piscataway, NJ) and the reverse order was used for Sample B. This produced four samples: ACy3, ACy5, BCy3 and BCy5. Unbound dye was removed with PD desalting columns (Amersham). Labeled proteins were incubated on the two slides of the antibody array kit: one slide was incubated with a mixture of ACy3 & BCy5 and the second slide with ACy5 & BCy3. After washing and drying by centrifugation, slides were scanned by an Axon Genepix scanner (Molecular Devices, Sunnyvale, CA). Measurement was performed with Genepix software which calculates readings based on averaging fluorescent readings (spot median) for both spots for each antibody on each slide.
Preliminary experiments with the microarray were conducted to examine the inter-experiment reliability of the platform. Aliquots of pooled tissue homogenates from two groups were run in four separate experiments and coefficient of variations (CV) were calculated for each of the protein measurements on the array. Average CV was 0.14 (SD=0.27, range 0.01 to 2.0)
2.5 Data Analysis
Group differences in demographic and clinicopathological variables were analyzed using ANOVA, Χ2 and Kruskall-Wallis tests, with post-hoc comparisons carried out as appropriate. All tests were two-tailed. Spearman correlation coefficients (r) and regression models were used to examine associations between AD lesions (Aβ plaques and PHFtau neurofibrillary tangles) and cellular and synaptic data, as well as potential confounds such as age, and postmortem interval, medications (e.g., psychotropic medications, hormone replacement therapy). These statistics were conducted using JMP version 9.0.0 (SAS, Cary, NC, USA).
For antibody microarray experiments, microarray quality was initially verified and normalized using R package “marray” (Yang, et al., 2007). We applied LOESS normalization within each slide and median normalization across slides. We then computed Internally Normalized Ratio (INR) according to manufacturer’s methods on log2 transformed protein expression measurements. INR takes advantage of the dye-swap experimental design to control for differences in fluorolabeling efficiency. In our experiment, each sample is measured twice: first labeled by Cy5 and then by Cy3 (the brain homogenate control is labeled using the other dye). With the two experiments each yielding two Cy5/Cy3 ratios R1 and R2, the INR is defined as √ (R1/R2). INR values for the replicate spots on the array were averaged.
Normalized INR values from all proteins with valid data were used in a principal components analysis (PCA) to find the largest protein variation directions among the cases and assess the degree to which they separate the groups. One-way ANOVA was applied to normalized INR values to identify the proteins that differentiated any one of three groups (AD-Dementia, AD-Resilient and NC) from the other two using R package “limma” (F-statistic p-value ≤ 0.01). Post hoc t-tests were conducted to identify those proteins that best distinguished the AD-Resilient and AD-Dementia groups (p-value ≤ 0.01). A heat map was generated using software “Clustering” (Eisen, et al., 1998) and “Java TreeView” (Saldanha, 2004).
3. Results
3.1 Clinical and Neuropathological Features
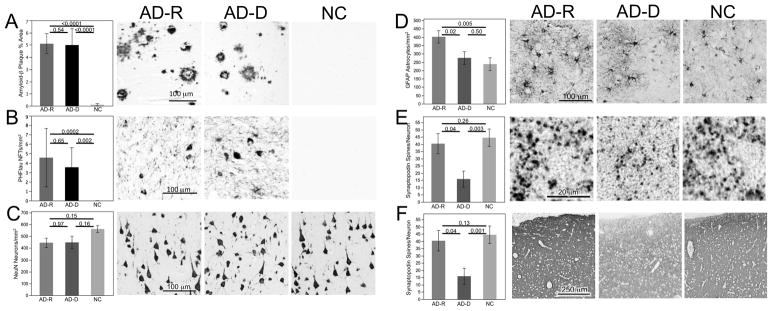
Cases were selected by algorithmic stratification from the 282 females of 458 ROS participants who died as of September 2009 and had complete diagnostic neuropathological data. Cases in each group had been selected for comparability and contrasting differences in target features of age, sex (all female), a composite cognition index and a composite AD pathology index (Table 1). All cases were fully dextral except 1 AD-Dementia subject who was ambidextrous. Statistical analyses further showed no significant differences among groups for education, use of hormone replacement therapy (only 1 normal comparison case had ever been on this), postmortem interval, brain weight, and presence or absence of macroscopic or microscopic infarcts, which if present, were in regions other than mid-frontal cortex. As expected, significant differences were found in frequency of apolipoprotein E ε4 allele, mini-mental state examination score, and CERAD and Braak scores. Immunohistochemically labeled amyloid-β plaque % areas and PHFtau neurofibrillary tangle densities in midfrontal cortex were comparable in the AD-Resilient and AD-Dementia groups and both were significantly greater in these groups than the NC group. Brain weights were less in the AD-Dementia group compared to the AD-Resilient and NC groups but differences were not statistically significant (Table 1 and Supplement Figure).
Table 1.
Demographic, Clinical and Diagnostic Neuropathological Characteristics of Comparison Groups
| Variable | Group Means (SD, Range) | Statistic | Post-Hoc Tukey HSD or Fisher’s Exact p-value | ||||
|---|---|---|---|---|---|---|---|
| AD-Resilient (n=10) | AD-Dementia (n=10) | Normal Comparison (n=10) | F, p or Fisher’s Exact p | AD-Resil vs. AD-Dem | AD-Resil vs. Normal | AD-Dem vs. Normal | |
| Age (yrs) | 84.8 (6.2, 76–94) | 87.6 (4.4, 81–94) | 86.2 (4.3, 78–93) | F[2,27]=0.73, p=0.49 | p=0.45 | p=0.82 | p=0.81 |
| Education (yrs) | 17.7 (1.6, 16–21) | 16.9 (3.4, 10–22) | 17.2 (2.9, 11–21) | F[2,27]=0.22, p=0.081 | p=0.79 | p=0.91 | p=0.97 |
| APOE ε4+ | 7/9 | 4/9 | 0/10 | * Fisher’s p=0.0017 | p=0.33 | p=0.0007 | p=0.033 |
| Antemortem Interval1 | 5.6 (3.7, 1.6–12.7) | 6.3 (3.7, 0.6–13) | 8.3 (4.7, 1.6–15.6) | F[2,27]=1.13, p=0.34 | p=0.92 | p=0.33 | p=0.54 |
| MMSE2 | 28.7 (1.1, 27–30) | 12.2 (7.6, 0–23) | 29.3 (1.2, 27–30) | F[2,27]=46.9, p<0.0001 | p<0.0001 | p=0.96 | p<0.0001 |
| Global Cognition3 | 0.0 (0.2, −0.23–0.31) | −2.3 (0.79, −3.7–−1.5) | 0.1 (0.30. −0.3–0.71) | F[2,27]=75.5, p<0.0001 | p<0.0001 | p=0.76 | p<0.0001 |
| Postmortem Interval4 | 10.4 (7.9, 0–24) | 5.3 (2.3, 1.8–9) | 6.5 (4.3, 2.6–17) | F[2,27]=2.47, p=0.10 | p=0.10 | p=0.25 | p=0.87 |
| Brain Weight (g) | 1169 (138, 1010–1420) | 1099 (94, 962–1285) | 1186 (100, 1060–1340) | F[2,27]=1.66, p=0.21 | p=0.37 | p=0.93 | p=0.21 |
| CERAD Score5 | 1.6 (0.2, 1–2) | 1.3 (0.5, 1–2) | 3.9 (0.1, 3–4) | * Fisher’s p<0.0001 | p=0.37 | p<0.0001 | p<0.0001 |
| Braak Score | 4.2 (0.6, 3–5) | 4.6 (0.5, 4–5) | 2.2 (0.9, 1–3) | * Fisher’s p<0.0001 | p=0.37 | p=0.0004 | p<0.0001 |
| Global Pathology6 | 1.06 (0.29, 0.75–1.63) | 1.07 (0.28, 0.81–1.54) | 0.06 (0.04, 0.01–0.13) | F[2,27]=60.1, p<0.0001 | p=0.95 | p<0.0001 | p<0.0001 |
| Infarcts (Macroscopic) | 1/10 | 3/10 | 4/10 | Fisher’s p=0.45 | p=0.58 | p=0.30 | p=1.00 |
| Infarcts (Microscopic) | 3/10 | 5/10 | 1/10 | Fisher’s p=0.23 | p=0.58 | p=0.30 | p=1.00 |
| Lewy bodies (any) | 1/10 | 0/10 | 1/10 | Fisher’s p=1.0 | p=0.58 | p=0.30 | p=1.00 |
| Hippocampal Sclerosis | x/10 | x/10 | x/10 | Fisher’s p=0.xx | p=0.58 | p=0.30 | p=1.00 |
Antemortem Interval = months between last assessment and death;
MMSE = Mini-Mental State Examination score;
Global Cognition = composite index of cognition based on average z-scores for 19 individual cognitive tests;
Postmortem Interval = time in hours between death and brain tissue fixation and freezing;
CERAD Score = Consortium to Establish a Registry for Alzheimer’s Disease plaque scores, where 1=frequent, 2=moderate, 3=sparse, and 4=none;
Global Pathology = composite index of AD-related pathological lesions - average scaled scores (counts/standard deviation) of neuritic plaques, diffuse plaques and neurofibillary tangles in Bielschowsky silver stained sections of 5 regions: hippocampal CA1, entorhinal cortex, middle temporal cortex, inferior parietal cortex and midforntal cortex. MFC = midfrontal cortex sections from same blocks used in cellular and synaptic studies here.
Fisher’s Exact used for ordinal variables.
Rare coincidental Lewy bodies were observed in one NC case and one AD-Resilient case. Hippocampal sclerosis was noted in 1 AD-Dementia case. Results from analyses of the main outcome variables of interest including or excluding these cases did not differ.
3.2 Cellular and Synaptic Features
As depicted in Figure 1 and described in Table 2, NeuN neuron density was slightly lower in the NC group compared to both the AD-Resilient and AD-Dementia groups, but differences were not significant. GFAP astrocyte densities differed among groups with post-hoc tests demonstrating increased density in the AD-Resilient group compared to both the AD-Dementia and NC groups. Synaptic integrity was assessed with measurement of postsynaptic synaptopodin-labeled spine count densities as well as presynaptic vesicle synaptophysin immunoreactivity. In accord with their expected relationship, pre- and postsynaptic measures were highly correlated (Spearman’s r = 0.58, p = 0.0009). Both synaptopodin spines and synaptophysin immunoexpression were preserved in AD-Resilient group, with levels comparable to those in the NC group. The AD-Dementia group exhibited lower measures compared to both AD-Resilient and NC groups.
Figure 1.
Comparison of densities of (A) amyloid-β plaques, (B) PHFTau neurofibrillary tangles, (C) NeuN neurons, (D) GFAP astrocytes, (E) synaptopodin spines and (F) synaptophsin expression among AD-Resilient (AD-R), AD-Demenita (AD-D) and Normal Comparison (NC) groups. Graphs portray means with standard error bars. Statistical probability of individual between group differences are indicatd with p-values from posthoc comparsions. Photomicrographs depict immunostained appearances of representative cases from each group. Photomicrographs A–E were from layer III while the lower magnification photomicrograph F encompasses layers I – IV.
Table 2.
Densities of AD Lesions, Neurons, Astrocytes, Postsynaptic Spines and Presynaptic Synaptophysin Immunoreactivity in Mid-Frontal Cortex
| Variable | Group Means (SD) | Statistic | Post-Hoc Comparison p-value | ||||
|---|---|---|---|---|---|---|---|
| AD-Resilient (n=10) | AD-Dementia (n=10) | Normal Comparison (n=10) | Kruskal-Wallis Χ2 or ANOVA F, p | AD-Resilient vs. AD-Dem | AD-Resilient vs. Normal | AD-Dementia vs. Normal | |
| Amyloid % Area | 5.1 (2.5) | 5.0 (4.3) | 0.1 (0.3) | Χ2[2]=20.1, p<0.0001 | p=0.54 | p<0.0001 | p<0.0001 |
| Tau NFTs/mm2 | 4.6 (9.8) | 3.5 (6.6) | 0.0 (0.0) | Χ2[2]=14.9, p=0.0006 | p=0.65 | p=0.0002 | p=0.002 |
| NeuN Neurons/mm2 | 445 (125) | 448 (168) | 562 (86) | F[2,27]=2.4, p=0.11 | p=0.97 | p=0.16 | p=0.15 |
| GFAP Astocytes/mm2 | 402 (117) | 276 (114) | 238 (115) | F[2,27]=5.3, p=0.011 | p=0.02 | p=0.005 | p=0.50 |
| Synaptopodin Spines/mm2 | 18997 (11431) | 8904 (10342) | 24426 (9006) | F[2,27]=5.6, p=0.009 | p=0.04 | p=0.26 | p=0.003 |
| Synaptophysin OD* | 1314 (217) | 1091 (276) | 1484 (199) | F[2,27]=6.7, p=0.004 | p=0.04 | p=0.13 | p=0.001 |
OD=optical density in gray scale units
While cases for each group had been algorithmically selected for comparability in demographic and potentially confounding variables, we still assessed for associations of NeuN, GFAP, synaptopodin spine and synaptophysin density measures with age, education, antemortem interval (time between last clinical assessment and autopsy), postmortem interval, hemisphere used, and exposure to potentially relevant medications (e.g., psychotropic, hormone replacement therapy) using correlation and t-test analyses within the whole cohort of 30 cases, and within each diagnostic category. We observed no significant associations of the anatomical variables with any of these potential confounds (Supplement Table).
3.3 Relation of Neuropathologic Indices to Cellular and Synaptic Features
Correlation analyses were used to examine how Aβ plaques and PHFtau neurofibrillary tangles, independent of clinical status, were related to the measures of neurons, astrocytes and synapses in the entire cohort of 30 cases. Aβ plaques were significantly associated with all cellular and synaptic features. They negatively correlated with NeuN neurons (ρ=−0.39, p=0.03), positively correlated with GFAP astrocytes (ρ=0.49, p=0.008), and negatively correlated with synaptopodin spines (ρ=−0.44, p=0.02) and synaptophysin immunoreactivity (ρ=−0.39, p=0.03). PHFtau neurofibrillary tangles were positively correlated with GFAP astrocytes (ρ=0.43, p=0.03) and negatively correlated with synaptopodin spines (ρ=−0.45, p=0.01). Whjle not statistically significant, PHFtau tangles negatively correlated with NeuN neurons (ρ=−0.31, p=0.10) and synaptophysin (ρ=−0.27, p=0.16).
3.4 Antibody Microarray Protein Expression Profiling
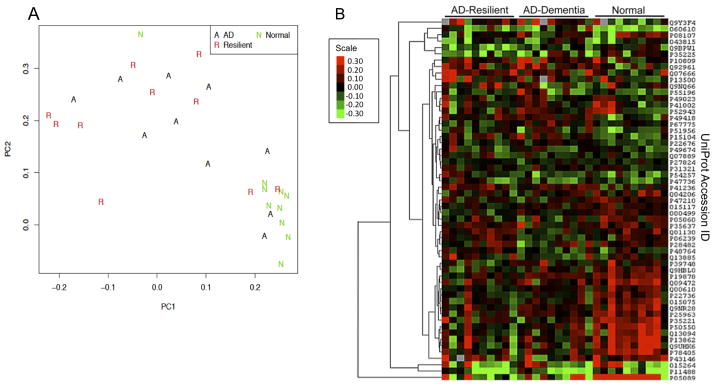
Our goal was to determine the degree to which the proteome represented on the Clontech Ab500 antibody microarray could distinguish AD-Resilient, AD-Dementia and NC groups and to identify candidate proteins that distinguished the AD-Resilient group from the AD-Dementia group. With PCA we observed tight clustering of NC cases while AD-Dementia and AD-Resilient cases were more dispersed (Figure 2a). This suggests that the protein expression profile of healthy brain tissues is distinct from those with abundant AD pathology and that AD pathology disrupts protein expression in non-cohesive ways. Using one-way ANOVA, we then identified 57 proteins that distinguished the groups (Table 3), and visualized the proteins in a heat map (Figure 2b). The NC group was especially distinct from AD-Dementia and AD-Resilient groups, but we did observe some differentiation in the expression pattern between AD-Dementia and AD-Resilient groups. Post-hoc comparisons identified 16 proteins that significantly differentiated AD-Resilient and AD-Dementia groups (Table 3). The analytic results for all proteins are presented in the Supplementary Spreadsheet.
Figure 2.
Antibody Microarray Analyses. A) Plot of principal components analysis applied to the antibody microarray full data set generated using the first (PC1) and second (PC2) components. Normal Comparison (N) cases clustered distinctly from AD-Dementia (A) and AD-Resilient (R) groups. Both AD-Resilient and AD-Dementia groups were more mixed and showed a more dispersed pattern on the plot. B) Heat map generated using 57 proteins differentially expressed in any of the three groups compared to the others (selected by one-way ANOVA, p-value ≤ 0.01). Hierarchical clustering was applied to the proteins to help visualize the differences. Euclidean distance and complete linkage were used when computing hierarchical clustering of proteins. Five missing values on the heat map are colored in dark gray. Color scale indicates ordinally induced log2 expression levels of proteins.
Table 3.
Proteins Identified in Antibody Microarray that Differentiated AD-Resilient and AD-Dementia Groups
| Protein UniProt ID | Name (Alphabetical) | Direction of Change in AD- Resilient vs AD-Dementia, p-value | Biological Class and Function |
|---|---|---|---|
| Q02410 | Amyloid beta A4 precursor protein- binding family A member 1 | Increased, 0.0037 | Transporter protein. Interacts and stabilizes amyloid precursor protein (APP), inhbiting production of proteolytic APP fragments including the amyloid-β; signal transduction and possible roles in synaptic vesicle exocytosis and neuronal cell adhesion |
| P30658 | Chromobox protein homolog 2 | Increased, 0.00045 | Adapter protein. Component of a Polycomb group (PcG) multiprotein PRC1-like complex, involved in chromatin remodeling and modification of histones |
| P30281 | Cyclin D3 | Decreased, 0.006 | Cell cycle protein. Regulated CDK Kinases; sensor of astrocyte gap junction communication and possible mitogen mediator of astrocytes |
| P33992 | DNA replication licensing factor MCM5 | Decreased, 0.0091 | Cell cycle protein. Chromatin binding protein, participates in cell cycle regulation |
| P55060 | Exportin-2 | Increased, 0.00019 | Transporter protein. Nuclear-cyoplasmic transport, apoptosis and proliferation |
| P08700 | Interleukin-3 | Increased, 0.0061 | Cell-cell signaling protein. Pleiotropic functions including neuroprotection, inhibition of neuron death induced by Ab, trophic effects in hippocampal neurons, microglial activator. |
| P06239 | Lymphocyte-specific protein tyrosine kinase | Increased, 0.00029 | Protein kinase. Src family tyrosine kinase expressed in neurons, tyrosine phosphorylates tau, down-regulated in AD, Lck gene locus risk for AD |
| Q9UPY8 | Microtubule-associated protein RP/EB family member 3 | Decreased, 0.0062 | Cell cycle protein. Involved in microtubule polymerization stabilization and anchoring; possible role in odor memory |
| P15172 | Myoblast determination protein 1 | Decreased, 0.0047 | Transcription regulator. Myogenic regulatory factor with early role in muscle differentiation |
| Q09013 | Myotonin protein kinase | Increased, 0.00069 | Protein kinase. Serine threonine kinase for myogenin, L-type calcium channels, phophlemman; role in long-term potentiation |
| Q01130 | Serine/arginine-rich splicing factor 2 | Increased, 0.00033 | Splicing factor. Regulates splicing, alternative translation and various RNA metbolism factors; regulates protein sumoylation |
| P67775 | Serine/threonine-protein phosphatase | Decreased, 0.0063 | Protein phosphatase. “PP2A” - Important phosphatase regulating activity of multiple signaling kinases and phosphorylation of proteins including tau |
| P48764 | Sodium/hydrogen exchanger 3 | Increased, 0.0031 | Ion transport. Major proton extruding system component with role in signal transduction |
| P15923 | Transcription factor 3 (E2alpha) | Increased, 1×10-6 | Transpcription rgulator. Regulates neuronal differentiation and survival, binds to sites affecting insulin and k-immunoglobulin genes, enhances expression of HES1 (target of Notch), prevents apoptosis |
| P25445 | Tumor necrosis factor receptor superfamily, member 6 | Decreased, 0.0027 | Apoptosis. Central role in the physiological regulation of programmed cell death via FADD, caspase 8 and caspase 10 |
4. Discussion
The allied concepts of “resilience,” “reserve,” “resistance, and “asymptomatic” are well recognized in clinical medicine (Riudavets, et al., 2007,Schmitt, et al., 2000). In AD, these terms reflect preserved cognitive functioning despite abundant AD pathology. Some refer to this as “pathological aging” (Dickson, et al., 1992) while others have used “preclinical AD” (Hubbard, et al., 1990,Price and Morris, 1999) implying that with time, this pathological state would lead to clinical dementia. Supporting this notion, Price et al. reported no measureable cognitive impairment per se in such preclinical AD cases, but degraded cognitive function in the presence of plaques and tangles (Price, et al., 2009). As new in vivo biomarker technologies allow us to identify AD pathology in asymptomatic people (Weiner, et al., 2011), it becomes increasingly important to understand the neurobiological basis of symptom expression in AD. Such understanding may point us to novel approaches to maintain cognitive functioning.
There have been relatively few reports describing cellular or molecular correlates of resilient brain aging. Early studies in the Religious Orders Study noted similarities in neuritic, astroglial and microglial constituents of amyloid plaques in AD patients (with dementia) compared with “nondemented controls with high amyloid plaque density,” but found the plaque densities to be higher in those with dementia compared to those without (Mochizuki, et al., 1996) as well as a complex relationship of choline acetyl transferase activity to severity of AD in normal controls, MCI, early and late AD (DeKosky, et al., 2002), although not all data in other studies have agreed with this (Beach, et al., 2000). Lovell and colleagues have also investigated preclinical AD, reporting alterations in zinc transporter proteins and oxidative damage, akin to abnormalities seen in AD with dementia (Bradley, et al., 2010,Lovell, et al., 2011,Lyubartseva, et al., 2010).
In contrast to the aforementioned studies which described similarities between resilient/preclinical AD and AD with dementia, Troncoso and colleagues have reported differences, including preserved numbers and larger neuronal sizes in the hippocampus and other brain regions in “asymptomatic AD” cases compared to normal, MCI and clinical AD control cases in the Baltimore Longitudinal Study of Aging (BLSA) and Nun Study cohorts (Iacono, et al., 2008,West, et al., 2004). They suggested that the larger neuron sizes may represent an early reaction to the effects of amyloid-β or PHFtau that could modify symptomatic progression of the disease. Other studies in the BLSA suggested intermediate changes in some presynaptic proteins but not others in pre-clinical or “early” AD (O’Brien, et al., 2009,Sze, et al., 1997).
We measured immunolabeled neurons, astrocytes, postsynaptic spines and presynaptic vesicle protein expression to extend these earlier findings, and characterize distinctive features of resilient aging. The three groups did not differ significantly in NeuN neuron densities. While this finding suggests no gross neuron loss in the mid-frontal cortex across these groups, we recognize that density may not reflect total number because reduction in the reference area/volume used to calculate density due to atrophy would attenuate differences.
We found that GFAP astrocytes were increased in the AD-Resilient group compared to both the AD-Dementia and NC groups. Astrocyte responses to injury are diverse and are regulated along context-specific spectra of molecular expression, morphological changes and neurochemical production. In insidious conditions like AD, there is increased expression of GFAP and hypertrophy of astrocyte cell bodies and processes. Cortical astrocytes maintain brain health through structural and biochemical support to neurons and endothelial cells, maintain extracellular ion balance, provide nutrients to neurons, regulate cortical blood flow, transmitter uptake and release, modulate synaptic neurotransmission and provide trophic and detoxifying support in response to injury (Sofroniew and Vinters, 2010). We speculate that astroglial activation may play an ameliorative role for brain functioning in the setting of AD pathology.
Synaptic loss is frequently cited as the best neuropathological correlate of dementia severity in AD (Terry, et al., 1991) and has been linked to the accumulation of both amyloid-β and tau pathology (Arendt, 2009). A majority of immunochemical studies have targeted presynaptic proteins, especially synaptophysin, a synaptic vesicle membrane protein in presynaptic terminals. Presynaptic terminals represent both intrinsic and extrinsic connections to the measured region of interest. Our findings are consistent with the literature wherein normal synaptophysin expression in midfrontal cortex in the AD-Resilient group indicates preserved connectivity while the reduced synaptophysin expression in the AD-Dementia group suggests decreased connectivity.
Dendritic spines are highly specialized micro-compartments of postsynaptic dendritic membrane with central roles in excitatory neurotransmission, signal transduction, long-term potentiation, neuroplasticity, learning and memory (Alvarez and Sabatini, 2007). Spines have received relatively little attention in AD (Baloyannis, 2009). We found that synaptopodin, an actin-associated protein enriched in postsynaptic densities is a robust marker of dendritic spines (Soetanto, et al., 2010). To our knowledge, only one study has examined synaptopodin in AD and reported its significant down-regulation (Reddy, et al., 2005). We found synaptopodin-labeled spine puncta density to be reduced in AD-Dementia as well, but densities were equivalent to normal in the AD-Resilient group. The postsynaptic synaptopodin and presynaptic synaptophysin measures were highly correlated, thus providing mutual confirmation of synaptic preservation in the AD-Resilient group.
An antibody microarray served as an unbiased measure of the relative expression levels of over 500 proteins that span a broad range of functional groups. It is noteworthy that PCA found tight clustering of component scores of the NC cases while the cases with pathological AD, both AD-Dementia and AD-Resilient, were more variable in their distributions. We speculate that this reflects a “cohesive” protein profile in normal brain and an erratically disrupted protein expression profile in the presence of pathological AD. Nonetheless, we identified some proteins that distinguished the AD-Resilient group from the AD-Dementia group. Differences were seen in amyloid-β precursor binding protein (which inhibits production of proteolytic APP fragments and protects against memory loss in AD transgenic mouse models (Mitchell, et al., 2010)), lymphocyte-specific protein tyrosine kinase (which phosphorylates tyrosine residues in tau, has been reported to be decreased in AD and genetically associated with AD (Scales, et al., 2011)), and serine/threonine-protein phosphatase (“PP2A”, an important phosphatase for tau (Iqbal, et al., 2009)). Several proteins that mediate or regulate apoptosis were also differentially expressed, including interleukin-3, tumor necrosis factor receptor, and transcription factor 3 (E2α), as well as other proteins with intracellular trafficking, cytoskeletal, transcriptional and cell cycle regulation.
It is important to note both the strengths and weaknesses of this study. Our sample stratification served to maximize contrast of AD-Resilient and AD-Dementia groups for the target features of interest (pathology × cognition). In many ways, the ROS is an ideal cohort for this given its participants’ relatively uniform education, nutrition, healthcare, cultural and other lifestyle factors that if more diverse, might confound a neurobiological study of resilience. However, generalizability to other populations may be limited. Another potential strength and weakness is our choice of methods for semi-quantitative histopathology. We utilized automated, computer-assisted analyses of fractional area determination, two-dimensional profile counting, and optical density. These minimize operator bias, lend themselves well to larger scale, high-throughput studies, and they successfully found between group differences. However, they may introduce systematic inaccuracies that might be obviated with reference volume-based stereological approaches. Third, we consider our antibody microarray experiments to be “discovery,” identifying candidate proteins that will require further validation and investigation. All microarrays present various challenges including false positives and negatives due variations in slide spotting and possible inconsistent labeling efficiency of Cy5 or Cy3 for a particular antigen. Our use of a pooled tissue standard as a reference in all experiments, dye swapping and calculated INR attenuated many potential pitfalls, but the challenge of multiple comparisons remains, especially in relatively small sample sizes. Finally, while postmortem neuropathology remains the best approach to investigate the neurobiology of uniquely human brain diseases, it only allows a snapshot of the brain as it was near the time of death, it can suggest, but not establish, causal relationships between clinical and brain data, and confounds such as agonal state and postmortem changes can alter the brains’ cellular and molecular integrity. It nonetheless remains noteworthy that resilient brain aging can be distinguished cellularly and biochemically and may serve as a conceptual framework for dementia pathophysiology, prevention and treatment strategies.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Aging (R01AG039478, R01AG15819, P30AG10161, P30AG10124) and the Marian S. Ware Alzheimer’s Program/National Philanthropic Trust. We are deeply indebted to all the volunteers in the Rush Religious Order Study, and the staff engaged in subject assessment, autopsy, and brain banking at Rush University Medical Center.
Footnotes
Disclosure statement
The authors report no conflicts of interest with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- AmericanPsychiatricAssociation. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Anderson K, Potter A, Baban D, Davies KE. Protein expression changes in spinal muscular atrophy revealed with a novel antibody array technology. Brain. 2003;126(Pt 9):2052–64. doi: 10.1093/brain/awg208. [DOI] [PubMed] [Google Scholar]
- Arendt T. Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118(1):167–79. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1(1):103–16. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42(3):722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloyannis SJ. Dendritic pathology in Alzheimer’s disease. J Neurol Sci. 2009;283(1–2):153–7. doi: 10.1016/j.jns.2009.02.370. [DOI] [PubMed] [Google Scholar]
- Beach TG, Kuo YM, Spiegel K, Emmerling MR, Sue LI, Kokjohn K, Roher AE. The cholinergic deficit coincides with Abeta deposition at the earliest histopathologic stages of Alzheimer disease. J Neuropathol Exp Neurol. 2000;59(4):308–13. doi: 10.1093/jnen/59.4.308. [DOI] [PubMed] [Google Scholar]
- Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S63–8. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006a;27(3):169–76. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006b;66(12):1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–84. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–8. doi: 10.1016/0197-4580(95)00021-6. discussion 8–84. [DOI] [PubMed] [Google Scholar]
- Bradley MA, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med. 2010;48(12):1570–6. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51(2):145–55. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13(1):179–89. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–8. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank DC, Arnold SE. Cool with Plaques and Tangles [editorial] New England Journal of Medicine. 2009;360(22):i2357–2359. doi: 10.1056/NEJMe0901965. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, Libow LS, Lesser GT, Maroukian M, Grossman HT. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65(9):1211–7. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatjiharissi E, Ngo H, Leontovich AA, Leleu X, Timm M, Melhem M, George D, Lu G, Ghobrial J, Alsayed Y, Zeismer S, Cabanela M, Nehme A, Jia X, Moreau AS, Treon SP, Fonseca R, Gertz MA, Anderson KC, Witzig TE, Ghobrial IM. Proteomic analysis of waldenstrom macroglobulinemia. Cancer Res. 2007;67(8):3777–84. doi: 10.1158/0008-5472.CAN-06-3089. [DOI] [PubMed] [Google Scholar]
- Hubbard BM, Fenton GW, Anderson JM. A quantitative histological study of early clinical and preclinical Alzheimer’s disease. Neuropathol Appl Neurobiol. 1990;16(2):111–21. doi: 10.1111/j.1365-2990.1990.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. Journal of Neuropathology & Experimental Neurology. 1997;56:1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, Troncoso JC. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009;73(9):665–73. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono D, O’Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC. Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol. 2008;67(6):578–89. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118(1):53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Recent advances in our understanding of neurodegeneration. J Neural Transm. 2009;116(9):1111–62. doi: 10.1007/s00702-009-0240-y. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Soman S, Bradley MA. Oxidatively modified nucleic acids in preclinical Alzheimer’s disease (PCAD) brain. Mech Ageing Dev. 2011;132(8–9):443–8. doi: 10.1016/j.mad.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubartseva G, Smith JL, Markesbery WR, Lovell MA. Alterations of zinc transporter proteins ZnT-1, ZnT-4 and ZnT-6 in preclinical Alzheimer’s disease brain. Brain Pathol. 2010;20(2):343–50. doi: 10.1111/j.1750-3639.2009.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mitchell JC, Perkinton MS, Yates DM, Lau KF, Rogelj B, Miller CC, McLoughlin DM. Expression of the neuronal adaptor protein X11alpha protects against memory dysfunction in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;20(1):31–6. doi: 10.3233/JAD-2009-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TW, Nissanov J, Han LY, Mufson EJ, Schneider JA, Cochran EJ, Bennett DA, Lee VM, Trojanowski JQ, Arnold SE. Novel method to quantify neuropil threads in brains from elders with or without cognitive impairment. J Histochem Cytochem. 2000;48(12):1627–38. doi: 10.1177/002215540004801206. [DOI] [PubMed] [Google Scholar]
- Mochizuki A, Peterson JW, Mufson EJ, Trapp BD. Amyloid load and neural elements in Alzheimer’s disease and nondemented individuals with high amyloid plaque density. Exp Neurol. 1996;142(1):89–102. doi: 10.1006/exnr.1996.0181. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999;158(2):469–90. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, Iacono D, Riudavets MA, Driscoll I, Price DL, Martin LJ, Troncoso JC. Neuropathologic Studies of the Baltimore Longitudinal Study of Aging (BLSA) J Alzheimers Dis. 2009;18(3):665–75. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr. 2008;13(1):45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026–36. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7(2):103–17. doi: 10.3233/jad-2005-7203. discussion 73–80. [DOI] [PubMed] [Google Scholar]
- Riudavets MA, Iacono D, Resnick SM, O’Brien R, Zonderman AB, Martin LJ, Rudow G, Pletnikova O, Troncoso JC. Resistance to Alzheimer’s pathology is associated with nuclear hypertrophy in neurons. Neurobiol Aging. 2007;28(10):1484–92. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ. Java Treeview--extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–8. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Scales TM, Derkinderen P, Leung KY, Byers HL, Ward MA, Price C, Bird IN, Perera T, Kellie S, Williamson R, Anderton BH, Reynolds CH. Tyrosine phosphorylation of tau by the SRC family kinases lck and fyn. Mol Neurodegener. 2011;6:12. doi: 10.1186/1750-1326-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55(3):370–6. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Soetanto A, Wilson RS, Talbot K, Un A, Schneider JA, Sobiesk M, Kelly J, Leurgans S, Bennett DA, Arnold SE. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry. 2010;67(5):448–57. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(8):933–44. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968;7(2):331–56. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- Tyas SL, Salazar JC, Snowdon DA, Desrosiers MF, Riley KP, Mendiondo MS, Kryscio RJ. Transitions to mild cognitive impairments, dementia, and death: findings from the Nun Study. Am J Epidemiol. 2007;165(11):1231–8. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2011;8(1 Suppl):S1–S68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25(9):1205–12. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- White L. Brain Lesions at Autopsy in Older Japanese-American Men as Related to Cognitive Impairment and Dementia in the Final Years of Life: A Summary Report from the Honolulu-Asia Aging Study. J Alzheimers Dis. 2009;18(3):713–25. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: overview and change in cognitive and motor speed. Aging Neuropschol Cogn. 2004;11:280–303. [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–8. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Yang YH, Paquet A, Dudoit S. marray: Exploratory analysis for two-color spotted microarray data. R package version 130. 2007 http://www.bioconductor.org/packages/release/bioc/html/marray.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.